Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Xueguang Shao | + 1697 word(s) | 1697 | 2022-01-18 09:11:08 | | | |
| 2 | Conner Chen | Meta information modification | 1697 | 2022-02-08 01:46:46 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Shao, X. Interaction of Water and Solutes. Encyclopedia. Available online: https://encyclopedia.pub/entry/19152 (accessed on 08 February 2026).
Shao X. Interaction of Water and Solutes. Encyclopedia. Available at: https://encyclopedia.pub/entry/19152. Accessed February 08, 2026.
Shao, Xueguang. "Interaction of Water and Solutes" Encyclopedia, https://encyclopedia.pub/entry/19152 (accessed February 08, 2026).
Shao, X. (2022, February 07). Interaction of Water and Solutes. In Encyclopedia. https://encyclopedia.pub/entry/19152
Shao, Xueguang. "Interaction of Water and Solutes." Encyclopedia. Web. 07 February, 2022.
Copy Citation
Water plays an important role in chemical and biological processes. The interaction of water and solutes is of great significance for understanding the properties of aqueous solutions or bio-systems.
water structure
quantitative analysis
1. Structural Information of Water
The structure of water has been an interesting subject in chemistry and biology for decades due to the complex and flexible patterns of hydrogen bonding [1][2][3][4]. The effect of the temperature on the NIR spectrum of liquid water was studied as early as in 1925 [5], and over the past few decades, a number of works reported the temperature dependency of the NIR spectra of water [6][7][8][9][10][11]. Most of the researches focused on the absorption band around 6900 cm−1 measured at different temperatures, where an isosbestic point can be observed, implying the variation of the overlapped spectral components with the change of the temperature. With the help of chemometric methods, the spectral features of water structures with different hydrogen bonds were obtained, and the relative abundances of different water structures that change with the temperature were found [10][11]. Thus, the temperature-dependent NIR spectroscopy combined with chemometrics provides an efficient way to explore the structure of water in aqueous systems.
To investigate the effect of the temperature on the NIR spectra of water, a method for selecting the temperature-dependent variables from the temperature-dependent NIR spectra was developed. Figure 1A shows the temperature-dependent NIR spectra of water measured from 30 to 60 °C in the spectral range of 6000–10,000 cm−1. It can be seen that with the increase of the temperature, a shift of the peak around 6900 cm−1 to a higher wavenumber was observed, which is caused by the change of the overlapped spectral components corresponding to different water structures. To obtain the temperature-dependent information from the spectra, a method combined CWT and Monte-Carlo uninformative variable elimination (MC-UVE) was proposed for the selection of the temperature-dependent variables (wavenumbers) from the NIR spectra measured at different temperatures [12]. CWT was used to decompose the spectra into the spectral components with different frequencies, and then MC-UVE was employed to evaluate the importance of the variables in the quantitative model of the spectra and temperature. Figure 1B shows the stability of the transformed water spectra obtained by MC-UVE, which is named as “fountain graph”. In the fountain for the peak around 6900 cm−1, seven variables with a significant temperature dependency can be found. This indicates the complexity of water structures and suggests that there are different water species of which the spectral features change differently with the temperature. Furthermore, the temperature-dependent NIR spectra of the aqueous solutions containing NaCl, glucose, and human serum albumin (HSA) were investigated. Figure 1C shows the transformed spectra of water and solutions by CWT and the locations of the selected variables. It can be seen that the selected variables are located at similar but not identical wavenumbers for different solutions, indicating that the variables can be used for the discrimination of different solutions. Furthermore, using the selected variables, quantification can also be achieved. The results indicate that temperature-dependent NIR spectra can be severed as a mirror to reflect the complexity of water structures and identify the aqueous solutions of different compositions.
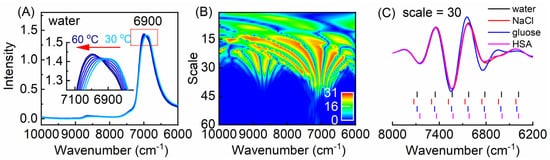
Figure 1. Temperature-independent near-infrared (NIR) spectra of water in the range of 6000–10,000 cm−1 measured from 30 to 60 °C (A), the fountain graph (B), and the selected variables for the samples of water, NaCl (5.8 g L−1), glucose (20 g L−1), and HSA (5.0 g L−1) solutions (C).
