Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Denis Prodius | + 2738 word(s) | 2738 | 2022-01-27 04:20:34 | | | |
| 2 | Denis Prodius | Meta information modification | 2738 | 2022-02-03 22:06:06 | | | | |
| 3 | Denis Prodius | + 17 word(s) | 2755 | 2022-02-03 22:09:35 | | | | |
| 4 | Denis Prodius | + 17 word(s) | 2755 | 2022-02-03 22:53:12 | | | | |
| 5 | Jason Zhu | -49 word(s) | 2706 | 2022-02-08 02:45:35 | | | | |
| 6 | Jason Zhu | + 48 word(s) | 2803 | 2022-02-08 02:48:30 | | | | |
| 7 | Jason Zhu | + 46 word(s) | 2849 | 2022-02-08 11:15:06 | | | | |
| 8 | Jason Zhu | + 1 word(s) | 2850 | 2022-02-08 11:24:17 | | | | |
| 9 | Jason Zhu | Meta information modification | 2850 | 2022-02-10 10:03:35 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Prodius, D. Application of Ionic Liquids for Metals. Encyclopedia. Available online: https://encyclopedia.pub/entry/19102 (accessed on 07 February 2026).
Prodius D. Application of Ionic Liquids for Metals. Encyclopedia. Available at: https://encyclopedia.pub/entry/19102. Accessed February 07, 2026.
Prodius, Denis. "Application of Ionic Liquids for Metals" Encyclopedia, https://encyclopedia.pub/entry/19102 (accessed February 07, 2026).
Prodius, D. (2022, February 03). Application of Ionic Liquids for Metals. In Encyclopedia. https://encyclopedia.pub/entry/19102
Prodius, Denis. "Application of Ionic Liquids for Metals." Encyclopedia. Web. 03 February, 2022.
Copy Citation
The recovery and separation of individual elements, critical materials and valuable metals from complex systems requires complex energy-consuming solutions with many hazardous chemicals used. Ionic liquids (ILs), also known as molten salts and future solvents, are endowed with unique features that have already had a promising impact on cutting-edge science and technologies. Functionalized ILs, solid extraction, Supported Ionic Liquid Phase (SILP), and agricultural waste-based IL show great promise for sustainable high-value metal recovery.
Battery Metals
Lithium
Ionic liquids
Metals for Electronic Devices and Optics
1. Battery Metals
The following metals to be discussed play a vital role in rechargeable battery technologies, of which the market is rapidly expanding in conjunction with the popularity of Electric Vehicles (EVs). Most EVs are powered by Li-ion batteries. The lithium-ion cell consists of an anode, cathode, current collectors, separator, and nonaqueous electrolyte. The anode is usually granite and the cathode a lithium-based oxide, such as LiCoO2, LiMn2O4, Li2MnO3, and LiNiMnCo2. Therefore Li, Mn, Co, and Ni markets have been evaluated to account for battery material consumption.
According to Olivetti et al. [1], most lithium-ion materials are expected to meet near future demands. However, concern over rapid adoption of electric vehicles may strain the supply of some battery-grade materials. In 2019, 100% of the U.S. manganese and 78% of cobalt supplies were imported, leaving the metal supply vulnerable to geopolitical factors, especially with the Democratic Republic of Congo producing 60% of the global supply of cobalt [2].
Lithium-ion batteries are popular due to their lightweight, high-energy density, and long-life cycles [2][3]. However, the technology is still improving, and the future of Li-ion batteries may require lower Ni content to optimize energy density. Consequently, the Global EV Outlook 2020 estimates that the amount of electric vehicle batteries retired by 2030 will be roughly equivalent to the current annual production of 100–120 GWh. They emphasize the need for battery reuse and recycling to decrease environmental liability and promote sustainable end-of-life practices [2].
