Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Corina Aurelia Zugravu | + 2183 word(s) | 2183 | 2022-02-08 03:37:18 | | | |
| 2 | Vivi Li | Meta information modification | 2183 | 2022-02-08 09:40:42 | | | | |
| 3 | Vivi Li | Meta information modification | 2183 | 2022-02-08 09:41:09 | | | | |
| 4 | Vivi Li | Meta information modification | 2183 | 2022-02-10 11:47:14 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zugravu, C. Antioxidants in Hops. Encyclopedia. Available online: https://encyclopedia.pub/entry/19196 (accessed on 07 February 2026).
Zugravu C. Antioxidants in Hops. Encyclopedia. Available at: https://encyclopedia.pub/entry/19196. Accessed February 07, 2026.
Zugravu, Corina. "Antioxidants in Hops" Encyclopedia, https://encyclopedia.pub/entry/19196 (accessed February 07, 2026).
Zugravu, C. (2022, February 08). Antioxidants in Hops. In Encyclopedia. https://encyclopedia.pub/entry/19196
Zugravu, Corina. "Antioxidants in Hops." Encyclopedia. Web. 08 February, 2022.
Copy Citation
Hop plant (Humulus lupulus L.) has been used by humans for ages, presumably first as a herbal remedy, then in the manufacturing of different products, from which beer is the most largely consumed. Female hops cones have different useful chemical compounds, an important class being antioxidants, mainly polyphenols.
hops
antioxidants
metabolic syndrome
bioavailability
1. Introduction
Hops Humulus Lupulus Linnaeus is a plant known and cultivated by humans for a very long time. Early evidence links its origin to ancient China, later found in most temperate areas of the world [1]. Though today we associate hops with beer production, and this was true for centuries, its initial use has been different. Hops were often used as medicinal plants in the popular pharmacopoeia, designed to relieve the symptoms of a large number of health problems [2]. It has been attributed anti-inflammatory and antimicrobial properties, as well as diuretic, digestive, sedative, progestogenic properties, even being considered a cure for insomnia. For this wide range of health benefits, it was regarded as a life prolonging plant [3].
The main component of the hops used today is the cone, the female inflorescence of the plant, but other areas of the hops plant also prove to be useful (the leaves, stems or rhizome). There are several bioactive molecules that underlie the sanogenetic effects of the plant [1][4][5]. For example, in the cone glands, the primary metabolites are the bitter resins and the aromatics. The secondary substances belong to three categories of substances: resins, oils and polyphenols (4–14% dried weight) [6][7] (Figure 1).
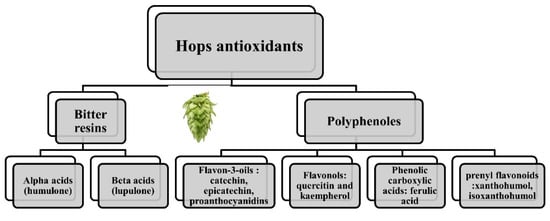
Figure 1. Antioxidants in hops.
There are two types of bitter resins: alpha acids (mainly humulone) and beta acids (mainly lupulone). As for polyphenols, they can be classified into: flavonols (quercitin and kaempherol), flavon-3-oils (the main ones being catechin, epicatechin, proanthocyanidins), phenolic carboxylic acids (ferulic acid) and, in lesser quantities, prenyl flavonoids such as xanthohumol (0.1–1% on dry weight) [8][9] and isoxanthohumol. Their very small quantities do not necessary preclude a biological effect. For example, isoxanthohumol is transformed in the intestine into prenylnaringenin, a phytoestrogen uptaken by the intestinal cell. Depending on the individual microbiota, it was estimated that one third of the population could produce high enough levels to reach the threshold for a biological effect [10].
