Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sanaz Behtaj | + 3749 word(s) | 3749 | 2022-01-27 04:40:37 | | | |
| 2 | Camila Xu | + 2 word(s) | 3751 | 2022-02-09 01:49:51 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Behtaj, S. Nerve Guidance Conduits. Encyclopedia. Available online: https://encyclopedia.pub/entry/19191 (accessed on 07 February 2026).
Behtaj S. Nerve Guidance Conduits. Encyclopedia. Available at: https://encyclopedia.pub/entry/19191. Accessed February 07, 2026.
Behtaj, Sanaz. "Nerve Guidance Conduits" Encyclopedia, https://encyclopedia.pub/entry/19191 (accessed February 07, 2026).
Behtaj, S. (2022, February 08). Nerve Guidance Conduits. In Encyclopedia. https://encyclopedia.pub/entry/19191
Behtaj, Sanaz. "Nerve Guidance Conduits." Encyclopedia. Web. 08 February, 2022.
Copy Citation
Nerve guidance conduits (NGCs) are tubular biostructures to bridge nerve injury sites via orienting axonal growth in an organized fashion as well as supplying a supportively appropriate microenvironment.
fibrous scaffold
neural tissue engineering
structural support
PNS
1. Introduction
Nerve guidance conduits (NGCs) are tubular biostructures with engineered biomaterials developed to supply a nourishing and supportive microenvironment for nerve regeneration and to orient axonal growth in a correct path across the nerve injury site [1][2]. NGCs have been used to supply various biochemical and physical factors, including neurotrophic factors, extracellular matrix (ECM) proteins, anisotropic gradients and diverse types of supporting cells in a range of in-vitro and in-vivo models [1]. Due to the ability to engineer NGCs with many different parameters, materials and cells, NGCs offer the ability to be customized to suit each individual injury site and patient variations [3][1]. However, successful implementation of NGCs calls for a fundamental consideration of their structural design, such as a three-dimensional (3D) fibrillar network structure and topographic cues that are capable of up-and downregulation of cell-conduit interactions in the ECM environment [4]. In particular, NGCs with similar morphological, physical, and mechanical properties to ECM might enhance their therapeutic efficacy. Additionally, the greatest potential lies in supplying NGCs with correctly selected biochemical, physical, and biological factors that can work synergistically with other nerve regeneration therapy strategies to activate the growth process [4], including the application of appropriate stimuli such as electrical stimulation [5].
2. Point to Consider for Developing Nerve Guidance Conduits
2.1. The Conditions of the Injury Site
The primary considerations in the treatment of nerve injuries are understanding the pathomechanisms behind nerve damage and associated soft tissue or vascular injuries [3]. The cellular and molecular responses to nerve injuries are dependent on many factors, particularly the location of the nerve, the time elapsed, and patient age [6]. With PNS injuries, physical trauma can lead to the death of the axons, demyelination of the supporting Schwann cells and production of myelin debris, and the infiltration of macrophages in response to changes in chemokine and cytokine secretion. Transplantation of NGCs into this hostile injury site must therefore consider that considerable cellular debris exists, and that the molecular environment may not be conducive to supporting growth of transplanted cells [7][8]. However, due to the numerous bioengineering characteristics that can be used, it is possible to design NGCs that can modulate various aspects of the injury site and thereby enhance integration and regeneration.
2.2. General NGC Requirements
From a clinical research standpoint, there are numerous physical characteristics that need to be considered. The NGC needs to be flexible so that it can be easily handled by surgeons during implantation without excessive manipulation or damage to the surrounding tissue. After implantation, the NGC needs to be non-toxic/biocompatible with the cellular environment and not stimulate any mutagenic, carcinogenic or cytotoxic behaviour, and be minimally immunogenic so that it does not exacerbate inflammatory responses [9]. The NGC needs to be permeable to some degree to facilitate fluid exchange but should have minimal swelling. Ultimately, the NGC should be biodegradable so that it does not require surgical removal. However, regulating the degradation rate is critical as the outcome of conduit transplantation can be negatively affected by too slow or too rapid a degradation process [10]. If the degradation occurs too quickly, then the regenerating axons may fail to cross the injury site, and if the degradation takes too long, then the remaining components of the NGC may hinder the endogenous cell reorganization and regeneration. In order to obtain the optimized degradation rate, various chemical and physical properties, including molecular weight, chemical structure, degree of crystallinity of the applied biomaterials, have to be considered. Additionally, the degradation rate could be influenced by the morphological structure features of the NGCs such as size, shape, density, and porosity, to name but a few [3][2][10][11]. As such, the optimum balance of the aforementioned features should be taken into consideration for the successful application of NGC in nerve regeneration therapy.
The mechanical properties of NGC also play a key role in the repair of nerve gaps. Some of the mechanical roles of an NGC is to create a barrier to protect new axons from the encroaching scar tissue, to prevent the surrounding tissue compressing against the regenerating cells and to provide stable structural support until there is sufficient regeneration of the nerve. In addition, the NGC also has to resist tearing from sutures if they are used. As peripheral nerves are subject to stretching, compression, and shearing forces, the NGC must also be flexible and have the ability to withstand these forces [9][12]. To generate NGCs of appropriate strength and flexibility, the mechanical properties of the target area have to be estimated [13] and, therefore, special attention must then be given to the selection of materials so that they match the required mechanical characteristics of the target nerves.