1.1. Lithium
Primary sources of lithium include pegmatites and brines with salt lakes being the top exploitable resource. However, lithium in salt lake brines is always accompanied by magnesium and separating the two elements poses quite a challenge due to the elements’ similar chemical properties. Current methods for Li/Mg separation are time and reagent intensive, generate large amounts of waste, and have low Li recovery efficiency. IL systems are being developed to improve lithium recovery process.
Li et al. showcased the use of binary IL extractants for Mg removal to cut down on acid and base consumption [3]. [A336][V10] was synthesized from Aliquat 336 and Versatic Acid (a mixture of carboxylic acids) to be used in a three-stage counter-current extraction that boasted almost complete removal of magnesium and only 10% co-extraction of Li. Versatic acid was used because of its ability to extract magnesium impurities from concentrated lithium solution in a previous study [3]. Accordingly, [V10]− acted as the de-protonated acidic extractant molecule.
The binary IL was diluted with p-cymene, a bioderived solvent and proved to be more efficient on original brine rather than concentrated brine in an acid-based extraction where the reactions are driven by pH. Whereas with binary extractants, the reactions are driven by the common ion effect and the ion in this case being chloride. The binary extractants dependency on the common ion effect has been the major obstacle for implementing binary extractants because the salt concentration of most feeds, including seawater is not sufficient to drive extraction. Salt lake brines, however, have high salt concentrations; hence, they present a desirable scenario for binary extractants.
Recent findings published in Angewandte Chemie (Applied Chemistry) [4] led to the development of novel macromolecules for targeted complex coordination and selective extraction. The Sessler group reported a hemispherand-strapped calix[4]pyrrole ligand for selective binding to Li+ salts. Further research demonstrated that the molecule could operate successfully in solid–liquid or liquid–liquid phase extraction.
The Sessler process was mentioned in a review on metal separation for recycling and the researchers only critique was the use of toxic solvents, such as chloroform [5]. The main accomplishment of Ionic liquids (ILs) is as a greener substitute for traditional organic solvents. Combining ILs with this novel extractant would make the perfect combination. Sadly, there are likely many scenarios where ILs could be applied for exceptional process optimization, but the relative novelty of the field has prevented it thus far.
The study of ILs is often paired with supported liquid membrane (SLM) systems to improve efficiency as seen by Zante al in their efforts to selectively recover Li from brine. SILs feature an organic phase immobilized into the pores of a polymeric support placed between the feed and stripping aqueous solutions. In this work, a hydrophobic porous polyvinylidene fluoride (PVDF) membrane was impregnated with a mixture of [C4min][Tf2N] and TBP. The benefits of an SLM include a continuous permeation situation where extraction and stripping occur simultaneously. However, the lack of stability of SML currently limits their scale-up potential [6].
As exhibited by the example of SMLs and the earlier magnetic nanoparticles, ILs can be placed in or on many different structures. Liu et al. attempted a similar approach with TBP [C4min][Tf2N] [7]. Rather than impregnating a SLM, they used an electrodialysis system with a sandwiched liquid membrane consisting of the organic lithium-ion carrier and introduced two cation exchange membranes. The inner membranes acted as physical barriers between the feeding brine and receiving solution (Figure 1) [7].
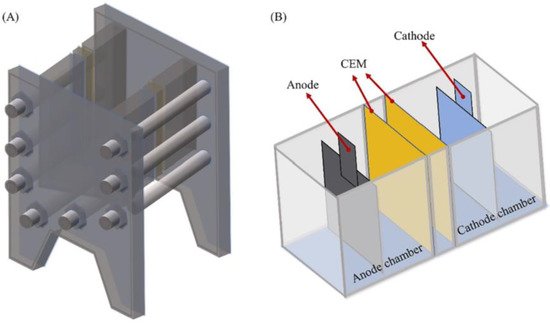
Figure 1. Schematic diagram of the electrodialysis device. (A) An assembled cell and (B) the placement of electrodes and CEMs in the cell. Reprinted from Desalination, 474, G. Liu, Z. Zhao, L. He, Highly selective lithium recovery from high Mg/Li ratio brines, 114185, Copyright (2020), with permission from Elsevier.