2. Bioavailability of the Active Substances
As for all foods, bioavailability is crucial when evaluating the practical consequences of hops components. Studies show that the bioavailability is rather low [11][12], and ways for increasing it were developed. One alternative is prenylation, which increases the biological activity of dietary flavonoids by enhancing their affinity for estrogen receptors, facilitate the interaction with cellular membranes and proteins. As a consequence, they tend to remain for longer time in certain target cells and have greater antioxidant effect [13]. The bioavailability is increased in the presence of the prenyl group, which decreases the polarity of flavonoids. Degradation of xanthohumol in the intestine is also influenced by the prenyl group. The presence of this group slows down the degradation of xanthohumol [13][14].
The bioavailability of prenylflavonoids is not only influenced by the presence or absence of functional groups. The human microbiota, as stated above, greatly influences the resulting metabolites (Figure 2), which may be of pharmaceutical importance as well. Numerous studies have been performed to identify them, and less to assess their bioavailability.
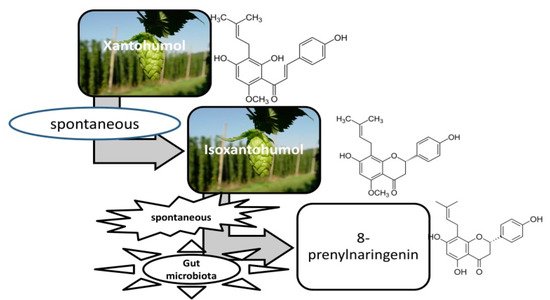
Figure 2. Transformation of xantohumol in the digestive system.
Depending on the predominant microorganisms in microbiota, the metabolic products may be different, with an activity similar or different to that of the initial substances [15].
Xanthohumol, the main substance studied among the many components of hops, has a low bioavailability to oral administration, which limits its sanogenetic effects [12]. Nookandeh et al. showed that more than 80% of the products of the intestinal degradation of the orally administered xanthohumol to laboratory animals was excreted in feces [16]. Similar results were found by Avula et al. who concluded that most of the administered xanthohumol is excreted in feces in the 24 hours after administration. Studies conducted on Caco-2 cells showed that xanthohumol accumulates in the cytosol, binds to the cellular proteins [17] and even has the ability to insert inside the lipid bilayer of cell membranes, which makes a rapid transmembrane transfer very difficult [18][19][20].
Novak et al. investigated the pharmacokinetics and bioavailability of pure xantohumiol or a hops extract, comparing the oral with the iv routes in rats. There were no differences due to sex, but the oral absorption was very low compared with the iv route. The probability of a hepato-entero-hepatic recirculation of the compounds was also postulated, based on the peaks of plasma levels after ingestion [21].
A blinded randomized study with different doses of xanthohumol given orally showed similarities with the animal experiments. This study highlighted that plasma xanthohumol significantly increases only after more than 60 mg. Two peaks in plasma xanthohumol (as observed in animal experiments), 1 h and 4 h after administration, were observed; the half time was around 20 h [22].
Low bioavailability was noted also for Iso-α-acids (IAA) and the reduced derivates, dihydro-iso-α-acids (DHIAA) and tetrahydro-iso-α-acids (THIAA). However, reduced metabolites had a higher bioavailability [23].
Cattoor et al. also carried out an analysis of the absorption of bitter acids from hops through Caco-2 monolayers. Membrane permeability was higher for alpha than for beta acids. Consequently, oral administration of beta acids had a low absorption, probably limited by the active transporters of P-gp and MRP-2 efflux and by phase II metabolism [24].
As low bioavailability is common to many plant bioactive substances, new forms of administration and new additives are being tested to improve or address this issue in the near future.
3. Hops Antioxidants and Cancer
Hops substances and cancer have been extensively researched during time and from the multitude of components, xanthohumol was frequently under scrutiny.
The substance was discovered in 1957 [25], its properties being brought to light only in the recent decades [26]. After ingestion, xanthohumol undergoes transformations, sometimes resulting in substances with a higher antioxidant power and a wider spectrum of action. It was noticed that xanthohumol is transformed by a non-enzymatic mechanism in isoxanthohumol and enzymatically, in 8 and 6-prenylnaringenin and in desmethylxanthohumol [27][28].