Size mismatching is a major challenge associated with the use of autografts [14]. The use of NGC has been investigated as a solution since they can be fabricated into size-matched structures. A successful NGC design have to specific to recipients and requires a concise consideration of the location of the nerve repair and size of the nerve gap. NGC morphological structure is another important area affecting nerve regeneration. For example, a lower wall thickness increases neuroma formation [15]. Additionally, the optimal internal diameter allows the damaged nerve to grow without compression while inhibiting ingress of surrounding tissues [1]. Therefore, optimizing NGC design before translating requires further extensive investigations. In this regard, rapid prototyping methods are leading towards advances in customized and personalized NGC design [14]. All the aforementioned factors are basic requirements in creating NGCs with the ability to provide the appropriate micro-environment to withstand the numerous molecular, cellular, and physical challenges of nerve repair [3].
2.3. NGC Structure
The morphological structure of NGCs plays an essential role in the successful regeneration of the injured nerve. As axons need to extend through the NGC, it is important that the morphological structure has a well-aligned orientation but also has porosity to offer axons the ability to seek new paths that may be more appropriate. Porosity is also important for allowing nutrient exchange and determining the appropriate balance of porosity versus structural alignment is one of the challenges [4][2]; as with too much porosity, axons may wander too widely and form a neuroma, whereas, with too much alignment, axons may be forced into inappropriate directions and therefore fail to make functional connections. Perhaps the most useful morphology to replicate is that of ECM, which is composed of a nanoscale network of proteins and glycosaminoglycans. These networks can make a barrier between tissues and provide a supportive meshwork around the cells to supply cell anchorage [7]. Therefore, studies have tried to develop scaffolds with similar features to ECM at the nanoscale level.
The manufacturing method is critical, as it can influence the resultant structure. There have been considerable advances in the range of methods that can be used to manufacture NGCs, including freeze-drying or lyophilization (low-temperature dehydration process under a vacuum), self-assembly (a process of association of system’s pre-existing components into an ordered structure or pattern), solvent casting (a process of manufacturing by immersing a mould in polymer solution), gas foaming (a high-temperature process for the production of foam-based polymer scaffold), and 3D printing (a creation of three-dimensional compartment under computer control) [11]. Despite the advantages associated with some of these techniques, the majority of them are incapable of developing nanofibrous substrates that mimic ECM, which is composed of fibres ranging in diameter from 50 to 500 nm [9]. For example, self-assembly is not scalable, and control over fibre dimensions is challenging. The low interconnectivity of porosity, small pore size, and irregular porosity are the main drawbacks of both gas foaming and freeze drying. The low mechanical strength limits the 3D printed scaffolds [7]. Being able to produce scaffolds that have various nanoscale fibres that mimic ECM may have the potential to increase cell-to-scaffold interactions and increase nutrient exchange that can stimulate the regeneration of neurons and associated supporting cells [16].
Among the aforementioned fabrication methods, electrospinning has generated considerable advances due to its ability to produce fibres of various scales and which have a large surface area with a three-dimensional (3D) porous structure resembling the native ECM network [7]. As such, electrospinning is one of the most widely studied techniques in fabricating nanofiber conduits [17][18]. Electrospinning offers the advantages of being a simple, low cost, controllable, and well-established technique for fabricating various fibrous meshes in different forms. Due to its ability to regulate the directional flow, it can create fibres of different orientations such as random fibres, aligned fibres, 3D fibrous scaffold, and core-shell fibres [19]. It also can use a wide variety of materials to produce continuous fibres with a range of properties, particularly those that confer the desired mechanical properties [9].
The electrospinning process can generate the various electrospun fibres via controlling the properties of the solution, processing, and environmental factors such as humidity and temperature (See Figure 1) [7]. Numerous parameters can affect the production process, including the polymer molecular weight, concentration, viscosity, electrical conductivity, elasticity, polarity, and surface tension in polymer solutions. From a fabrication point of view, the feed flow rate, applied field of voltage, nozzle-to-collector distance, the geometry of round collector, and collector rotation speed have key roles in generating the structure and alignment of the nanofibrous biomaterials [7][20][21]. As such, the diameter and the morphology of electrospun nanofibers are affected by different properties of electrospinning solution, process, and environment [7]. For example, higher-molecular-weight polymers and polymer concentrations in electrospinning solute result in larger fibre diameters, owing to higher viscosity and surface tension in polymer solutions during electrospinning, whereas the increase of applied voltage at the nozzle and the temperature results in decreasing fibre diameter [7][20].
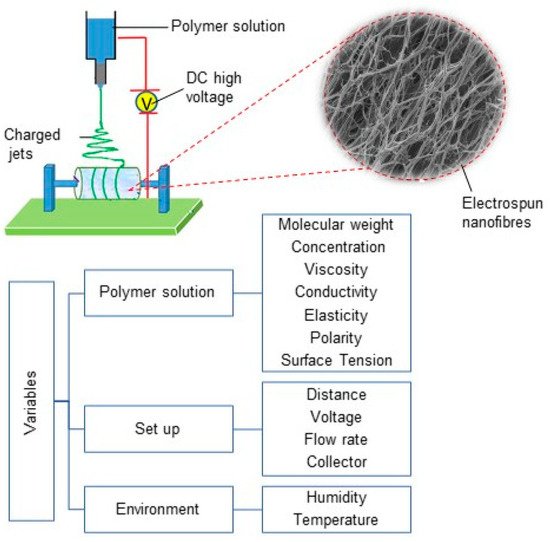
Figure 1. Electrospinning set-up diagram, electrospun nanofibres and different variables in electrospinning process.
