Human cytomegalovirus (HCMV) is a double-stranded DNA virus that belongs to the β-herpesvirus family and infects 40–90% of the adult population worldwide. HCMV infection is usually asymptomatic in healthy individuals but causes serious problems in immunocompromised people. It is found that a series of HCMV-encoded miRNAs (e.g., miR-UL112 and miR-UL148D) are explicitly involved in the regulation of viral DNA replication, immune evasion, as well as host cell fate. MiRNA-targeted therapies have been explored for the treatment of atherosclerosis, cardiovascular disease, cancer, diabetes, and hepatitis C virus infection. It is feasible to develop an alternative vaccine to restart peripheral immunity or to inhibit HCMV activity, which may contribute to the antiviral intervention for serious HCMV-related diseases.
1. Introduction
Human cytomegalovirus (HCMV) is a β-herpesvirus with double-stranded DNA, and it has the largest genome (approximately 230 kb) among its family members. In developed countries, 40–60% of adults are HCMV infected, and the seroprevalences in some developing countries approach 100%
[1][2]. The pathophysiological process of HCMV invasion in the human body causes a multilayered cascade of immune reactions, as follows: it aims at various signaling transduction pathways involving the whole peripheral immune system, leading to the impaired antigen recognition ability of CD8
+ T cells, the abnormal differentiation of CD4
+ T cells, the inhibition of the natural killer (NK) cell killing effect, or the reduction in proinflammatory cytokine secretion, rather than a single immune cell or a specific immune molecule. In addition, the immunosuppression status caused by HCMV infection may lead to a secondary infection by other pathogens and further deteriorate the host’s immune function.
Upon HCMV infection, it first contacts the cell membrane, then fuses with the cell membrane and enters the cytoplasm. The nucleocapsid containing viral DNA enters the nucleus through the activities of a series of viral proteins. At this time, if the HCMV genome can initiate transcription of the immediate early (IE) genes by the main immediate early promoter (MIEP), the newly assembled progeny virus will lyse the host cell and release to establish a lytic infection. The HCMV genome encodes a variety of regulatory proteins (such as IE2, IE72, and glycoproteins) that regulate viral DNA replication and virus offspring propagation
[3][4]. In addition, the proteins maintaining its structure (such as viral tegument protein phosphoprotein 65 (pp65), glycoprotein B, and glycoprotein H) also play indispensable roles in the regulation of viral transcription, the cell cycle, and even the immune response
[5][6]. Otherwise, HCMV infection will be latent, which is very important for virus long-term survival
[7].
HCMV latent infection, defined by the maintenance of viral genomes in the absence of new virus production, causes serious clinical symptoms in immunocompromised individuals, such as those undergoing solid organ or hematopoietic stem cell transplants. These patients are susceptible to HCMV reactivation from latency and virus replication in numerous tissues and organs, causing significant morbidity and mortality
[8]. To date, the treatment of latent HCMV infection is still a dilemma because all currently optional therapies are designed for viruses during replication
[9]. Thus, it is important to understand the pathological mechanisms of HCMV latency and to clarify a series of targets that may be involved in the conversion process to prevent serious cytomegalovirus disease.
Viruses encode short noncoding RNAs (microRNAs, miRNAs) that cooperate with viral proteins to regulate the expression of viral and host genes. MiRNAs are long noncoding RNAs (measuring ~22 nucleotides) that are encoded by eukaryotes and viruses (
Figure 1). They are incorporated into the multiprotein RNA-induced silencing complex (RISC). RISC leads to the transcriptional repression of messenger RNAs (mRNAs) through complementarity between nucleotides 2–8 of the miRNAs (the seed region) and 3′-untranslated regions (3′-UTR) of the mRNAs
[10][11][12]. The first miRNA was identified by Lee and colleagues in Caenorhabditis elegans in 1993
[13]. Pfeffer first identified miRNAs in Epstein–Barr virus (EBV)
[14]. More than 200 virus-encoded miRNAs have been identified in double-stranded DNA viruses, of which 26 HCMV-encoded miRNAs and their potential targets have been reported to play various roles in viral replication, immune evasion, and host-cell fate
[15][16]. Interestingly, in contrast to other herpes viruses, genes coding miRNAs are scattered throughout HCMV genome which implies each HCMV-encoded miRNA may be regulated by its own regulatory sequence
[16].