The positive action of xanthohumol in neoplasms was shown for tumors with various localizations, from lung or digestive system (colon, pancreas), to endocrine (thyroid) or genito-urinary (cervical, ovarian), head and neck, skin, malignant melanoma or leukemia [29][30].
The ways in which xanthohumol acts were the subject of a large number of studies are summarized by Jiang et al. in a 2018 article [29].
The action of xanthohumol is probably achieved by the inhibitory action on two signaling pathways with an essential role in maintaining the appearance of malignancy and in achieving metastases: Akt and NF-κB. Xanthohumol induces apoptosis of cancer cells by stimulating pro-apoptotic proteins (Bax, PARP, AIF- caspase-3, -8, -9) and by inhibiting proliferation, achieved by inhibiting Notch1, mTOR, STAT3. Xanthohumol also acts on the migration and invasion of neoplastic cells, probably by inhibiting FAK and MMP-2 expression [29]. Moreover, xanthohumol seems to act synergistically with the usual chemotherapeutic treatments, which is certainly a positive factor because it could theoretically allow for a decrease in the doses administered. Jiang et al. note that xanthohumol influences various proteins that bear upon proliferation, migration, invasion, apoptosis or resistance to many chemotherapeutics in cancer patients, without the exact mechanisms being known at this time.
Girisa et al. conclude that hops have an important anticancer potential, acting by modifying both signaling pathways (Akt, AMPK, ERK, IGFBP2, NF-κB, and STAT3), and by modulating proteins (Notch1, caspases, MMPs, Bcl-2, cyclin D1), oxidative stress markers, miRNAs and tumor suppressor proteins [31].
A very interesting aspect is related to the antioxidant action of xanthohumol. It is known that in the etiology of neoplasms, from cell proliferation to local invasion and metastases, oxidative stress is involved, on the regulation of which many oncological treatments are based. It is just that the accumulation of free radicals is not only a cause, but also a factor that determines the sensitivity to treatment [32], many chemotherapeutic agents acting precisely to stimulate the formation of free radicals in neoplastic cells [33]. In this context, it was shown that, although it has a high antioxidant power, xanthohumol also induces an important production of free radicals in various neoplastic cell lines, causing their apoptosis [34][35][36] (Figure 3).
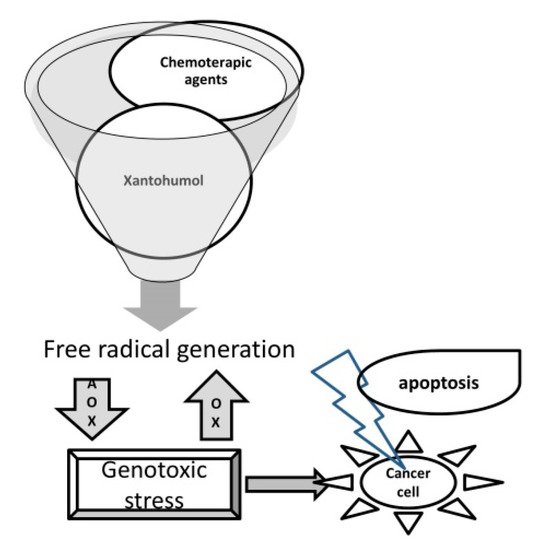
Figure 3. The oxidative stress: a common mechanism of action of xantohumol and chemotherapeutics on cancer cells.
Wei et al. concluded that xanthohumol also causes an imbalance between intracellular antioxidants, such as SOD, and the production of free radicals, which potentiates xanthohumol’s apoptotic and antimetastatic, antiproliferative action. High levels of free radicals, which are easily reached by depressing cellular antioxidant systems, reduce NF-κB activity, leading to cell death [37].