In NGC fabrication, the repeatability and reproducibility of the synthesizing process is key. Although electrospinning has been demonstrated as a well-suited technique capable of producing desired nanofibers via modifying electrospinning process parameters, obtaining identical properties, such as pore size, porosity, and fibre direction, are still major challenges and require precise controlling over the fabrication process and ambient parameters [7][14][22].
2.4. Application of Electrospun Substrates for Repairing the Nervous System
Numerous nanofibrous scaffolds have been extensively used in neural regeneration applications. For example, Debski et al. examined a nanofiber-based nerve conduit composed of poly (L-lactic acid)-co-poly(caprolactone), collagen and polyaniline (PANI) in a rat model. This conduit presented muscle atrophy decrease and was suggested as a mean for axonal regeneration support and managing nerve gap as in peripheral nerve [23]. Polycaprolactone/collagen VI electrospun conduits were assessed by Lv et al. in a 15-mm-long sciatic nerve defect in rats and reported the sustained release of collagen VI enhanced the recruitment of macrophages and their polarization toward the pro-healing (M2) phenotype [24]. Another study of the application of nanofibrous in nerve conduits offered polyvinyl alcohol (PVA)/carbon nanotubes (CNT) electrospun films as a suitable material for nerve conduits [25]. In a recent work by Wu et al., two different fabrication methods, lyophilization and electrospinning, for preparing chitosan scaffolds were compared for Schwann-cell transplantation in rat models, and they reported the electrospun scaffolds more favourable for cell–cell interactions in PNS repair [26]. Table 1 lists recent studies to examine the application of electrospun nerve conduits for repairing the nervous system.
As previously mentioned, for successful application of electrospun scaffolds in NGC, these fibres should meet some morphological requirements, including appropriate diameter sizes and porosity distribution. The fibrous meshes obtained by electrospinning show a range of 100–1100 nm fibre diameter. Fibre diameter is known to have an impact on neural cell growth and Schwann-cell (SC) migration. To illustrate, a work by Wang et al. reported the neurite length on scaffolds with the intermediate and large fibres were higher than those with thinner fibres [27]. However, the optimum size has to be measured; for example, another study reported that Schwann cells presented lower elongation lengths on the fibrous scaffolds with average diameters of 5 and 8 µm compared with those with an average diameter of 1 µm [28]. It also reported that scaffolds with larger fibre diameters could allow increased cell penetration [7].
Table 1. Recent studies focusing on the use of electrospun nerve conduits for regenerating peripheral nerves.
| Biomaterial | Cells | ES Parameters | Stimulating Agents | Stimulating Patterns | In Vivo | Refs. | ||
|---|---|---|---|---|---|---|---|---|
| Voltage (kV) | Flow Rate (mL·h−1) | Distance (cm) | ||||||
| chitosan | Schwann cells | 4 | 3 | - | BDNF & VEGF | aligned fibres | sciatic nerve defects in rats | [29] |
| PLA/PPy | rat hippocampal progenitor | 15 | - | 10 | PPy-coating | external stimulus (200 mV/cm) | - | [30] |
| PCL/chitosan | Schwann cells, PC12 cells and dorsal root ganglia | 15 | 1.5 | - | - | aligned fibres | sciatic nerve in adult female Sprague–Dawley rats | [31] |
| PLCL | murine macrophage cell line and rat Schwann cells | 16 | 2 | 10 | - | oriented microfiber-bundle cores and randomly organized nanofiber in wall of NGC | rat sciatic nerve injury | [32] |
| PCL | Schwann cells | 14 | 0.2 | - | sodium alginate hydrogel covalently cross-linked with N,N′-disuccinimidyl carbonate (DSC) | bilayer cylindrical conduit | sciatic nerves in a rat model | [33] |
| polyvinyl alcohol (PVA)/carbon nanotubes (CNT) | fibroblasts | 19 and 21 | 0.06–0.08 | 10 | - | providing conductivity via CNT | - | [25] |
| poly (lactide-co-trimethylene carbonate) (PLATMC) | Schwann cells | - | - | - | - | shape memory nanofibers | rat sciatic nerve defects | [34] |
| poly (L/D-lactic acid) (PLDLA) and phosphate glass microfibers (PGFs) | dorsal root ganglion | 1.5 kV cm−1 | 0.1 mL min–1 | - | CNTs chemically attached on the surface of the NGC | - | transected rat sciatic nerve | [35] |
| PCL | bone marrow stem cells (BMSCs) | 12 | 1 | - | - | honeycomb structure | - | [36] |
| PLLA | dorsal root ganglion | 15 | 1 | 10 | porcine decellularized nerve matrix hydrogel | aligned fibres | rat sciatic nerve defect model | [37] |
| PCL | PC-12 | 11 | 0.25 | 5.5 | cross-linking laminin | aligned fibres | rat sciatic nerve gap | [38] |
| poly (lactic-co-glycolic acid) (PLGA) | - | 10 | 0.4 | 15 | collagen sponge | intraluminal sponge fillers | rat sciatic nerve | [39] |
| poly (L-lactic acid)-co-poly(€-caprolactone), collagen (COL), polyaniline (PANI) | adipose-derived stem cells (ASCs) | 15 | 1 | 8 | - | - | rat model | [23] |
| PCL/collagen VI | macrophages | 15 | 4 | 18 | sustained release of collagen VI | - | rat sciatic nerve | [25] |
| chitosan | Schwann cell | 15 | 1 | 10 | - | - | - | [26] |
| PVA/gelatin/gellan | neural cells | 19 | 0.8 | 15 | quercetin | patterned hybrid of aligned fibres scaffold | - | [40] |
Creating proper porosity size and distribution to allow entry of nutrients and waste removal and guide axonal growth is another important area in NGC design. It is reported that the NGC with high porosity (>80%) supplies enough permeability for the nutrient flows from the outside to the inside of the conduit leading to improved nerve regeneration and simultaneously preventing infiltration of unwanted tissue into the NGC [15]. However, worth to mentioning much uncertainty still exists about selecting a proper method that is able to produce precise measurements of the porosity through an electrospun mesh.