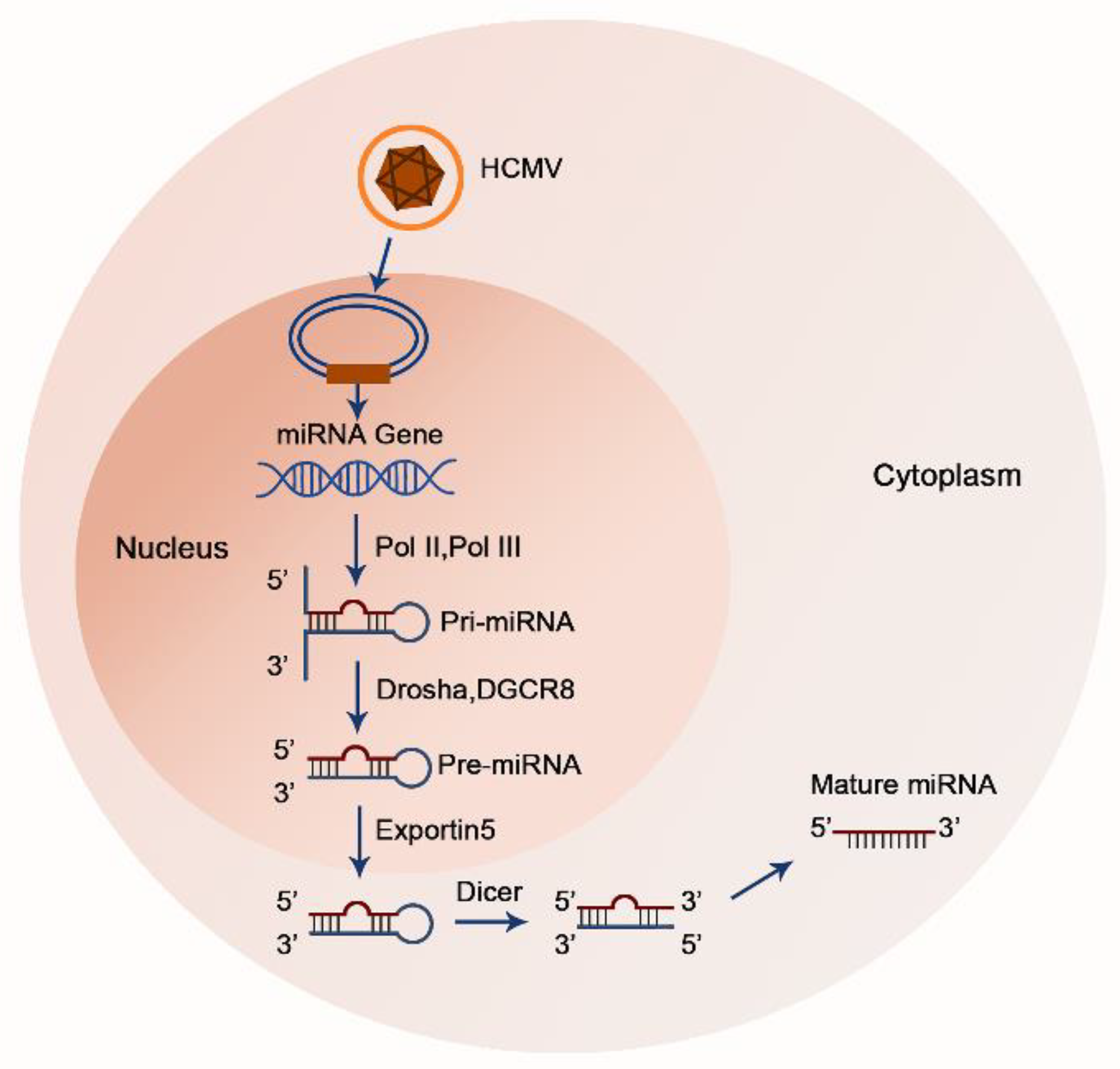
Figure 1. A sketch of HCMV-encoded miRNA biosynthesis model. Mature miRNAs are generated from hairpin secondary structures that arise from longer RNA polymerase II or polymerase III transcripts. In the nucleus, primary (pri-) miRNAs are cleaved into precursor (pre-) miRNAs via the microprocessor complex, consisting of DGCR8 and the ribonuclease Drosha. Next, pre-miRNAs are transported from the nucleus to the cytoplasm. After reaching the cytoplasm, pre-miRNAs are recognized and processed into their mature form by another RNase III, Dicer.
