
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ioannis Parodis | + 1275 word(s) | 1275 | 2022-01-19 09:46:22 |
Video Upload Options
Systemic lupus erythematosus (SLE) is a chronic autoimmune disorder that has detrimental effects on patient’s health-related quality of life (HRQoL).
1. Introduction
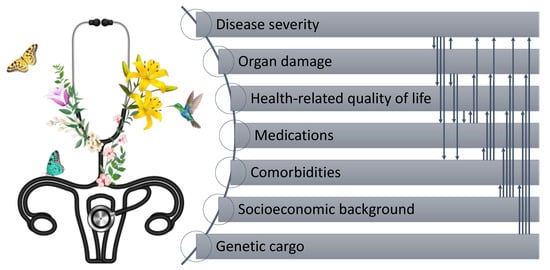
Systemic lupus erythematosus (SLE) is a rheumatic condition that is challenging to diagnose and treat, mainly owing to its immense heterogeneity of clinical symptoms and complexity with regard to comorbidity burden, often necessitating interdisciplinary care, with the goal being the best possible quality of life and long-term outcomes [1][2]. With the premise that preventing is better than restoring, early diagnosis and treatment initiation is imperative [3], a need of particular urgency given that up to 10% of SLE patients develop life-threatening conditions or complications, such as end-stage kidney disease [4][5]. Patient-reported outcome measures (PROMs) receive increasing attention within the lupus research community, especially PROMs addressing HRQoL [6]. Even though this signifies a shift of the current paradigm towards increasing patient participation in their care, more distance has to be bridged before PROMs are an integral part of the evaluation in clinical practice.
2. PROMs in the Treat-to-Target Context
3. The Matter of Not Only Optimal Choice but Also Optimal Use of PROMs
References
- Piga, M.; Arnaud, L. The Main Challenges in Systemic Lupus Erythematosus: Where Do We Stand? J. Clin. Med. 2021, 10, 243.
- Kaul, A.; Gordon, C.; Crow, M.K.; Touma, Z.; Urowitz, M.; Van Vollenhoven, R.; Ruiz-Irastorza, G.; Hughes, G. Systemic lupus erythematosus. Nat. Rev. Dis. Prim. 2016, 2, 16039.
- Kernder, A.; Richter, J.G.; Fischer-Betz, R.; Winkler-Rohlfing, B.; Brinks, R.; Aringer, M.; Schneider, M.; Chehab, G. Delayed diagnosis adversely affects outcome in systemic lupus erythematosus: Cross sectional analysis of the LuLa cohort. Lupus 2021, 30, 431–438.
- Moe, S.R.; Haukeland, H.; Molberg, Ø.; Lerang, K. Long-Term Outcome in Systemic Lupus Erythematosus; Knowledge from Population-Based Cohorts. J. Clin. Med. 2021, 10, 4306.
- Anders, H.-J.; Saxena, R.; Zhao, M.-H.; Parodis, I.; Salmon, J.E.; Mohan, C. Lupus nephritis. Nat. Rev. Dis. Prim. 2020, 6, 1–25.
- Annapureddy, N.; Devilliers, H.; Jolly, M. Patient-reported outcomes in lupus clinical trials with biologics. Lupus 2016, 25, 1111–1121.
- Van Vollenhoven, R.F.; Mosca, M.; Bertsias, G.; Isenberg, D.; Kuhn, A.; Lerstrøm, K.; Aringer, M.; Bootsma, H.; Boumpas, D.; Bruce, I.N.; et al. Treat-to-target in systemic lupus erythematosus: Recommendations from an international task force. Ann. Rheum. Dis. 2014, 73, 958–967.
- Steiman, A.J.; Urowitz, M.B.; Ibanez, D.; Papneja, A.; Gladman, D.D. Prolonged clinical remission in patients with systemic lupus erythematosus. J. Rheumatol. 2014, 41, 1808–1816.