2.5. NGC Material
In addition to the nanofibrous structure of NGC, their functionality and effectiveness are strongly influenced by material selection. The definition of the American National Institute of Health (NIH) for biomaterial is “any substance or combination of substances, other than drugs, synthetic or natural in origin, which can be used for any period of time, which augments or replaces partially or totally any tissue, organ or function of the body, in order to maintain or improve the quality of life of the individual” [41].
The extensive research in tissue engineering has been indicated that correctly chosen biomaterials can support the integrity and regeneration of cells without inducing inflammation [42]. Materials of the NGC should support the physical, chemical, and biological environment surrounding the neural and glial cells [6]. Both natural and synthetic biopolymers have been used in NGC production. Natural polymers possess a diverse property reflective of naturally occurring tissues and are popular carriers in tissue engineering. For instance, collagen is an abundant structural protein of various connective tissues, and its desirable properties, such as high biocompatibility, make it a popular natural polymer for NGCs [19]. Chitosan, silk fibroin, and extracellular matrix components are other natural materials utilized for neural scaffolds [43]. However, there are several limitations with the application of natural biopolymers, including the variability of mechanical properties, low environmental stability, cell-mediated immune responses, and risk of infection. In contrast to natural biomaterials, synthetic ones can be designed so that they have higher mechanical strength and stiffness, and the fabrication process can be tightly controlled to ensure uniformity [44]. Silicone was one of the earliest synthetic biomaterials used for synthetic nerve conduits mostly due to its elasticity. However, poor nutrient transfer, fibrotic host response and the irritation at the site of surgery, as well as the need for scarred tissue removal, were the common drawbacks of the silicon-based devices [19][45].
A wide range of synthetic polymeric materials has been used in nerve conduit fabrication among which conduits constructed from aliphatic polyester-based polymers, such as polycaprolactone polylactic-co-glycolic acid (PLGA), polyglycolic acid (PGA), poly(ε-caprolactone) (PCL) and poly (DL-lactic acid –co-ε-caprolactone) (P(DLLAco-CL)) have been investigated thoroughly as promising candidates in some clinical trials [13]. However, for many synthetic biomaterials, a considerable drawback is that cells do not readily attach to the synthetic biomaterial, and inflammatory reactions to the biomaterial material can limit their use. In addition, biomaterials with high rigidity can cause mechanical trauma at the injury site, fistula formation, and extrusion, which drive the search for variations in structural properties to identify synthetic biomaterials with more flexibility. Considering that natural and synthetic biomaterials offer benefits in different ways, a solution can be the combination of natural and synthetic biomaterials to work synergistically to provide an optimal mix of biocompatibilities, desired mechanical properties and degradation patterns [19]. For example, the natural polymer in a composites scaffold can improve the biocompatibility and biodegradability of the mixture via providing a biochemical interaction between cells and the scaffold [46]. An alternative strategy is to add some natural polymers to the surface of synthetic scaffolds, which can aid cell attachment, since the upper-most surface of the scaffold can have a major impact on cell attachment [44].
2.6. NGC Surface
A surface of the scaffold that promotes the adhesion of cells is key in achieving functional cell/scaffold interactions. Numerous characteristics can impact the biochemical mechanisms and the properties of the surface to influence cell adhesion, including the topographical features, stiffness, functional groups, hydrophilic/hydrophobic properties, and interfacial free energy [7][44]. As such, changing the surface chemistry, including bioactive molecule immobilization, has been broadly applied on the biomaterial surface. For example, gelatine treatment of an electrospun poly (lactic-co-glycolic acid) (PLGA) conduit improved the adhesion of mouse embryonic stem cells [11]. Additionally, surface modification with polydopamine has been shown to improve the hydrophilicity and stability of the material surface, leading to effective cell growth, adhesion, proliferation, and differentiation relevant for applications for peripheral nerve regeneration [47]. Functionalizing with conductive compounds, crosslinking of nano bioglass, and nerve growth factor immobilization are other common chemical surface modification approaches [48].
