| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Bartłomiej Gaida | + 493 word(s) | 493 | 2020-08-30 11:30:25 |
Video Upload Options
Carbohydrate-derived ionic liquids with at least one ionic counterpart derived from carbohydrate precursor have been explored as bio-alternatives to conventional ionic liquids for over a decade. Since their discovery, significant progress has been made regarding synthetic methods, understanding their environmental impact, and developing perspectives on their potential applications. Carbohydrates can be converted into cations or anions for ILs through standard reactions that are already applied widely in carbohydrate chemistry. Most of the research on sugar-based ILs has focused on cation synthesis, though the most promising synthetic route towards ILs (in terms of the number of required synthetic steps) involves transforming carbohydrates into anions such as gluconate, glucuronate, or galacturonate. Moreover, functionalization of poly(ionic liquid)s (PILs) with sugar moieties has also recently been investigated. The most common precursors for carbohydrate-derived ionic liquids include glucose, galactose, fructose, ribose, xylose, arabinose, isomannide, isosorbide, ribitol, and mannitol.
1. Introduction
Ionic liquids (ILs), which are composed of organic cations and organic or inorganic anions, show negligible vapor pressure, high thermal stability, and very low flammability. These properties initially classified them as “green” solvents, representing them a promising alternative to VOCs[1].Simultaneously, their chemical stability and often high solubility in water, may question their environmentally benign character. The improvement of this aspect has been proposed by the development of biomass-derived ILs that originate from compounds commonly existing in nature such as amino acids, carbohydrates, carboxylic acids, or choline. Biomass-derived ILs (bio-ILs) demonstrate lower toxicity and higher biocompatibility than their fossil fuel-derived analogues[2][3].
Among bio-ILs, considerable progress has recently been made regarding the use of sugars or carbohydrates, which are abundant, inexpensive, renewable, and environmentally friendly. Ionic liquids and salts produced using carbohydrates have been investigated for over a decade, with initial efforts focused on their functionalization and how the introduced functionalities relate to their physicochemical characteristics.[4] Notably, such systems have been recognized for their applicability as organocatalysts and solvents in ways that take advantage of their chirality[5]. Currently, broadened perspectives regarding potential IL applications span electrochemistry[6], energy storage[7][8], anti-bacterial systems[9][10], herbicides[11], and biomass conversion[12]. Carbohydrates have also recently been shown to reduce toxicity when incorporated into common imidazolium IL structures, thereby further enhancing their biocompatible character[13]. Although sugars are an economically favorable starting material, the process of manufacturing their ionic derivatives was originally complicated because of the required multiple-step synthetic method[4]. Therefore, recent research efforts aimed at improving and simplifying the synthesis of carbohydrate-derived ILs have focused on implementing protocols to reduce the number of synthetic steps and/or integrate green chemistry approaches. Such improvements have highlighted a more economically promising perspective regarding carbohydrate-based ILs and their applications.