2. The Regulation Mechanisms of Latent HCMV on Host Immune Cells
First, HCMV-encoded miRNAs can escape immune surveillance in a nonimmunogenic manner to favor intracellular latency. They reduce viral replication by downregulating IE expression or by silencing major viral proteins, or they suppress viral particle formation by targeting multiple host genes related to cell cycle control (e.g., cyclin E2 (CCNE2), collagenase stimulatory factor (CD147), BRCA1/BRCA2-containing complex, subunit 3 (BRCC3), EP300 interacting inhibitor of differentiation 1 (EID1), microtubule-associated proteins, RP/EB family member 2 (MAPRE2), and histone proteins (H3F3B)) without recognition by host immune cells
[17]. More importantly, HCMV-encoded miRNAs can target signaling pathways involved in cell apoptosis, immune response, and proinflammatory cytokine production to avoid elimination by the host and to achieve immune evasion. However, virus activation is inevitable when the host immune system is compromised or when obligatory differentiation stimuli occur, which initiates instantaneous host damage and fatal consequences (
Table 1). The importance of HCMV-encoded miRNAs is only beginning to be elucidated.
Table 1. Summary of HCMV-encoded miRNAs.
| Infection Stage |
Function |
HCMV-Encoded miRNA |
Targets |
References |
| Latency |
Limit viral gene expression |
miR-UL112-3p |
HCMV IE72 |
[4] |
| HCMV UL112/113 |
[18] |
| HCMV UL120/121 |
|
| UL114 |
[19] |
| miR-UL148D |
IER5 |
[20] |
| miR-US25-1-5p |
Cyclin E2 |
[21] |
| TRIM28 |
[21] |
| EID1 |
[21] |
| MAPRE2 |
[21] |
| miR-US25-2-3p |
eIF4A1 |
[22] |
| miR-US33-5p |
CCND1 |
[23] |
| STX3 |
[24] |
| Escape immune response |
miR-UL112-3p |
MICA; NK cells |
[25] |
| MICB; NK cells |
[26] |
| IRF1; innate immune cells |
[27] |
| miR-UL112-5p |
ERAP1; CD8+ T cells |
[28] |
| miR-US4-5p |
ERAP1; CD8+ T cells |
[29] |
| miR-UL59 |
ULBP1; NK cells |
[30] |
| miR-US5-1 |
HCMV US7; multiple immune cells |
[31] |
| miR-US5-2-3p |
HCMV US7; multiple immune cells |
[31] |
| |
miR-US33as-5p |
IFNAR1; innate immune cells |
[32] |
| Inhibit autophagy |
miR-UL112-3p |
ATG5; HFFs |
[24] |
| miR-US22-5p |
ATG5; HFFs |
[24] |
| miR-US29-5p |
ATG5; HFFs |
[24] |
| Inhibit apoptosis |
miR-US4-5p |
CASP2; HFFs |
[24] |
| miR-UL112-5p |
CASP3; HFFs |
[24] |
| miR-UL22A-5p |
CASP3; HFFs |
[24] |
| miR-US25-2-3p |
CASP3; HFFs |
[24] |
| miR-UL148D |
IEX-1; HEK293 cells |
[33] |
| PHAP1; HeLa cell S-100 |
[34] |
| ERN1; HeLa cell S-100 |
[34] |
| miR-UL22A-3p |
CASP7; HFFs |
[24] |
| miR-UL36-3p |
FAS; HFFs |
[24] |
| miR-US5-1 |
FAS; HFFs |
[24] |
| miR-US5-2-3p |
FAS; HFFs |
[24] |