Apart from providing chemical cues, cellular behaviours such as adhesion, migration and differentiation, as well as the regeneration of new tissues, can be influenced by topographical characteristics [49][50]. Cells’ receptor clustering or curvatures on the cell membrane can be affected by properties of the scaffold; therefore, imposed surface topography can have a huge impact on cellular responses, organization, and function, and have been reviewed in detail in the following reviews [6][51][52]. For example, the surface stiffness (or hardness) and the surface roughness have ability to affect the secretion of specific channel proteins from the cells leading to induce the desired signalling events and control neural cell development [4]. Thus, the topographical modification of the scaffold, such as changing the types, sizes, and spacing of surface patterns can alter surface energy improving the adsorption and bioactivation of the ECM proteins. In-depth consideration of the topographical modification effects on the nerve regeneration process is beyond the scope of this study. In this regard, the effect of topological structures in peripheral nerve repair was extensively reviewed by Ma et al. [53]. It can be concluded that providing proper contact guidance via chemical and topographical cues can be an important area of NGC design.
2.7. NGC Topographic Structures
To date, NGCs have been fabricated in a range of structures, such as cylindrical tubes with internal channels or intraluminal guidance, porous walls with electrospun outer conduits, or combinatorial techniques [11]. Earlier works fabricated NGCs with a single hollow shape to resemble the tube-like structure of nerves, which mainly fabricated by injection moulding, melt extrusion (a melted polymer is extruded within the nozzle), physical film rolling (a polymer mat is rolled around a mandrel, the edges of the roll are overlapped, sealed and compressed), braiding, and crosslinking (a cross-linking agent adds to a polymer mixture then loaded into a cylindrical mould) [2][14][43]. However, without internal architecture, regenerating axons were unable to navigate appropriately and often, the distribution of axons across the graft was limited with the result that the axons became misdirected and did not innervate their appropriate targets or the axons branched and innervated multiple targets [4][54]. These limitations led to the design of NGCs with an architecture that mimics the natural structure of nerve organization [12][43]. In this regard, multichannel nerve conduits have typically achieved better outcomes in axonal regeneration, compared to nerve conduits with a single lumen; as, with a higher internal surface area available, there is increased cell adhesion and migration and reduced axon dispersion [55]. A study focusing on developing NGC with a similar structure to the PNS anatomy designed a multi-tubular conduit made of electrospun polycaprolactone (PCL) fibres with a honeycomb structure seeded by BMSCs. They reported the BMSCs migrated and proliferated in all the small tubes and transdifferentiated into Schwann-like cells [36].
However, due to the multilayers or channels within these complex conduits, there are additional limitations that need to be overcome. Fluid permeability and nutrient exchange can be low, particularly in the internal regions, and the structural rigidity can be higher, which can increase cell death rates due to the lack of adequate nourishing sources and the cell metabolic waste removal [4][43].
To overcome some of the challenges faced in the implantation of the multichannel conduits, NGCs have been produced with physical lumen fillers: physical fillers, which fill the internal space of the nerve tube. A wide range of filler materials with a diverse geometrical property, such as hydrogel matrix (typically made of polysaccharides, ECM molecules, proteins, and peptides) and micro-/nanofilaments (fabricated via electrospinning and rapid prototyping) have been used as physical lumen fillers (See Figure 2) [56]. Among these fillers, microfilaments and nanofibers offer the possibility for resembling the nature structure of the nerve and have the potential to be used as filler material in the NGCs [57]. This generation of NGC represents a promising frontier in nerve repair, where neurites grow and orient with the incorporated luminal fillers. As the regenerating axons and supporting, cells can grow across and along all regions of the lumen filler, there is more opportunity for the axons to sort out and navigate according to axon guidance cues [43].
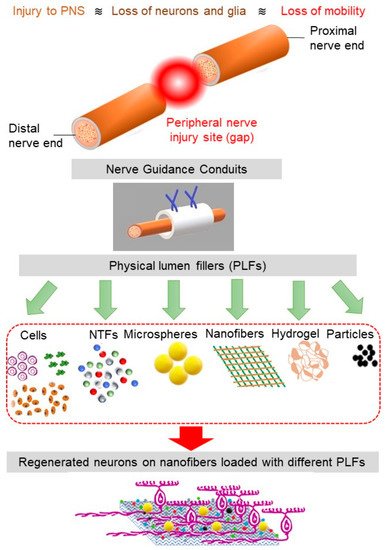
Figure 2. The schematics of PNS damage, NGC, and various physical lumen fillers (PLFs). The nanofibers loaded with the appropriate combination of PLFs facilitate cell regeneration.
References
- Stewart, C.E.; Kan, C.F.K.; Stewart, B.R.; Sanicola, H.W.; Jung, J.P.; Sulaiman, O.A.R.; Wang, D. Machine intelligence for nerve conduit design and production. J. Biol. Eng. 2020, 14, 1–19.
- Jahromi, M.; Razavi, S.; Bakhtiari, A. The advances in nerve tissue engineering: From fabrication of nerve conduit to in vivo nerve regeneration assays. J. Tissue Eng. Regen. Med. 2019, 13, 2077–2100.