Xanthohumol stimulated the anticancer actions of high ROS by the NF-κB signaling pathway. Future studies should show the extent to which classical neoplastic treatments could benefit from the associated administration of xanthohumol products in clinical practice.
An important activity of xanthohumol is related to metastasis formation. After xanthohumol proved to be effective in pulmonary adenocarcinoma, Sławińska-Brych et al. also confirmed its effectiveness in inhibiting metastatic processes. The mechanisms involved in the antiinvasive action appear to be the inhibition of the ERK/MAPK pathway and the suppression of FAK and PI3/AKT signaling [38].
Other mechanisms seem to be common to xanthohumol, iso-xanthohumol and 8-prenylnaringenin, as antioxidant and anticancer agents. They refer to the action on Aldose reductase (AKR1B1) and a counterpart, AKR1B10. Both substances are overexpressed in neoplasms with various localizations, from lung and prostate, to breast. This makes them potential treatment targets. Seliger et al. showed that xanthohumol, iso-xanthohumol and 8-prenylnaringenin substantially and indiscriminately inhibit B1 and B10 [39].
Recently, new studies reveal the action of xanthohumol in neoplasms with localizations that had not been previously followed. One of these is the stomach. Gastric cancer is a form of cancer with a high incidence and a very poor prognosis. Wei et al.’s 2018 study confirms that xanthohumol has a deleterious effect on gastric cancer cells, but not on healthy gastric cells. Xanthohumol inhibits proliferation, induces apoptosis and reduces cancer cell metastases, most likely by free radical overproduction [36].
An interesting subject could be the effects of xanthohumol in colorectal cancer, from the perspective of the previously mentioned reduced bioavailability, which leaves most of the ingested substance in contact with the terminal areas of the digestive system. Scagliarini et al. show that xanthohumol, applied on cancer cell cultures, can activate cell cycle disruptions, even in non-toxic concentrations, by reducing cyclins A and B and concomitantly increasing cyclin E [40]. Apoptosis was found in some of the most resistant cell lines 48 h after the debut of treatment with xanthohimol, and their sensitization to the action of usual chemotherapeutics was also noticed. Xanthumol activated p53 and p21, which recommends the substance as a pre-treatment of chemotherapy, not only to increase its effectiveness, but also to reduce the therapeutical dose and, consequently, the side effects. Taking into account the conclusions of Ambroz’s study [41], which reports antagonistic effects on concomitant administration of xanthohumol/chemotherapy, it was concluded that administration should be staged, in order to avoid decreasing the efficacy of the chemotherapeutics. It should be noted that other studies confirmed the cytotoxic action of other prenylflavonoids in hops on colonic proliferative cells, but xanthohumol had the most active contribution [42].
Harish et al. [11] summarized the mechanisms in which xanthohumol exerts its antineoplastic action in vitro, as follows: induction or inhibition of apoptosis, modulator of oncogenic signaling pathways, the free radical formation, and the ERK1/2, NF-κB, and Akt signals. The major courses of action differ after the location of the cancer. For example, in breast cancer it acts by decreasing the expression of Notch1, survivin and Ki-67, and by potentiating the expression of capsase 3, simultaneously inhibiting STAT3 and MDR 1. Another neoplastic localization in which xanthohumol was effective is the prostate, where it induced apoptosis via TNF-Related Apoptosis Inducing Ligand as well as by depolarizing mitochondria by activating procaspases 3, 8 and 9. One has to take into consideration difficulty of in vivo assessments, especially due to the reduced bioavailability of oral administration.
Recently, minor components of the antioxidant profile of hops were highlighted and investigated, having synergistic effects and being sometimes more active than xanthohumol. One of the minor natural compounds is xanthohumol C, which inhibits mammary neoplastic cell lines more intensely than xanthohumol or xanthumol-enriched hops extract. The mechanism of action of xanthohumol C appears to be different from that of xanthohumol; it seems to be involved in endoplasmic reticulum stress and in altering the adhesion of cells to each other [43]. Xanthohumol C is a chalcone with various types of action, including cytotoxic [44] and antioxidant [7][45] manifested in the context of neoplasms. Xanthohumol C was described by Stevens et al. [46] and due to difficulties in obtaining pure substances and, consequently, to the interference of other minor compounds, they noticed a lower antiproliferative action in certain neoplastic cell lines (breast, colon, prostate) than the one of xanthohumol [44].