This system displays a great perspective on the application for Li recovery from Mg/Li brines and offered potential minimal impact on the environment with excellent Li ion selectivity. Although, in both cases, a non-fluorinated, cheaper IL with similar efficiencies could have been used. The electrodialysis can also be applied towards lithium extraction from seawater, which, in the past, has not been considered feasible due to the low salt concertation [7].
1.2. Cobalt
Cobalt (Co) is the most critical element in lithium-ion batteries due to its vulnerable supply chain. Due to this, there have been many papers published focusing on cobalt recovery from battery cathodes and mixed cobalt and nickel waste.
Most leaching processes, at least for nickel laterites and sulfides, extract both cobalt, nickel and result in a mixed Co/Ni solution that needs to be separated [8]. A favored industrial process for the selective solvent extraction of cobalt from nickel is the use of phosphinic acid derivatives, such as Cyanex 272. However, Cyanex 272 tends to extract impurities, such as Cu, Zn, Fe, Cd, Ca, Mg, and Mn along with cobalt [8], and thus there is a need for more selective processes.
1.3. Nickel and Manganese
Cobalt, nickel, and manganese are also components of lithium battery cathodes. The next few studies have taken a more holistic approach to lithium-ion battery recycling, attempting to separate all potential critical metals from simulated end-of-life batteries. Othman et al. [9] created a feed solution consisting of Cu, Mn, Ni, and Li, as reflecting typical cathode material. Tetraoctylphosphonium oleate [P₈₈₈₈][Oleate], a functionalized fatty-acid-based IL with selective extractive ability influenced by pH, was chosen due to the hydrophobic nature of both the cation and anion.
The bulky nature of the oleate-ion means that even when deprotonated, the ion remains very hydrophobic, which prevents the loss of the anion to the aqueous phase, a common problem in IL chemistry. At pH 5, cobalt, manganese and nickel were extracted with nearly 100% efficiency through interaction with the oleate ions, and Li was not extracted at all. At pH values lower than zero, Co and Mn maintained their high extraction efficiencies, but Ni was no longer extracted.
This phenomenon is due to the formation of tetrachloro-anion complexes in the presence of excess Cl− ions. Mn and Co were separated using mixed salt regeneration solutions, and Ni was selectively precipitated from Li in the form of NiCO3. Overall, [P₈₈₈₈][Oleate] successfully facilitated the separation and recovery of Co, Mn, Ni, and Li from simulated HCl-based Li-ion leachate [9]. Although this method was acidic and reagent intensive, it transformed a rather messy mixture of metals and separated them with one IL based on pH dependence. It would be of interest to more thoroughly understand the pH dependence of ILs as they present a better atom economy than a multi solvent-based system.
Another IL based Mn, Co, Ni, Li separation system [10] involved the following: first, manganese extraction via N,N,N′,N′-tetra(n-octyl) diglycolamide (TODGA) dissolved in imidazolium-based ILS. TODGA is an extractant commonly used in metal recovery and has an affinity for trivalent metals. Next, cobalt removal using a phosphonium-based IL, such as [P66614][Cl]. Lastly, nickel/lithium separation with a DES made from carboxylic acids and lidocain (DecA:Lid [2:1]). Although this process used significantly less acid than the previously mentioned process, it still required large quantities of solvents and extractants and needs optimization and compactization.
2. Metals for Electronic Devices and Optics
Indium, gallium, and germanium are used in technologies, including light-emitting diodes (LEDs), fiber optics, infrared optics, and solar cells. Indium and germanium are often grouped together due to their similar uses, and because both are recovered as byproducts from the same ore. Demand for these metals has increased in the past few years as new telecommunication networks compete to have the fastest transmission speeds. Indium is used mainly for production of tin-doped indium oxide used in flat-panel displays and touchscreens, as well as in the form of indium phosphide for lasers and receivers, used in fiber optic networks application [11].