- Chrząszcz, P.; Derbisz, K.; Suszyński, K.; Miodoński, J.; Trybulski, R.; Lewin-Kowalik, J.; Marcol, W. Application of peripheral nerve conduits in clinical practice: A literature review. Neurol. I Neurochir. Polska 2018, 52, 427–435.
- Behtaj, S. Neuron-fibrous scaffold interfaces in the peripheral nervous system: A perspective on the structural requirements. Neural Regen. Res. 2021.
- Zarrintaj, P.; Zangene, E.; Manouchehri, S.; Amirabad, L.M.; Baheiraei, N.; Hadjighasem, M.R.; Farokhi, M.; Ganjali, M.R.; Walker, B.W.; Saeb, M.R.; et al. Conductive biomaterials as nerve conduits: Recent advances and future challenges. Appl. Mater. Today 2020, 20, 100784.
- Yang, C.-Y.; Huang, W.-Y.; Chen, L.-H.; Liang, N.-W.; Wang, H.-C.; Lu, J.; Wang, X.; Wang, T.-W. Neural tissue engineering: The influence of scaffold surface topography and extracellular matrix microenvironment. J. Mater. Chem. B 2020, 9, 567–584.
- Rahmati, M.; Mills, D.K.; Urbanska, A.M.; Saeb, M.R.; Venugopal, J.R.; Ramakrishna, S.; Mozafari, M. Electrospinning for tissue engineering applications. Prog. Mater. Sci. 2021, 117, 100721.
- López-Cebral, R.; Silva-Correia, J.S.; Reis, R.L.; Silva, T.H.; Oliveira, J.M. Peripheral Nerve Injury: Current Challenges, Conventional Treatment Approaches, and New Trends in Biomaterials-Based Regenerative Strategies. ACS Biomater. Sci. Eng. 2017, 3, 3098–3122.
- Asadian, M.; Chan, K.V.; Norouzi, M.; Grande, S.; Cools, P.; Morent, R.; De Geyter, N. Fabrication and Plasma Modification of Nanofibrous Tissue Engineering Scaffolds. Nanomaterials 2020, 10, 119.
- Biggi, S.; Bassani, G.A.; Vincoli, V.; Peroni, D.; Bonaldo, V.; Biagiotti, M.; Belli, R.; Alessandrino, A.; Biasini, E.; Freddi, G. Characterization of Physical, Mechanical, and Biological Properties of SilkBridge Nerve Conduit after Enzymatic Hydrolysis. ACS Appl. Bio Mater. 2020, 3, 8361–8374.
- Castro, V.O.; Merlini, C. Aligned electrospun nerve conduits with electrical activity as a strategy for peripheral nerve regeneration. Artif. Organs 2021, 45, 813–818.
- Yu, X.; Zhang, T.; Li, Y. 3D Printing and Bioprinting Nerve Conduits for Neural Tissue Engineering. Polymers 2020, 12, 1637.
- Pinho, A.C.; Fonseca, A.; Serra, A.; Santos, J.D.; Coelho, J.F.J. Peripheral Nerve Regeneration: Current Status and New Strategies Using Polymeric Materials. Adv. Healthc. Mater. 2016, 5, 2732–2744.
- Meena, P.; Kakkar, A.; Kumar, M.; Khatri, N.; Nagar, R.K.; Singh, A.; Malhotra, P.; Shukla, M.; Saraswat, S.K.; Srivastava, S.; et al. Advances and clinical challenges for translating nerve conduit technology from bench to bed side for peripheral nerve repair. Cell Tissue Res. 2021, 383, 617–644.
- Houshyar, S.; Bhattacharyya, A.; Shanks, R. Peripheral Nerve Conduit: Materials and Structures. ACS Chem. Neurosci. 2019, 10, 3349–3365.
- Seidlits, S.K.; Lee, J.Y.; Schmidt, C.E. Nanostructured scaffolds for neural applications. Nanomedicine 2008, 3, 183–199.
- Yen, C.M.; Shen, C.C.; Yang, Y.C.; Liu, B.S.; Lee, H.T.; Sheu, M.L.; Tsai, M.H.; Cheng, W.Y. Novel electrospun poly(ϵ-caprolactone)/type i collagen nanofiber conduits for repair of peripheral nerve injury. Neural Regen. Res. 2019, 14, 1617–1625.
- Mu, Y.; Wu, F.; Lu, Y.; Wei, L.; Yuan, W. Progress of electrospun fibers as nerve conduits for neural tissue repair. Nanomedicine 2014, 9, 1869–1883.
- Tian, L.; Prabhakaran, M.P.; Ramakrishna, S. Strategies for regeneration of components of nervous system: Scaffolds, cells and biomolecules. Regen. Biomater. 2015, 2, 31–45.
- Behtaj, S.; Karamali, F.; Masaeli, E.; Anissimov, Y.G.; Rybachuk, M. Electrospun PGS/PCL, PLLA/PCL, PLGA/PCL and pure PCL scaffolds for retinal progenitor cell cul-tivation. Biochem. Eng. J. 2021, 166, 107846.
- Amini, S.; Salehi, H.; Setayeshmehr, M.; Ghorbani, M. Natural and synthetic polymeric scaffolds used in peripheral nerve tissue engineering: Advantages and disadvantages. Polym. Adv. Technol. 2021, 32, 2267–2289.