- Van Vollenhoven, R.; Voskuyl, A.; Bertsias, G.; Aranow, C.; Aringer, M.; Arnaud, L.; Askanase, A.; Balážová, P.; Bonfa, E.; Bootsma, H.; et al. A framework for remission in SLE: Consensus findings from a large international task force on definitions of remission in SLE (DORIS). Ann. Rheum. Dis. 2016, 76, 554–561.
- Gladman, D.D.; Ibañez, D.; Urowitz, M.B. Systemic lupus erythematosus disease activity index 2000. J. Rheumatol. 2002, 29, 288–291.
- Franklyn, K.; Lau, C.S.; Navarra, S.V.; Louthrenoo, W.; Lateef, A.; Hamijoyo, L.; Wahono, C.S.; Chen, S.L.; Jin, O.; Morton, S.; et al. Definition and initial validation of a Lupus Low Disease Activity State (LLDAS). Ann. Rheum. Dis. 2015, 75, 1615–1621.
- Luijten, K.; Tekstra, J.; Bijlsma, J.; Bijl, M. The Systemic Lupus Erythematosus Responder Index (SRI); A new SLE disease activity assessment. Autoimmun. Rev. 2012, 11, 326–329.
- Wallace, D.J.; Kalunian, K.; Petri, M.A.; Strand, V.; Houssiau, F.A.; Pike, M.; Kilgallen, B.; Bongardt, S.; Barry, A.; Kelley, L.; et al. Efficacy and safety of epratuzumab in patients with moderate/severe active systemic lupus erythematosus: Results from EMBLEM, a phase IIb, randomised, double-blind, placebo-controlled, multicentre study. Ann. Rheum. Dis. 2013, 73, 183–190.
- Felson, D.; Smolen, J.S.; Wells, G.; Zhang, Y.; van Tuyl, L.; Funovits, J.; Aletaha, D.; Allaart, C.F.; Bathon, J.; Bombardieri, S.; et al. American College of Rheumatology/European League Against Rheumatism Provisional Definition of Remission in Rheumatoid Arthritis for Clinical Trials. Ann. Rheum. Dis. 2011, 70, 404–413.
- EULAR. Outcome Measures Library. Available online: http://oml.eular.org/ (accessed on 9 April 2020).
- Weldring, T.; Smith, S.M. Article Commentary: Patient-Reported Outcomes (PROs) and Patient-Reported Outcome Measures (PROMs). Health Serv. Insights 2013, 6, HSI.S11093–68.
- Studenic, P.; Radner, H.; Smolen, J.S.; Aletaha, D. Discrepancies between patients and physicians in their perceptions of rheumatoid arthritis disease activity. Arthritis Care Res. 2012, 64, 2814–2823.
- Studenic, P.; Smolen, J.S.; Aletaha, D. Near misses of ACR/EULAR criteria for remission: Effects of patient global assessment in Boolean and index-based definitions. Ann. Rheum. Dis. 2012, 71, 1702–1705.
- Studenic, P.; Felson, D.; De Wit, M.; Alasti, F.; Stamm, T.A.; Smolen, J.S.; Aletaha, D. Testing different thresholds for patient global assessment in defining remission for rheumatoid arthritis: Are the current ACR/EULAR Boolean criteria optimal? Ann. Rheum. Dis. 2020, 79, 445–452.
- Jones, B.; Flurey, C.A.; Proudman, S.; Ferreira, R.J.; Voshaar, M.; Hoogland, W.; Chaplin, H.; Goel, N.; Hetland, M.L.; Hill, C.; et al. Considerations and priorities for incorporating the patient perspective on remission in rheumatoid arthritis: An OMERACT 2020 special interest group report. Semin. Arthritis Rheum. 2021, 51, 1108–1112.
- Heijke, R.; Björk, M.; Thyberg, I.; Kastbom, A.; McDonald, L.; Sjöwall, C. Comparing longitudinal patient-reported outcome measures between Swedish patients with recent-onset systemic lupus erythematosus and early rheumatoid arthritis. Clin. Rheumatol. 2021, 1–8.