Gallium Arsenide (GaAs) device consumption has also increased due to the telecommunications growth, as GaAs is used to manufacture integrated circuits and optoelectronic devices [12]. Germanium is mainly used in optics due to its transparency to part of the infrared electromagnetic spectrum, high refractive index, and low chromatic dispersion. Germanium also can catalyze the polymerization of PET resin without undesirable coloring.
2.1. Indium
Some ILs studied for the leaching and extraction of In(III) from aqueous solution include Cyphos® IL 101 [13], Aliquat®336 [14], [A324H+][Cl−] in Solvesso 100 [15], and [Hbet][Tf2N] in ascorbic acid [16]. The experiment using [Hbet][Tf2N] [16] was performed on crushed liquid crystal displays (LCDs). Ascorbic acid was added to decrease the distribution rate of iron and promote selective indium extraction. Overall, extraction of indium from this process reached up to 98.63%.
Indium was also selectively extracted from Ni(II) and Zn(II), by tri-butyl-phosphate (TBP) in n-hexyl-trimethylammonium bis(trifluoromethylsulfonyl)amide [N1116][TFSA] and then recovered by direct electrodeposition of the loaded organic phase [17]. The aqueous solution consisted of Fe(II), Ni(II), Zn(II), In(III), and Sn(II) ions in H[TFSA]. Nitric acid was added to oxidize Sn(II)/Sn(IV) and Fe(II)/Fe(III), which were then precipitated as hydroxide and oxide, respectively.
TBP, a neutral extractant soluble in ILs, was contacted with the aqueous phase to extract indium via a cation exchange mechanism. The electrodeposition of [In(TBP)33+]/In(0) under different potentiation conditions was then performed. The reduction peak at −1.0 V was assigned at the [In(TBP)33+]/In(0) couple and resulted in the electrodeposition of most pure In metal [17].
Binnemans’ group recently published studies on the electrodeposition of indium from ILs. The electrochemical deposition of indium from aqueous solutions has the unfortunate consequences of simultaneously releasing hydrogen gas. Ionic liquids present a way to circumvent hydrogen evolution by offering a wider electrochemical window than water. In the case of electrodeposition from an IL, the IL acts as a liquid cathode, which is advantageous over solid cathode because liquid cathodes offer a constant electrode surface and promote more regular crystal growth. The electrodeposition of In in Cyphos IL 101 involved two steps: the reduction of In(III) to In(I) followed by the reduction of In(I) to In(0). The electrodeposition of InCl3 and In(Tf2N)3 in DME or PEG400 was also tested. A combination of these electrolytes seemed to form an electroactive complex [18].
Indium can be found in goethite residue from zinc production. However, indium is only present in small concentrations, which means other higher concentrated elements, such as iron, are often leached together with indium. Supported Ionic Liquid Phase (SILP) extractions have also been examined for recovery of indium, as well as germanium, from iron-rich solutions.
SILPs consists of a thin IL layer covalently anchored or impregnated onto a solid support, usually a porous resin. SILPs hold great potential for industrial scale operations due to the ease in which the solid phase can be separated from the aqueous phase at the completion of the reaction. Both indium and germanium were selectively recovered from iron rich solutions using Aliquat336- based ionic liquids impregnated on Amberlite XAD-16N, with SFIn/Fe equal to 5400 and SFGe/Fe equal to 34,400 [14][19]. Aliquat 336 is a commercial liquid anion exchanger that consists of a quaternary ammonium salt and is a popular choice for IL cations.
2.2. Germanium
As aforementioned, one of the main sources of Ge used in industrial processes is by-product from zinc ore processing. Another major source of Ge is coal fly ash from power plants. Coal ash can be leached with water; however, the resulting leachate contains many elements and germanium in the form of GeO2 and GeS2, therefore, solvent extraction is a popular method for separation [20]. Extraction agents Alamine 336, Aliquat 336, and Cynaex 923 have been used for Ge separation, with the aid of tartaric acid and oxalic acid complexing agents [21]. Aliquat 336 has also been used in conjugation with a flat sheet and hollow fiber supported ionic liquid membrane (HFSLM) system for selective germanium(IV) extraction [22].