| miR-UL36-5p |
SLC25A6 (ANT3); HEK293 cells, U373 cells and HELF cells |
[35] |
| miR-UL70-3p |
MOAP1; HEK293T cells |
[35] |
| miR-US4-5p |
QARS; CD8+ T cells |
[29] |
| miR-US22-5p |
US22; human fibroblast cells |
[36] |
| Reduce inflammatory cytokine production |
miR-UL112-3p |
IKKα/IKKβ; fibroblasts |
[37] |
| miR-US5-1 |
IKKα/IKKβ; fibroblasts |
[37] |
| miR-UL112-3p |
IL-32; NK cells |
[16] |
| TLR2; NK cells |
[38] |
| miR-UL112-3p |
Vamp3; NK cells |
[39] |
| miR-US5-1 |
Vamp3; NK cells |
[39] |
| miR-US5-2-3p |
Vamp3; NK cells |
[39] |
| miR-UL112-3p |
Rab5c; NK cells |
[40] |
| miR-US5-1 |
Rab5c; NK cells |
[40] |
| miR-US5-2-3p |
Rab5c; NK cells |
[40] |
| miR-UL112-3p |
Rab11a; NK cells |
[39] |
| miR-US5-1 |
Rab11a; NK cells |
[39] |
| miR-US5-2-3p |
Rab11a; NK cells |
[39] |
| miR-UL112-3p |
SNAP23; NK cells |
[39] |
| miR-US5-1 |
SNAP23; NK cells |
[39] |
| miR-US5-2-3p |
SNAP23; NK cells |
[39] |
| miR-UL112-3p |
CDC42; NK cells |
[39] |
| miR-US5-1 |
CDC42; NK cells |
[39] |
| miR-US5-2-3p |
CDC42; NK cells |
[39] |
| miR-UL148D |
ACVR1B; NK cells |
[40] |
| RANTES; NK cells |
[41] |
| miR-US25-1-5p |
CD147; HEK293 cells |
[42] |
| Suppress cell cycle progression |
miR-UL36-3p |
CDK6; HFFs |
[24] |
| miR-US5-1 |
CDK6; HFFs |
[24] |
| miR-US5-2-3p |
CDK6; HFFs |
[24] |
| miR-US25-1-3p |
CDK6; HFFs |
[24] |
| miR-US25-2-3p |
CDK6; HFFs |
[24] |
| Induce myelosuppression |
miR-UL22A-3p |
SMAD3; CD34+ HPCs |
[43] |
| miR-UL22A-5p |
SMAD3; CD34+ HPCs |
[43] |
| miR-US5-2-3p |
NAB1; CD34+ HPCs |
[43] |
| Reactivation |
Promote viral gene expression |
miR-UL112-3p |
BclAF1 |
[44] |
| miR-UL36-5p |
HCMV UL138 |
[45] |
| miR-US5-1 |
Geminin |
[46] |
| Induce cell differentiation |
miR-US22-5p |
EGR1; HEK293 cells, NHDF |
[47] |
| Promote apoptosis |
miR-US4-5p |
PAK2; HEK293, HELF and THP-1 cells |
[48] |
| miR-US25-1-5p |
BRCC3; EAhy926 cells |
[49] |
| Others |
N |
miR-UL22A-5p |
BMPR2 |
[50] |
| miR-US4-3p |
CASP7 |
[29] |
| CDK6 |
[24] |
| ERAP1 |
[29] |
| miR-US22-3p |
US22 |
[36] |
| miR-US33-3p |
US29 |
[51] |
| miR-UL69 |
N |
|
| miR-UL70-5p |
N |
|
| miR-US5-2-5p |
N |
|
| miR-US25-2-5p |
N |
|
| miR-US29-3p |
N |
|
During the latent stage, HCMV encodes miRNAs to inhibit its own DNA synthesis in infected cells and to reduce the production of inflammatory mediators synthesized by host cells mainly through directly suppressing their transcription or interfering with the signaling pathways that stimulate their production, thereby enabling long-term coexistence in host cells. Moreover, HCMV-encoded miRNAs also blunt the host immune response by weakening immune cells (Figure 2).
 Figure 2.