Other hops substances were studied in relation to neoplasms, especially the bitter acids. Humulon shows anticancer and anti-inflammatory effects on skin cancer [47]. Lupulon activates apoptotic pathways including apoptotic TRAIL-receptors, in human cancer colon cells and in the equivalent metastatic cells, even in TRAIL resistant cancer cells [48][49]. Both substances show promising effects for cancer prophylaxis and treatment.
References
- Zanoli, P.; Zavatti, M. Pharmacognostic and pharmacological profile of Humulus lupulus L. J. Ethnopharmacol. 2008, 116, 383–396.
- Koetter, U.; Biendl, M. Hops (Humulus lupulus): A Review of its Historic and Medicinal Uses. HerbalGram 2010, 87, 44–57.
- Dimpfel, W.; Suter, A. Sleep improving effects of a single dose administration of a valerian/hops fluid extract—A double blind, randomized, placebo-controlled sleep-EEG study in a parallel design using electrohypnograms. Eur. J. Med. Res. 2008, 13, 200–204.
- Muzykiewicz, A.; Nowak, A.; Zielonka-Brzezicka, J.; Florkowska, K.; Duchnik, W.; Klimowicz, A. Comparison of antioxidant activity of extracts of hop leaves harvested in different years. Herba Pol. 2019, 65, 1–9.
- Astray, G.; Gullón, P.; Gullón, B.; Munekata, P.E.; Lorenzo, J.M. Humulus lupulus L. as a natural source of functional biomolecules. Appl. Sci. 2020, 10, 5074.
- Steenackers, B.; De Cooman, L.; De Vos, D. Chemical transformations of characteristic hop secondary metabolites in relation to beer properties and the brewing process: A review. Food Chem. 2015, 172, 742–756.
- Nikolić, D.; van Breemen, R.B. Analytical methods for quantitation of prenylated flavonoids from hops. Curr. Anal. Chem. 2013, 9, 71–85.
- Stevens, J.F.; Page, J.E. Xanthohumol and related prenylflavonoids from hops and beer: To your good health! Phytochemistry 2004, 65, 1317–1330.
- Roehrer, S.; Behr, J.; Stork, V.; Ramires, M.; Médard, G.; Frank, O.; Kleigrewe, K.; Hofmann, T.; Minceva, M. Xanthohumol C, a minor bioactive hop compound: Production, purification strategies and antimicrobial test. J. Chromatogr. B 2018, 1095, 39–49.
- Possemiers, S.; Bolca, S.; Grootaert, C.; Heyerick, A.; Decroos, K.; Dhooge, W.; De Keukeleire, D.; Rabot, S.; Verstraete, W.; Van de Wiele, T. The prenylflavonoid isoxanthohumol from hops (Humulus lupulus L.) is activated into the potent phytoestrogen 8-prenylnaringenin in vitro and in the human intestine. J Nutr. 2006, 136, 1862–1867.
- Harish, V.; Haque, E.; Śmiech, M.; Taniguchi, H.; Jamieson, S.; Tewari, D.; Bishayee, A. Xanthohumol for Human Malignancies: Chemistry, Pharmacokinetics and Molecular Targets. Int. J. Mol. Sci. 2021, 22, 4478.
- Venturelli, S.; Burkard, M.; Biendl, M.; Lauer, U.M.; Frank, J.; Busch, C. Prenylated chalcones and flavonoids for the prevention and treatment of cancer. Nutrition 2016, 32, 1171–1178.