- Xue, J.; Xie, J.; Liu, W.; Xia, Y. Electrospun Nanofibers: New Concepts, Materials, and Applications. Acc. Chem. Res. 2017, 50, 1976–1987.
- Dębski, T.; Kijeńska-Gawrońska, E.; Zołocińska, A.; Siennicka, K.; Słysz, A.; Paskal, W.; Włodarski, P.; Święszkowski, W.; Pojda, Z. Bioactive Nanofiber-Based Conduits in a Peripheral Nerve Gap Management—An Animal Model Study. Int. J. Mol. Sci. 2021, 22, 5588.
- Lv, D.; Zhou, L.; Zheng, X.; Hu, Y. Sustained release of collagen VI potentiates sciatic nerve regeneration by modulating macrophage phe-notype. Eur. J. Neurosci. 2017, 45, 1258–1267.
- Jhang, J.-C.; Lin, J.-H.; Lou, C.-W.; Chen, Y.-S. Biodegradable and conductive PVA/CNT nanofibrous membranes used in nerve conduit applications. J. Ind. Text. 2021.
- Qu, W.; Wu, Y.-X.; Ma, H.; Wang, J.-L. Production of chitosan scaffolds by lyophilization or electrospinning: Which is better for peripheral nerve regeneration? Neural Regen. Res. 2021, 16, 1093–1098.
- Wang, H.B.; Mullins, M.E.; Cregg, J.M.; McCarthy, C.W.; Gilbert, R.J. Varying the diameter of aligned electrospun fibers alters neurite outgrowth and Schwann cell mi-gration. Acta Biomater. 2010, 6, 2970–2978.
- Daud, M.F.; Pawar, K.; Claeyssens, F.; Ryan, A.; Haycock, J.W. An aligned 3D neuronal-glial co-culture model for peripheral nerve studies. Biomaterials 2012, 33, 5901–5913.
- Rao, F.; Wang, Y.; Zhang, D.; Lu, C.; Cao, Z.; Sui, J.; Wu, M.; Zhang, Y.; Pi, W.; Wang, B.; et al. Aligned chitosan nanofiber hydrogel grafted with peptides mimicking bioactive brain-derived neu-rotrophic factor and vascular endothelial growth factor repair long-distance sciatic nerve defects in rats. Theranostics 2020, 10, 1590–1603.
- Sudwilai, T.; Ng, J.J.; Boonkrai, C.; Israsena, N.; Chuangchote, S.; Supaphol, P. Polypyrrole-coated electrospun poly(lactic acid) fibrous scaffold: Effects of coating on electrical conductivity and neural cell growth. J. Biomater. Sci. Polym. Ed. 2014, 25, 1240–1252.
- Zhao, Q.; Lu, S.-B.; Quan, Q.; Meng, H.-Y.; Chang, B.; Liu, G.-B.; Cheng, X.-Q.; Tang, H.; Wang, Y.; Peng, J. Aligned fibers enhance nerve guide conduits when bridging peripheral nerve defects focused on early repair stage. Neural Regen. Res. 2019, 14, 903–912.
- Dong, X.; Liu, S.; Yang, Y.; Gao, S.; Li, W.; Cao, J.; Wan, Y.; Huang, Z.; Fan, G.; Chen, Q.; et al. Aligned microfiber-induced macrophage polarization to guide schwann-cell-enabled peripheral nerve regeneration. Biomaterials 2021, 272, 120767.
- Askarzadeh, N.; Nazarpak, M.H.; Mansoori, K.; Farokhi, M.; Gholami, M.; Mohammadi, J.; Mottaghitalab, F. Bilayer Cylindrical Conduit Consisting of Electrospun Polycaprolactone Nanofibers and DSC Cross-Linked Sodium Alginate Hydrogel to Bridge Peripheral Nerve Gaps. Macromol. Biosci. 2020, 20, e2000149.
- Wang, J.; Xiong, H.; Zhu, T.; Liu, Y.; Pan, H.; Fan, C.; Zhao, X.; Lu, W.W. Bioinspired Multichannel Nerve Guidance Conduit Based on Shape Memory Nanofibers for Potential Application in Peripheral Nerve Repair. ACS Nano 2020, 14, 12579–12595.
- Ahn, H.-S.; Hwang, J.-Y.; Kim, M.S.; Lee, J.-Y.; Kim, J.-W.; Kim, H.-S.; Shin, U.S.; Knowles, J.C.; Kim, H.-W.; Hyun, J.K. Carbon-nanotube-interfaced glass fiber scaffold for regeneration of transected sciatic nerve. Acta Biomater. 2015, 13, 324–334.
- Xue, J.; Li, H.; Xia, Y. Nanofiber-Based Multi-Tubular Conduits with a Honeycomb Structure for Potential Application in Peripheral Nerve Repair. Macromol. Biosci. 2018, 18, 1800090.
- Zheng, C.; Yang, Z.; Chen, S.; Zhang, F.; Rao, Z.; Zhao, C.; Quan, D.; Bai, Y.; Shen, J. Nanofibrous nerve guidance conduits decorated with decellularized matrix hydrogel facilitate peripheral nerve injury repair. Theranostics 2021, 11, 2917–2931.