Since 2016, at least 30% of the germanium in the world has been provided through recycled germanium materials, such as electronic devices and optical fibers. According to Ruiz, Sola, and Palmerola the recycling efficiency for fiber-optics scrap is higher than 80% [23].
After extraction of germanium from end-of-life products, it can be reprocessed through electrodeposition, which not only guarantees a high purity but also the ability to form special structures, such as nanoparticles and nanowires (for semiconductors). One study reported the deposition of germanium from GeCl4 saturated [BMIM][PF6] onto gold [24]. Another study reported electrochemical deposition gallium nanoparticles and germanium nanostructures from [EMIm]Tf2N using pulsed laser assistance [25].
2.3. Gallium
Gallium can be found in semiconductors in the forms of GaN and GaAs, accompanied by InAs. Semiconductors and LEDs, despite containing valuable sources of gallium and indium, are often not recycled due to recycling difficulties. Plastic casings and dismantling small parts are examples of some challenges. Acid can be used to effectively leach semiconductors; however, the simultaneous formation of lethal arsine (AsH3) gas poses a significant health and safety hazard.
ILs offer an alternative leaching method that is significantly safer. Trihalide ILs are capable of dissolving metals and alloys without the formation of any gases. van de Bossche et al. used [P44410][Br3] to demonstrate its effectiveness in leaching semiconductor compounds and real LEDs [26]. After the metals were saturated in the organic phase, Arsenic was stripped using NaBr salt, gallium was stripped with water, and indium was recovered using Cyphos 101 to form an indium(III) hydroxide precipitate. Unfortunately, GaN could not be dissolved through this process.
Gallium does not readily form minerals in which it is the major component; consequently, most gallium is recovered as a by-product from the processing of other raw materials. Like germanium, gallium can be found in zinc ore residue, coal ash, bauxite residues and iron mine tailings. Bauxite is the major ore from aluminum production. During the Bayer process, the bauxite is digested by sodium hydroxide, and aluminum is crystallized out.
The leftover Bayer leach liquor contains a mixed cocktail of gallium, vanadium, silica, residual ammonium, and other impurities. In the 1990s, the extraction of gallium with Kelex 100 was explored but determined to be impractically slow to be employed on an industrial scale. However, Raigual et al. recently studied the behavior of Kelex 100 in 1,2,3- triazolium IL diluents [27].
ILs were superior to kerosene systems because they equilibrated faster, bonded to gallium stronger, and avoided the use of volatile and flammable organic solvents. At optimal conditions, 4-ethyl-5-methyl-1,3-dihexyl-1,2,3-triazolium bis(trifluoromethylsulfonyl)imide [HHT23][Tf2N] reached Ga/Al distribution ratios as high as 1000 and yielded a pure gallium product. This process shows promise for industrial application, especially since it does not require decomposing the Bayer liquids before extraction.
Kinsmen et al. [28] attempted to recover gallium from iron mine tailings using methyltriocytlammonia [MTOA][I] (where [MTOA] = [A336]), which serves the dual purposed of reducing iron(III) to a less soluble state and extracting gallium. Using [MTOA][I] as a reductant and reactant proved to be operationally simple and cost effective. This experiment exemplifies Green Chemistry principles with environmentally viable reagents, a regenerable organic phase, and reduction of waste streams compared to traditional methods. [MTOA][I] was diluted in toluene and water was used as a stripping agent. The next steps would be using pure [MTOA][I] without a diluent as was proven possible in the experiment done with water saturated [HTOA][NO3] extraction of Pd mentioned earlier in this paper [28][29].
References
- Olivetti, E.A.; Ceder, G.; Gaustad, G.G.; Fu, X. Lithium-Ion Battery Supply Chain Considerations: Analysis of Potential Bottlenecks in Critical Metals. Joule 2017, 1, 229–243.