- Mukai, R. Prenylation enhances the biological activity of dietary flavonoids by altering their bioavailability. Biosci. Biotechnol. Biochem. 2018, 82, 207–215.
- Wunderlich, S.; Zürcher, A.; Back, W. Enrichment of xanthohumol in the brewing process. Mol. Nutr. Food Res. 2005, 49, 874–881.
- Paraiso, I.L.; Plagmann, L.S.; Yang, L.; Zielke, R.; Gombart, A.F.; Maier, C.S.; Sikora, A.E.; Blakemore, P.R.; Stevens, J.F. Reductive Metabolism of Xanthohumol and 8-Prenylnaringenin by the Intestinal Bacterium Eubacterium ramulus. Mol. Nutr. Food Res. 2019, 63, e1800923.
- Nookandeh, A.; Frank, N.; Steiner, F.; Ellinger, R.; Schneider, B.; Gerhäuser, C.; Becker, H. Xanthohumol metabolites in faeces of rats. Phytochemistry 2004, 65, 561–570.
- Pang, Y.; Nikolic, D.; Zhu, D.; Chadwick, L.R.; Pauli, G.F.; Farnsworth, N.R.; van Breemen, R.B. Binding of the hop (Humulus lupulus L.) chalcone xanthohumol to cytosolic proteins in Caco-2 intestinal epithelial cells. Mol. Nutr. Food Res. 2007, 51, 872–879.
- Arczewska, M.; Kamiński, D.M.; Górecka, E.; Pociecha, D.; Rój, E.; Sławińska-Brych, A.; Gagoś, M. The molecular organization of prenylated flavonoid xanthohumol in DPPC multibilayers: X-ray diffraction and FTIR spectroscopic studies. Biochim. Biophys. Acta 2013, 1828, 213–222.
- Wesołowska, O.; Gąsiorowska, J.; Petrus, J.; Czarnik-Matusewicz, B.; Michalak, K. Interaction of prenylated chalcones and flavanones from common hop with phosphatidylcholine model membranes. Biochim. Biophys. Acta 2014, 1838, 173–184.
- Avula, B.; Ganzera, M.; Warnick, J.E.; Feltenstein, M.W.; Sufka, K.J.; Khan, I.A. High-performance liquid chromatographic determination of xanthohumol in rat plasma, urine, and fecal samples. J. Chromatogr. Sci. 2004, 42, 378–382.
- Nowak, B.; Poźniak, B.; Popłoński, J.; Bobak, Ł.; Matuszewska, A.; Kwiatkowska, J.; Dziewiszek, W.; Huszcza, E.; Szeląg, A. Pharmacokinetics of xanthohumol in rats of both sexes after oral and intravenous administration of pure xanthohumol and prenylflavonoid extract. Adv. Clin. Exp. Med. 2020, 29, 1101–1109.
- Legette, L.; Karnpracha, C.; Reed, R.L.; Choi, J.; Bobe, G.; Christensen, J.M.; Rodriguez-Proteau, R.; Purnell, J.Q.; Stevens, J.F. Human pharmacokinetics of xanthohumol, an antihyperglycemic flavonoid from hops. Mol. Nutr. Food Res. 2014, 58, 248–255.
- Cattoor, K.; Remon, J.P.; Boussery, K.; Van Bocxlaer, J.; Bracke, M.; De Keukeleire, D.; Deforce, D.; Heyerick, A. Bioavailability of hop-derived iso-α-acids and reduced derivatives. Food Funct. 2011, 2, 412–422.
- Cattoor, K.O.; Bracke, M.; Deforce, D.; De Keukeleire, D.; Heyerick, A. Transport of hop bitter acids across intestinal Caco-2 cell monolayers. J. Agric. Food Chem. 2010, 58, 4132–4140.
- Verzele, M.; Stockx, J.; Fontijn, F.; Anteunis, M. Xanthohumol, a new natural chalkone. Bull. Sociétés Chim. Belg. 1957, 66, 452–475.