- Chang, W.; Shah, M.B.; Zhou, G.; Walsh, K.; Rudraiah, S.; Kumbar, S.G.; Yu, X. Polymeric nanofibrous nerve conduits coupled with laminin for peripheral nerve regeneration. Biomed. Mater. 2020, 15, 035003.
- Hou, Y.; Wang, X.; Zhang, Z.; Luo, J.; Cai, Z.; Wang, Y.; Li, Y. Repairing Transected Peripheral Nerve Using a Biomimetic Nerve Guidance Conduit Containing Intraluminal Sponge Fillers. Adv. Healthc. Mater. 2019, 8, 1900913.
- Vashisth, P.; Kar, N.; Gupta, D.; Bellare, J.R. Three Dimensional Quercetin-Functionalized Patterned Scaffold: Development, Characterization, and In Vitro Assessment for Neural Tissue Engineering. ACS Omega 2020, 5, 22325–22334.
- Bergmann, C.P.; Stumpf, A. Biomaterials. In Dental Ceramics Topics in Mining, Metallurgy and Materials Engineering; Springer: Berlin/Heidelberg, Germany, 2013; pp. 9–13.
- Muheremu, A.; Ao, Q. Past, Present, and Future of Nerve Conduits in the Treatment of Peripheral Nerve Injury. BioMed Res. Int. 2015, 2015, 1–6.
- Yi, S.; Xu, L.; Gu, X. Scaffolds for peripheral nerve repair and reconstruction. Exp. Neurol. 2019, 319, 112761.
- Behtaj, S.; Öchsner, A.; Anissimov, Y.G.; Rybachuk, M. Retinal Tissue Bioengineering, Materials and Methods for the Treatment of Glaucoma. Tissue Eng. Regen. Med. 2020, 17, 253–269.
- Katiyar, K.S.; Das, S.; Burrell, J.C.; Cullen, D.K. Scaffolds for bridging sciatic nerve gaps. In Handbook of Tissue Engineering Scaffolds: Volume Two; Elsevier: Amsterdam, The Netherlands, 2019; pp. 67–93.
- Zha, F.; Chen, W.; Zhang, L.; Yu, D. Electrospun natural polymer and its composite nanofibrous scaffolds for nerve tissue engineering. J. Biomater. Sci. Polym. Ed. 2019, 31, 519–548.
- Yan, J.; Wu, R.; Liao, S.; Jiang, M.; Qian, Y. Applications of Polydopamine-Modified Scaffolds in the Peripheral Nerve Tissue Engineering. Front. Bioeng. Biotechnol. 2020, 8, 590998.
- Ghane, N.; Khalili, S.; Khorasani, S.N.; Neisiany, R.E.; Das, O.; Ramakrishna, S. Regeneration of the peripheral nerve via multifunctional electrospun scaffolds. J. Biomed. Mater. Res. Part A 2021, 109, 437–452.
- Chen, C.; Kong, X.; Lee, I.-S. Modification of surface/neuron interfaces for neural cell-type specific responses: A review. Biomed. Mater. 2015, 11, 014108.
- Bramini, M.; Rocchi, A.; Benfenati, F.; Cesca, F. Neuronal Cultures and Nanomaterials. In Glutamate and ATP at the Interface of Metabolism and Signaling in the Brain; Springer: Singapore, 2019; Volume 22, pp. 51–79.
- Roach, P.; Parker, T.; Gadegaard, N.; Alexander, M. Surface strategies for control of neuronal cell adhesion: A review. Surf. Sci. Rep. 2010, 65, 145–173.
- Zhang, W.; Yang, Y.; Cui, B. New perspectives on the roles of nanoscale surface topography in modulating intra-cellular signaling. Curr. Opin. Solid State Mater. Sci. 2021, 25, 100873.
- Ma, Y.; Gao, H.; Wang, H.; Cao, X. Engineering topography: Effects on nerve cell behaviors and applications in peripheral nerve repair. J. Mater. Chem. B 2021, 9, 6310–6325.
- Nguyen, T.Y.; Liu, H. A Review of Current Advances in Biomaterials for Neural Tissue Regeneration. Recent Patents Biomed. Eng. 2013, 6, 29–39.
- Ekram, B.; El-Hady, B.M.A.; El-Kady, A.M.; Fouad, M.T.; Sadek, Z.I.; Amr, S.M.; Gabr, H.; Waly, A.I.; Guirguis, O.W. Enhanced mesenchymal stem cells growth on antibacterial microgrooved electrospun zinc chloride/polycaprolactone conduits for peripheral nerve regeneration. J. Bioact. Compat. Polym. 2021, 36, 152–168.
- Sarker, M.D.; Naghieh, S.; McInnes, A.D.; Schreyer, D.J.; Chen, X. Strategic Design and Fabrication of Nerve Guidance Conduits for Peripheral Nerve Regeneration. Biotechnol. J. 2018, 13, e1700635.
- Sarker, M.D.; Naghieh, S.; McInnes, A.D.; Schreyer, D.J.; Chen, X. Regeneration of peripheral nerves by nerve guidance conduits: Influence of design, biopolymers, cells, growth factors, and physical stimuli. Prog. Neurobiol. 2018, 171, 125–150.
More
Information
Subjects:
Engineering, Biomedical
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Entry Collection:
Neurodegeneration
Revisions:
2 times
(View History)
Update Date:
09 Feb 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