- Abergel, T.; Bunsen, T.; Gorner, M.; Leduc, P. Global EV Outlook 2020: Entering the Decade of Electric Drive? OECD Publishing: Paris, France, 2020.
- Li, Z.; Mercken, J.; Li, X.; Riaño, S.; Binnemans, K. Efficient and Sustainable Removal of Magnesium from Brines for Lithium/Magnesium Separation Using Binary Extractants. ACS Sustain. Chem. Eng. 2019, 7, 19225–19234.
- He, Q.; Williams, N.J.; Oh, J.H.; Lynch, V.M.; Kim, S.K.; Moyer, B.A.; Sessler, J.L. Selective Solid-Liquid and Liquid-Liquid Extraction of Lithium Chloride Using Strapped CalixPyrroles. Angew. Chem. 2018, 130, 12100–12104.
- Nelson, J.J.M.; Schelter, E.J. Sustainable Inorganic Chemistry: Metal Separations for Recycling. Inorg. Chem. 2019, 58, 979–990.
- Zante, G.; Boltoeva, M.; Masmoudi, A.; Barillon, R.; Trébouet, D. Lithium Extraction from Complex Aqueous Solutions Using Supported Ionic Liquid Membranes. J. Membr. Sci. 2019, 580, 62–76.
- Liu, G.; Zhao, Z.; He, L. Highly Selective Lithium Recovery from High Mg/Li Ratio Brines. Desalination 2020, 474, 114185.
- A New Process for Cobalt—Nickel Separation. Available online: https://www.teck.com/media/CESL-Publication-Nickel-New-Process-Cobalt-Nickel-Separation-2010.pdf (accessed on 29 November 2021).
- Othman, E.A.; van der Ham, A.G.J.; Miedema, H.; Kersten, S.R.A. Recovery of Metals from Spent Lithium-Ion Batteries Using Ionic Liquid . Sep. Purif. Technol. 2020, 252, 117435.
- Zante, G.; Braun, A.; Masmoudi, A.; Barillon, R.; Trébouet, D.; Boltoeva, M. Solvent Extraction Fractionation of Manganese, Cobalt, Nickel and Lithium Using Ionic Liquids and Deep Eutectic Solvents. Miner. Eng. 2020, 156, 106512.
- Shanks, W.C.P., III; Kimball, B.E.; Tolcin, A.C.; Guberman, D.E. Germanium and Indium. In Critical Mineral Resources of the United States—Economic and Environmental Geology and Prospects for Future Supply; Schulz, K.J., DeYoung, J.H., Jr., Seal, R.R., II, Bradley, D.C., Eds.; U.S. Geological Survey Professional Paper; U.S. Geological Survey: Reston, VA, USA, 2017; pp. 1–27.
- PGMs Retain their Sheen. Available online: https://www.recyclingtoday.com/article/platinum-group-metals-commodity-focus/ (accessed on 29 November 2021).
- Deferm, C.; Onghena, B.; Nguyen, V.T.; Banerjee, D.; Fransaer, J.; Binnemans, K. Non-Aqueous Solvent Extraction of Indium from an Ethylene Glycol Feed Solution by the Ionic Liquid Cyphos IL 101: Speciation Study and Continuous Counter-Current Process in Mixer-Settlers. RSC Adv. 2020, 10, 24595–24612.
- van Roosendael, S.; Regadío, M.; Roosen, J.; Binnemans, K. Selective Recovery of Indium from Iron-Rich Solutions Using an Aliquat 336 Iodide Supported Ionic Liquid Phase (SILP). Sep. Purif. Technol. 2019, 212, 843–853.
- Alguacil, F.J.; Escudero, E. Solvent Extraction of Indium (III) from HCl Solutions by the Ionic Liquid (A324H+) (Cl−) Dissolved in Solvesso 100. Hydrometallurgy 2019, 189, 105104.