- Liu, M.; Yin, H.; Qian, X.; Dong, J.; Qian, Z.; Miao, J. Xanthohumol, a Prenylated Chalcone from Hops, Inhibits the Viability and Stemness of Doxorubicin-Resistant MCF-7/ADR Cells. Molecules 2016, 22, 36.
- Legette, L.; Ma, L.; Reed, R.L.; Miranda, C.L.; Christensen, J.M.; Rodriguez-Proteau, R.; Stevens, J.F. Pharmacokinetics of xanthohumol and metabolites in rats after oral and intravenous administration. Mol. Nutr. Food Res. 2012, 56, 466–474.
- Possemiers, S.; Heyerick, A.; Robbens, V.; De Keukeleire, D.; Verstraete, W. Activation of proestrogens from hops (Humulus lupulus L.) by intestinal microbiota; conversion of isoxanthohumol into 8-prenylnaringenin. J. Agric. Food Chem. 2005, 53, 6281–6288.
- Jiang, C.H.; Sun, T.L.; Xiang, D.X.; Wei, S.S.; Li, W.Q. Anticancer activity and mechanism of xanthohumol: A prenylated flavonoid from hops (Humulus lupulus L.). Front. Pharmacol. 2018, 9, 530.
- Bolton, J.L.; Dunlap, T.L.; Hajirahimkhan, A.; Mbachu, O.; Chen, S.N.; Chadwick, L.; Nikolic, D.; van Breemen, R.B.; Pauli, G.F.; Dietz, B.M. The Multiple Biological Targets of Hops and Bioactive Compounds. Chem. Res. Toxicol. 2019, 32, 222–233.
- Girisa, S.; Saikia, Q.; Bordoloi, D.; Banik, K.; Monisha, J.; Daimary, U.D.; Verma, E.; Ahn, K.S.; Kunnumakkara, A.B. Xanthohumol from Hop: Hope for cancer prevention and treatment. IUBMB Life 2021, 73, 1016–1044.
- Sosa, V.; Moliné, T.; Somoza, R.; Paciucci, R.; Kondoh, H.; Lleonart, M.E. Oxidative stress and cancer: An overview. Ageing Res. Rev. 2013, 12, 376–390.
- Afzal, S.; Jensen, S.A.; Sørensen, J.B.; Henriksen, T.; Weimann, A.; Poulsen, H.E. Oxidative damage to guanine nucleosides following combination chemotherapy with 5-fluorouracil and oxaliplatin. Cancer Chemother. Pharmacol. 2012, 69, 301–307.
- Zhang, B.; Chu, W.; Wei, P.; Liu, Y.; Wei, T. Xanthohumol induces generation of reactive oxygen species and triggers apoptosis through inhibition of mitochondrial electron transfer chain complex I. Free Radic. Biol. Med. 2015, 89, 486–497.
- Festa, M.; Capasso, A.; D’Acunto, C.W.; Masullo, M.; Rossi, A.G.; Pizza, C.; Piacente, S. Xanthohumol induces apoptosis in human malignant glioblastoma cells by increasing reactive oxygen species and activating MAPK pathways. J. Nat. Prod. 2011, 74, 2505–2513.
- Wei, S.; Sun, T.; Du, J.; Zhang, B.; Xiang, D.; Li, W. Xanthohumol, a prenylated flavonoid from Hops, exerts anticancer effects against gastric cancer in vitro. Oncol. Rep. 2018, 40, 3213–3222.
- Fouani, L.; Kovacevic, Z.; Richardson, D.R. Targeting Oncogenic Nuclear Factor Kappa B Signaling with Redox-Active Agents for Cancer Treatment. Antioxid. Redox Signal. 2019, 30, 1096–1123.
- Sławińska-Brych, A.; Mizerska-Kowalska, M.; Król, S.K.; Stepulak, A.; Zdzisińska, B. Xanthohumol Impairs the PMA-Driven Invasive Behaviour of Lung Cancer Cell Line A549 and Exerts Anti-EMT Action. Cells 2021, 10, 1484.