To understand water structures in liquid water and aqueous solutions, Gaussian fitting was adopted to analyze the temperature-dependent NIR spectra of water. Six spectral components were used to fitting the spectra, corresponding to different water species with no (S0), one (S1), two (S2), three (S3), and four (S4) hydrogen bonds, as well as the rotation vibration (Sr) of the water molecule [13]. To describe the complex water structures more exactly, a model was proposed by denoting the proton acceptor (oxygen) with A and the proton donor (hydrogen) with D. The water molecule with m hydrogen bonds on oxygen atom and n hydrogen bonds on hydrogen atom is represented by AmDn, where m and n equal to 0, 1, or 2. Therefore, nine water structures can be defined, i.e., A0D0, A0D1, A1D0, A0D2, A1D1, A2D0, A1D2, A2D1, and A2D2. Ten spectral components corresponding to the nine water structures and Sr were obtained from the temperature-dependent spectra of water and glucose solutions by Gaussian fitting with a knowledge-based genetic algorithm [14]. Figure 2 is one of the results obtained by the fitting. It can be seen that the fitted spectrum coincides well with the measured one. The integral intensity of the 10 peaks was investigated using the NIR spectra measured at different temperatures. With the increase of the temperature, the content of A0D0 increases, while that of A2D2 decreases, indicating the weakening of the hydrogen bonds and the dissociation of the water structures with more hydrogen bonds into that with less hydrogen bonds. Furthermore, through the variation of the spectral components of these water structures with the glucose concentration, the enhancement of the ordered (tetrahedral) hydrogen-bonded water structures induced by the interaction of water and glucose were found, providing a proof for the explanation of the protective effect of glucose on the bio-molecules in aqueous solutions.
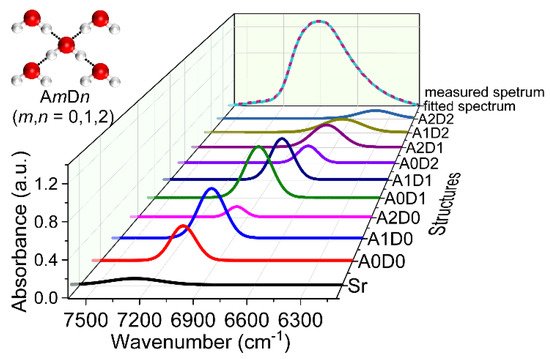
Figure 2. Result of Gaussian fitting for the NIR spectra of water measured at 30 °C.
CWT has been proven to be a powerful tool for the resolution enhancement in the spectral analysis [15][16][17][18]. To analyze the spectral features of water in mixtures of water and ethanol, the fourth-order derivative of the temperature-dependent NIR spectra of the mixtures was calculated by CWT [19]. The overlapped peaks were separated, and the spectral features of OH and CH with various intermolecular interactions were identified in the fourth derivative spectra. By fitting the derivative spectra of the mixtures by those of pure water and ethanol, the obtained coefficients for ethanol show a linear relation with the content, but those for water exhibit a non-linear relation, which provides clear evidence for the interactions of ethanol and water in the mixtures. Furthermore, from the residual spectra after the fitting, the structures of water species, aggregations of ethanol, hetero clusters of ethanol–water, and their variation with the content were analyzed. The residual spectra calculated by high-order derivatives provide a very good way to uncover the spectral information about the interactions. These results indicate that the spectral information of water structures with different hydrogen bonds can be extracted from the temperature-dependent NIR spectra, and the temperature-induced spectral features can be a probe to reveal the structural changes and interactions in aqueous solutions.
2. Interaction of Water and Solutes
Water plays an important role in chemical and biological processes. The interaction of water and solutes is of great significance for understanding the properties of aqueous solutions or bio-systems [20]. Due to the sensitivity of NIR spectra to water structures, the spectral changes of water under different perturbations can be a probe to reveal the interactions in aqueous solutions. The interaction of water and bio-organisms including carbohydrate molecules and oligopeptide was investigated using the NIR spectra of aqueous solutions measured at different concentrations and temperatures [13][14][21][22][23][24]. The spectral components related to different water structures were obtained from the NIR spectra. Through the variation of these structures with the temperature and the solute concentration, an increase of the tetrahedrally hydrogen-bonded water structure induced by bio-molecules was observed, showing that the thermal stability of water structures may be enhanced in bio-systems.