- Golder, V.; Kandane-Rathnayake, R.; Hoi, A.Y.-B.; Huq, M.; Louthrenoo, W.; An, Y.; Li, Z.G.; Luo, S.F.; Sockalingam, S.; Lau, C.S.; et al. Association of the lupus low disease activity state (LLDAS) with health-related quality of life in a multinational prospective study. Arthritis Res. Ther. 2017, 19, 1–11.
- Elefante, E.; Tani, C.; Stagnaro, C.; Signorini, V.; Parma, A.; Carli, L.; Zucchi, D.; Ferro, F.; Mosca, M. Articular involvement, steroid treatment and fibromyalgia are the main determinants of patient-physician discordance in systemic lupus erythematosus. Arthritis Res. 2020, 22, 1–8.
- Azizoddin, D.R.; Jolly, M.; Arora, S.; Yelin, E.; Katz, P. Patient-Reported Outcomes Predict Mortality in Lupus. Arthritis Rheum. 2018, 71, 1028–1035.
- Louthrenoo, W.; Kasitanon, N.; Morand, E.; Kandane-Rathnayake, R. Associations between physicians’ global assessment of disease activity and patient-reported outcomes in patients with systemic lupus erythematosus: A longitudinal study. Lupus 2021, 30, 1586–1595.
- Strand, V.; Gladman, D.; Isenberg, D.; Petri, M.; Smolen, J.; Tugwell, P. Endpoints: Consensus recommendations from OMERACT IV. Lupus 2000, 9, 322–327.
- Yen, J.C.; Neville, C.; Fortin, P.R. Discordance between patients and their physicians in the assessment of lupus disease activity: Relevance for clinical trials. Lupus 1999, 8, 660–670.
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483.
- Yellen, S.B.; Cella, D.F.; Webster, K.; Blendowski, C.; Kaplan, E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J. Pain Symptom Manag. 1997, 13, 63–74.
- U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research; U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research; U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health. Guidance for industry: Patient-reported outcome measures: Use in medical product development to support labeling claims: Draft guidance. Health Qual. Life Outcomes 2006, 4, 1–79.
- Strand, V.; Simon, L.S.; Meara, A.S.; Touma, Z. Measurement properties of selected patient-reported outcome measures for use in randomised controlled trials in patients with systemic lupus erythematosus: A systematic review. Lupus Sci. Med. 2020, 7, e000373.
- Strand, V.; Levy, R.A.; Cervera, R.; Petri, M.A.; Birch, H.; Freimuth, W.W.; Zhong, Z.J.; Clarke, A.E. Improvements in health-related quality of life with belimumab, a B-lymphocyte stimulator-specific inhibitor, in patients with autoantibody-positive systemic lupus erythematosus from the randomised controlled BLISS trials. Ann. Rheum. Dis. 2013, 73, 838–844.
- The EuroQol Group. EuroQol-a new facility for the measurement of health-related quality of life. Health Policy 1990, 16, 199–208.
- Aggarwal, R.; Wilke, C.T.; Pickard, A.S.; Vats, V.; Mikolaitis, R.; Fogg, L.; Block, J.A.; Jolly, M. Psychometric Properties of the EuroQol-5D and Short Form-6D in Patients with Systemic Lupus Erythematosus. J. Rheumatol. 2009, 36, 1209–1216.
- Lindblom, J.; Gomez, A.; Borg, A.; Emamikia, S.; Ladakis, D.; Matilla, J.; Pehr, M.; Cobar, F.; Enman, Y.; Heintz, E.; et al. EQ-5D-3L full health state discriminates between drug and placebo in clinical trials of systemic lupus erythematosus. Rheumatology 2021, 60, 4703–4716.
- Györi, N.; Giannakou, I.; Chatzidionysiou, K.; Magder, L.; Van Vollenhoven, R.F.; Petri, M. Disease activity patterns over time in patients with SLE: Analysis of the Hopkins Lupus Cohort. Lupus Sci. Med. 2017, 4, e000192.