- Seliger, J.M.; Misuri, L.; Maser, E.; Hintzpeter, J. The hop-derived compounds xanthohumol, isoxanthohumol and 8-prenylnaringenin are tight-binding inhibitors of human aldo-keto reductases 1B1 and 1B10. J. Enzym. Inhib. Med. Chem. 2018, 33, 607–614.
- Scagliarini, A.; Mathey, A.; Aires, V.; Delmas, D. Xanthohumol, a Prenylated Flavonoid from Hops, Induces DNA Damages in Colorectal Cancer Cells and Sensitizes SW480 Cells to the SN38 Chemotherapeutic Agent. Cells 2020, 9, 932.
- Ambroz, M.; Lnenickova, K.; Matouskova, P.; Skalova, L.; Bousova, I. Antiproliferative Effects of Hop-derived Prenylflavonoids and Their Influence on the Efficacy of Oxaliplatine, 5-fluorouracil and Irinotecan in Human ColorectalC Cells. Nutrients 2019, 11, 879.
- Lněničková, K.; Šadibolová, M.; Matoušková, P.; Szotáková, B.; Skálová, L.; Boušová, I. The Modulation of Phase II Drug-Metabolizing Enzymes in Proliferating and Differentiated CaCo-2 Cells by Hop-Derived Prenylflavonoids. Nutrients 2020, 12, 2138.
- Roehrer, S.; Stork, V.; Ludwig, C.; Minceva, M.; Behr, J. Analyzing bioactive effects of the minor hop compound xanthohumol C on human breast cancer cells using quantitative proteomics. PLoS ONE 2019, 14, e0213469.
- Popłoński, J.; Turlej, E.; Sordon, S.; Tronina, T.; Bartmańska, A.; Wietrzyk, J.; Huszcza, E. Synthesis and Antiproliferative Activity of Minor Hops Prenylflavonoids and New Insights on Prenyl Group Cyclization. Molecules 2018, 23, 776.
- Forino, M.; Pace, S.; Chianese, G.; Santagostini, L.; Werner, M.; Weinigel, C.; Rummler, S.; Fico, G.; Werz, O.; Taglialatela-Scafati, O. Humudifucol and Bioactive Prenylated Polyphenols from Hops (Humulus lupulus cv. “Cascade”). J. Nat. Prod. 2016, 79, 590–597.
- Stevens, J.F.; Taylor, A.W.; Nickerson, G.B.; Ivancic, M.; Henning, J.; Haunold, A.; Deinzer, M.L. Prenylflavonoid variation in Humulus lupulus: Distribution and taxonomic significance of xanthogalenol and 4’-O-methylxanthohumol. Phytochemistry 2000, 53, 759–775.
- Yasukawa, K.; Takeuchi, M.; Takido, M. Humulon, a bitter in the hop, inhibits tumor promotion by 12-O-tetradecanoylphorbol-13-acetate in two-stage carcinogenesis in mouse skin. Oncology 1995, 52, 156–158.
- Lamy, V.; Roussi, S.; Chaabi, M.; Gossé, F.; Lobstein, A.; Raul, F. Lupulone, a hop bitter acid, activates different death pathways involving apoptotic TRAIL-receptors, in human colon tumor cells and in their derived metastatic cells. Apoptosis Int. J. Program Cell Death 2008, 13, 1232–1242.
- Lamy, V.; Bousserouel, S.; Gossé, F.; Minker, C.; Lobstein, A.; Raul, F. Lupulone triggers p38 MAPK-controlled activation of p53 and of the TRAIL receptor apoptotic pathway in human colon cancer-derived metastatic cells. Oncol. Rep. 2011, 26, 109–114.
More
Information
Subjects:
Nutrition & Dietetics; Food Science & Technology; Others
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
4 times
(View History)
Update Date:
10 Feb 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