The interaction of water and ethanol was studied using the temperature-dependent NIR spectroscopy with high-order chemometric algorithms, including NPCA, PARAFAC, and ATLD [25]. The spectral features of water and ethanol in the mixtures were obtained by the three methods. Through the variation of the spectra with the concentration, it was revealed that ethanol promotes the formation of water clusters. To obtain more spectral information of the interactions of water and ethanol, a new method was proposed based on the rotation of the loadings in principal component analysis (PCA) [26]. The calculated spectra were found to be more reliable to reflect the structures in the mixture, from which the spectral features of different water species (S0–S4), ethanol clusters, and the interaction of OH and CH groups were observed. Through the difference between the calculated and experimental spectra, it was found that, when ethanol is added into water, the contents of large water clusters with two, three, and four hydrogen bonds increase, and the interaction of water and ethanol varies nonlinearly with the concentration.
The structure of water at low temperatures is related to many special phenomena. For example, the freezing point of water reduces when an antifreeze is added, and water in polar fish does not freeze below the freezing point. The structure of water at low temperatures and the mechanism of the cryoprotectant dimethyl sulfoxide (DMSO) in reducing the freezing point of water were investigated using the NIR spectra measured at low temperatures [27]. CWT was adopted to enhance the resolution of the NIR spectra. The spectral features reflecting the interaction of DMSO and water were found from the resolution-enhanced spectra, and two hydrogen-bonded DMSO–water structures (DW2 and D2W) were identified in the mixtures with different DMSO/water ratios. Through the variation of the spectral features, it was found that DW2 structure inhibits the formation of tetrahedral water structures at low temperatures, which may be the reason for DMSO reducing the freezing point of the mixture. To further understand the effect of protein on the antifreezing performance of the DMSO–water system, the effect of formamide (FA) on the hydrogen bonding of DMSO and water was studied [28]. From the resolution-enhanced spectra by CWT, the spectral feature of the interaction of DMSO and water (S=O…H–O) was observed. When FA exists in the mixture, the intensity of the peak decreases with the increase of FA content, indicating that FA may replace the water molecules to form the hydrogen bond of S=O and H–N. Furthermore, the spectra of the three-component mixtures were analyzed by ATLD. Two varying spectral features due to water and DMSO were obtained, but the spectral feature variation with the content of FA was not found. This result implies that, although FA may reduce slightly the antifreezing effect, DMSO is still the key component to prevent water from icing.
References
- Ball, P. Water-an enduring mystery. Nature 2008, 452, 291–292.
- Naserifar, S.; Goddard, W.A. Liquid water is a dynamic polydisperse branched polymer. Proc. Natl. Acad. Sci. USA 2019, 116, 1998–2003.
- Perakis, F.; De Marco, L.; Shalit, A.; Tang, F.; Kann, Z.R.; Kühne, T.D.; Torre, R.; Bonn, M.; Nagata, Y. Vibrational spectroscopy and dynamics of water. Chem. Rev. 2016, 116, 7590–7607.
- Loerting, T.; Fuentes-Landete, V.; Tonauer, C.M.; Gasser, T.M. Open questions on the structures of crystalline water ices. Commun. Chem. 2020, 3, 109.
- Collins, J.R. Change in the infra-red absorption spectrum of water with temperature. Phys. Rev. 1925, 26, 771–779.
- Waggener, W.C. Absorbance of liquid water and deuterium oxide between 0.6 and 1.8 microns. comparison of absorbance and effect of temperature. Anal. Chem. 1958, 30, 1569–1570.
- Segtnan, V.H.; Sasic, S.; Isaksson, T.; Ozaki, Y. Studies on the structure of water using two-dimensional near-infrared correlation spectroscopy and principal component analysis. Anal. Chem. 2001, 73, 3153–3161.
- Sasic, S.; Segtnan, V.H.; Ozaki, Y. Self-modeling curve resolution study of temperature-dependent near-infrared spectra of water and the investigation of water structure. J. Phys. Chem. A 2002, 106, 760–766.
- Czarnik-Matusewicz, B.; Pilorz, S. Study of the temperature-dependent near-infrared spectra of water by two-dimensional correlation spectroscopy and principal components analysis. Vib. Spectrosc. 2006, 40, 235–245.