Figure 2. The interaction between HCMV-infected cells and immune cells. Innate immune response to early HCMV infection is mediated by APCs including DCs, NK cells through releasing multiple inflammatory cytokines. For example, HCMV-infected cells can directly trigger DCs to enhance antiviral IFN-I and secrete IL-6 and IL-10 through engagement of the TLR7 and/or TLR9 pathways. Activated NK cells promote anti-HCMV effect through binding NKG2D with its ligands to produce proinflammatory cytokines (e.g., TNF-α and IFN-γ) and cytotoxic granules containing effector molecules (e.g., granzyme B and perforin) to cause lysis or apoptosis of infected cells. NK cells also directly kill transformed and infected cells via antibody-dependent cellular cytotoxicity (ADCC). Besides, the control of HCMV viral replication and viral spreading is mediated by adaptive immune responses. For instance, CD4
+ and CD8
+ T cells recognize viral peptides presented by MHC-II and MHC-I molecules, respectively. Activated CD4
+ T cells secret large amounts of IL-2, IFN-γ and TNF-α to promote the killing effect of CD8
+ T cells and to assist B cells in producing antibodies. CD8
+ T cells lyse infected cells through secreting granzyme B, perforin and IFN-γ. CD8
+ T cells also produce CCL4 and CCL5 to recruit other immune cells to amplify local inflammation. Conversely, HCMV presents a series of miRNA-mediated strategies to resist immune attack and realize its latent infection. Herein take miR-UL112 as a typical example. In brief, miR-UL112 silences MICB mRNA translation thereby decreasing MICB binding to NKG2D and inhibiting differentiation of NK cells. Additionally, it attenuates NK cell-mediated cytotoxicity by downregulating inflammatory cytokines such as IL-32 and IFN, and TLR2-mediated NF-κB signaling. MiR-UL112-5p targets ERAP1, thereby inhibiting the processing and presentation of the HCMV pp65495-503 peptide to specific CTLs, and acts to reduce FOXO3 activity and BCL2L11 expression to promote infected cells’ survival. MiR-UL112 and other HCMV-encoded miRNAs also play essential roles in inhibiting viral DNA replication and apoptosis of host cells.
3. Conclusions and Outlook
HCMV is prevalent worldwide and causes lifelong latent infection, while it mainly contributes to the adverse prognosis of immunosuppressed patients upon reactivation. Due to minimal viral transcripts expressed, there are some troubles for technicians to raise a first alert to infection using present detection techniques; when HCMV is reactivated, it is intractable for doctors to assess cytomegalovirus disease stages due to its rapid progression and the suboptimal therapeutic choices thus far. It is far from enough to only detect nucleic acids for negative and positive results. Therefore, a comprehensive understanding of the HCMV-dominant molecular mechanisms is the key to prevention and treatment.
The development of HCMV vaccines has been going on for many years, some of which utilize viral protein antigens to elicit immune response, such as glycoprotein B/microfluidized adjuvant 59 (gB/MF59) vaccines, transgenic disabled infection single-cycle (DISC) vaccines, enveloped virus-like particle (eVLP) gB HCMV vaccines. There are also DNA-based HCMV vaccines (encoding both gB and pp65) which being conducted in preclinical or clinical trials and have achieved promising results
[52]. Previous studies have proposed the insertion of miR-let-7b or miR-93 target sequences into the genome of influenza virus strain H1N1 or H5N1 in order to attenuate the virus
[53][54]. Herein highlighted a series of HCMV-encoded miRNAs which are explicitly involved in HCMV reactivation (e.g., miR-UL148D) or in both latency and reactivation (e.g., miR-UL112). Therefore, development strategies of small molecule drug, as suggested by Abdalla
[4], will be more directed and they are ideal for congenital infection intervene and cytomegalic inclusion disease (CID) treatment. In the meantime, multiple concomitant regulatory factors (e.g., IL-6 and TNF-α) are also underlined. Step-by-step validation in clinical laboratory is urgent, and solid experimental data need to be further analyzed for their promising value in clinical application
[55].