- Luo, D.; Zhu, N.; Li, Y.; Cui, J.; Wu, P.; Wang, J. Simultaneous Leaching and Extraction of Indium from Waste LCDs with Acidic Ionic Liquids. Hydrometallurgy 2019, 189, 105146.
- Matsumiya, M.; Sumi, M.; Uchino, Y.; Yanagi, I. Recovery of Indium Based on the Combined Methods of Ionic Liquid Extraction and Electrodeposition. Sep. Purif. Technol. 2018, 201, 25–29.
- Monnens, W.; Deferm, C.; Sniekers, J.; Fransaer, J.; Binnemans, K. Electrodeposition of Indium from Non-Aqueous Electrolytes. Chem. Commun. 2019, 55, 4789–4792.
- van Roosendael, S.; Roosen, J.; Banerjee, D.; Binnemans, K. Selective Recovery of Germanium from Iron-Rich Solutions Using a Supported Ionic Liquid Phase (SILP). Sep. Purif. Technol. 2019, 221, 83–92.
- Nguyen, T.H.; Lee, M.S. A Review on Germanium Resources and Its Extraction by Hydrometallurgical Method. Miner. Process. Extr. Metall. Rev. 2020, 42, 406–426.
- Kamran Haghighi, H.; Irannajad, M.; Fortuny, A.; Sastre, A.M. Recovery of Germanium from Leach Solutions of Fly Ash Using Solvent Extraction with Various Extractants. Hydrometallurgy 2018, 175, 164–169.
- Kamran Haghighi, H.; Irannajad, M.; Fortuny, A.; Sastre, A.M. Selective Separation of Germanium (IV) from Simulated Industrial Leachates Containing Heavy Metals by Non-Dispersive Ionic Extraction. Miner. Eng. 2019, 137, 344–353.
- Ruiz, A.G.; Sola, P.C.; Moreno Palmerola, N.M. Germanium: Current and Novel Recovery Processes. In Advanced Material and Device Applications with Germanium; Lee, S., Ed.; InTechOpen: London, UK, 2018; Available online: https://doi.org/10.5772/intechopen.77997 (accessed on 29 November 2021).
- Endres, F.; Zein El Abedin, S. Nanoscale Electrodeposition of Germanium on Au (111) from an Ionic Liquid: An in Situ STM Study of Phase Formation. Part II. Ge from GeCl4. Phys. Chem. Chem. Phys. 2002, 4, 1649–1657.
- Yu, Z.; Meng, X.; Yin, M.; Sun, M.; Yuan, M.; Li, H. Pulsed Laser-Assisted Ionic Liquid Electrodeposition of Gallium Nanoparticles and Germanium Nanostructures for Energy Storage. Chem. Phys. Lett. 2018, 698, 181–186.
- van den Bossche, A.; Vereycken, W.; vander Hoogerstraete, T.; Dehaen, W.; Binnemans, K. Recovery of Gallium, Indium, and Arsenic from Semiconductors Using Tribromide Ionic Liquids. ACS Sustain. Chem. Eng. 2019, 7, 14451–14459.
- Raiguel, S.; Dehaen, W.; Binnemans, K. Extraction of Gallium from Simulated Bayer Process Liquor by Kelex 100 Dissolved in Ionic Liquids. Dalton Trans. 2020, 49, 3532–3544.
- Kinsman, L.M.M.; Morrison, C.A.; Ngwenya, B.T.; Love, J.B. Reducing the Competition: A Dual-Purpose Ionic Liquid for the Extraction of Gallium from Iron Chloride Solutions. Molecules 2020, 25, 4047.
- Katsuta, S.; Tamura, J. Extraction of Palladium (II) and Platinum (IV) from Hydrochloric Acid Solutions with Trioctylammonium Nitrate Ionic Liquid without Dilution. J. Solut. Chem. 2018, 47, 1293–1308.
More
Information
Subjects:
Chemistry, Applied
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
9 times
(View History)
Update Date:
10 Feb 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