- Renati, P.; Kovacs, Z.; De Ninno, A.; Tsenkova, R. Temperature dependence analysis of the NIR spectra of liquid water confirms the existence of two phases, one of which is in a coherent state. J. Mol. Liq. 2019, 292, 111449.
- Xu, J.; Dorrepaal, R.M.; Martinez-Gonzalez, J.A.; Tsenkova, R.; Gowen, A.A. Near-infrared multivariate model transfer for quantification of different hydrogen bonding species in aqueous systems. J. Chemom. 2020, 34, 3274.
- Cui, X.; Zhang, J.; Cai, W.; Shao, X. Selecting temperature-dependent variables in near-infrared spectra for aquaphotomics. Chemom. Intell. Lab. Syst. 2018, 183, 23–28.
- Cui, X.; Cai, W.; Shao, X. Glucose induced variation of water structure from temperature dependent near infrared spectra. RSC Adv. 2016, 6, 105729–105736.
- Tan, J.; Sun, Y.; Ma, L.; Feng, H.; Guo, Y.; Cai, W.; Shao, X. Knowledge-based genetic algorithm for resolving the near-infrared spectrum and understanding the water structures in aqueous solution. Chemom. Intell. Lab. Syst. 2020, 206, 104150.
- Leung, A.K.-M.; Chau, F.-T.; Gao, J.-B. Wavelet transform: A method for derivative calculation in analytical chemistry. Anal. Chem. 1998, 70, 5222–5229.
- Shao, X.; Cai, W. Wavelet analysis in analytical chemistry. Rev. Anal. Chem. 1998, 17, 235–283.
- Shao, X.; Leung, A.K.M.; Chau, F.-T. Wavelet: A new trend in chemistry. Accounts Chem. Res. 2003, 36, 276–283.
- Sun, Y.; Cui, X.; Cai, W.; Shao, X. Understanding the complexity of the structures in alcohol solutions by temperature–dependent near–infrared spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 229, 117864.
- Shao, X.; Cui, X.; Wang, M.; Cai, W. High order derivative to investigate the complexity of the near infrared spectra of aqueous solutions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 213, 83–89.
- Ball, P. Water as an active constituent in cell biology. Chem. Rev. 2008, 108, 74–108.
- Beganović, A.; Moll, V.; Huck, C.W. Comparison of multivariate regression models based on water-and carbohydrate-related spectral regions in the near-infrared for aqueous solutions of glucose. Molecules 2019, 24, 3696.
- Beganović, A.; Beć, K.B.; Grabska, J.; Stanzl, M.T.; Brunner, M.E.; Huck, C.W. Vibrational coupling to hydration shell–Mechanism to performance enhancement of qualitative analysis in NIR spectroscopy of carbohydrates in aqueous environment. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 237, 118359.
- Dong, Q.; Guo, X.; Li, L.; Yu, C.; Nie, L.; Tian, W.; Zhang, H.; Huang, S.; Zang, H. Understanding hyaluronic acid induced variation of water structure by near-infrared spectroscopy. Sci. Rep. 2020, 10, 1387.
- Cheng, D.; Cai, W.; Shao, X. Understanding the interaction between oligopeptide and water in aqueous solution using temperature-dependent near-infrared spectroscopy. Appl. Spectrosc. 2018, 72, 1354–1361.
- Cui, X.; Zhang, J.; Cai, W.; Shao, X. Chemometric algorithms for analyzing high dimensional temperature dependent near infrared spectra. Chemom. Intell. Lab. Syst. 2017, 170, 109–117.
- Shao, X.; Cui, X.; Liu, Y.; Xia, Z.; Cai, W. Understanding the molecular interaction in solutions by chemometric resolution of near−infrared spectra. ChemistrySelect 2017, 2, 10027–10032.
- Zhao, H.T.; Sun, Y.; Guo, Y.C.; Cai, W.S.; Shao, X.G. Near infrared spectroscopy for low-temperature water structure analysis. Chem. J. Chin. Univ. 2020, 41, 1968–1974.
- Su, T.; Sun, Y.; Han, L.; Cai, W.S.; Shao, X.G. Revealing the interactions of water with cryoprotectant and protein by near-infrared spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 266, 120417.
More
Information
Subjects:
Chemistry, Analytical; Spectroscopy; Others
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.7K
Revisions:
2 times
(View History)
Update Date:
08 Feb 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