 Figure 2. The interaction between HCMV-infected cells and immune cells. Innate immune response to early HCMV infection is mediated by APCs including DCs, NK cells through releasing multiple inflammatory cytokines. For example, HCMV-infected cells can directly trigger DCs to enhance antiviral IFN-I and secrete IL-6 and IL-10 through engagement of the TLR7 and/or TLR9 pathways. Activated NK cells promote anti-HCMV effect through binding NKG2D with its ligands to produce proinflammatory cytokines (e.g., TNF-α and IFN-γ) and cytotoxic granules containing effector molecules (e.g., granzyme B and perforin) to cause lysis or apoptosis of infected cells. NK cells also directly kill transformed and infected cells via antibody-dependent cellular cytotoxicity (ADCC). Besides, the control of HCMV viral replication and viral spreading is mediated by adaptive immune responses. For instance, CD4+ and CD8+ T cells recognize viral peptides presented by MHC-II and MHC-I molecules, respectively. Activated CD4+ T cells secret large amounts of IL-2, IFN-γ and TNF-α to promote the killing effect of CD8+ T cells and to assist B cells in producing antibodies. CD8+ T cells lyse infected cells through secreting granzyme B, perforin and IFN-γ. CD8+ T cells also produce CCL4 and CCL5 to recruit other immune cells to amplify local inflammation. Conversely, HCMV presents a series of miRNA-mediated strategies to resist immune attack and realize its latent infection. Herein take miR-UL112 as a typical example. In brief, miR-UL112 silences MICB mRNA translation thereby decreasing MICB binding to NKG2D and inhibiting differentiation of NK cells. Additionally, it attenuates NK cell-mediated cytotoxicity by downregulating inflammatory cytokines such as IL-32 and IFN, and TLR2-mediated NF-κB signaling. MiR-UL112-5p targets ERAP1, thereby inhibiting the processing and presentation of the HCMV pp65495-503 peptide to specific CTLs, and acts to reduce FOXO3 activity and BCL2L11 expression to promote infected cells’ survival. MiR-UL112 and other HCMV-encoded miRNAs also play essential roles in inhibiting viral DNA replication and apoptosis of host cells.
Figure 2. The interaction between HCMV-infected cells and immune cells. Innate immune response to early HCMV infection is mediated by APCs including DCs, NK cells through releasing multiple inflammatory cytokines. For example, HCMV-infected cells can directly trigger DCs to enhance antiviral IFN-I and secrete IL-6 and IL-10 through engagement of the TLR7 and/or TLR9 pathways. Activated NK cells promote anti-HCMV effect through binding NKG2D with its ligands to produce proinflammatory cytokines (e.g., TNF-α and IFN-γ) and cytotoxic granules containing effector molecules (e.g., granzyme B and perforin) to cause lysis or apoptosis of infected cells. NK cells also directly kill transformed and infected cells via antibody-dependent cellular cytotoxicity (ADCC). Besides, the control of HCMV viral replication and viral spreading is mediated by adaptive immune responses. For instance, CD4+ and CD8+ T cells recognize viral peptides presented by MHC-II and MHC-I molecules, respectively. Activated CD4+ T cells secret large amounts of IL-2, IFN-γ and TNF-α to promote the killing effect of CD8+ T cells and to assist B cells in producing antibodies. CD8+ T cells lyse infected cells through secreting granzyme B, perforin and IFN-γ. CD8+ T cells also produce CCL4 and CCL5 to recruit other immune cells to amplify local inflammation. Conversely, HCMV presents a series of miRNA-mediated strategies to resist immune attack and realize its latent infection. Herein take miR-UL112 as a typical example. In brief, miR-UL112 silences MICB mRNA translation thereby decreasing MICB binding to NKG2D and inhibiting differentiation of NK cells. Additionally, it attenuates NK cell-mediated cytotoxicity by downregulating inflammatory cytokines such as IL-32 and IFN, and TLR2-mediated NF-κB signaling. MiR-UL112-5p targets ERAP1, thereby inhibiting the processing and presentation of the HCMV pp65495-503 peptide to specific CTLs, and acts to reduce FOXO3 activity and BCL2L11 expression to promote infected cells’ survival. MiR-UL112 and other HCMV-encoded miRNAs also play essential roles in inhibiting viral DNA replication and apoptosis of host cells.