2. Applications of carbohydrate-derived ILs
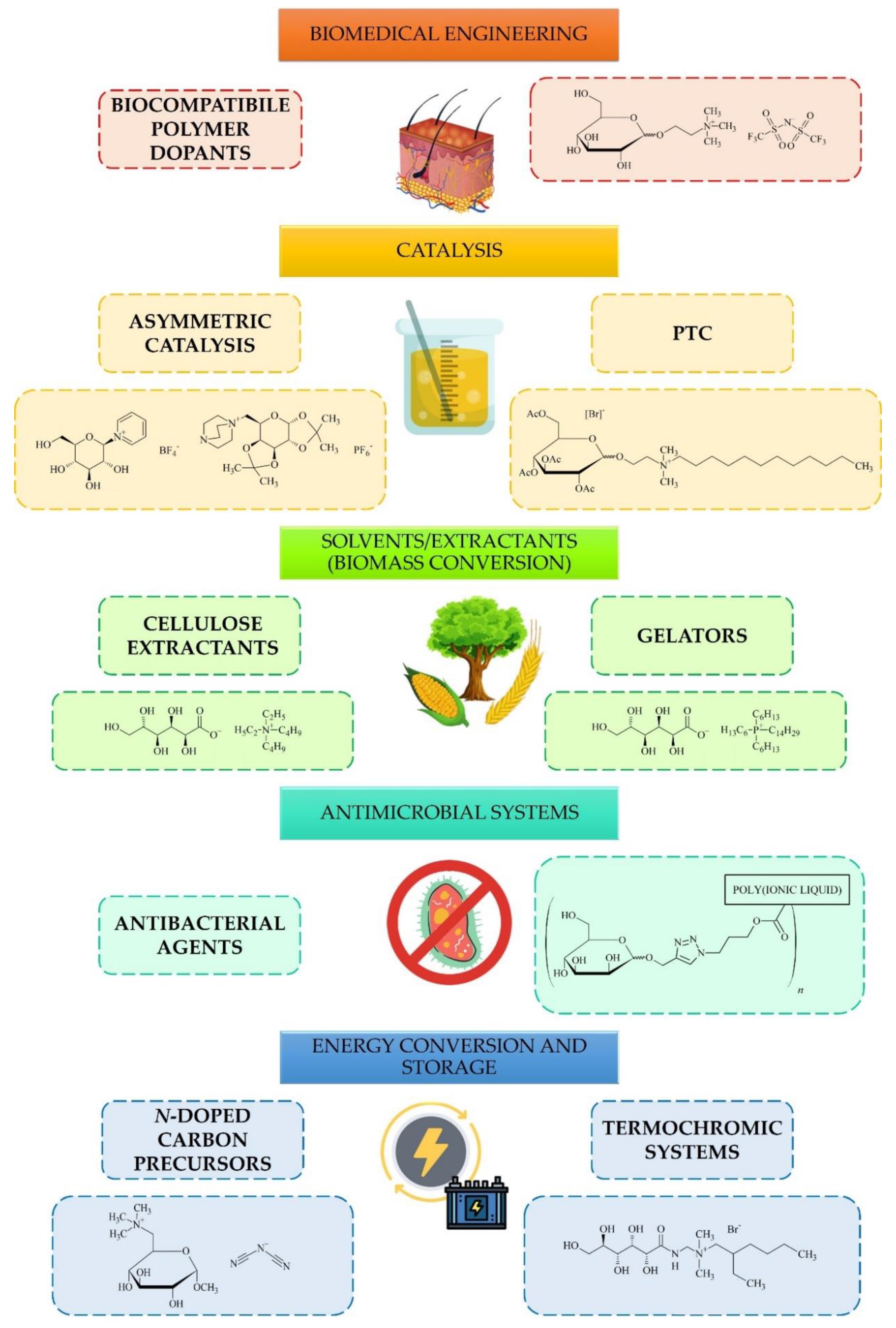
Due to versatile features such as hydrogen bond-rich structure, chirality, low-toxicity or high biodegradability, carbohydrate-derived ILs have been explored for a number of applications that span catalysis[14][15][16][17][18], biomedicine[6] and ecology[11], energy conversion and storage[7][8] or application as solvents[12][19] (Figure 1).
Figure 1. Applications and future perspectives for carbohydrate-derived ILs.
References
- Tom Welton; Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis. Chemical Reviews 1999, 99, 2071-2084, 10.1021/cr980032t.
- Joris Hulsbosch; Dirk E. De Vos; Koen Binnemans; Rob Ameloot; Biobased Ionic Liquids: Solvents for a Green Processing Industry?. ACS Sustainable Chemistry & Engineering 2016, 4, 2917-2931, 10.1021/acssuschemeng.6b00553.
- Joana Margarida Gomes; Simone S. Silva; Rui L. Reis; Biocompatible ionic liquids: fundamental behaviours and applications. Chemical Society Reviews 2019, 48, 4317-4335, 10.1039/c9cs00016j.
- Bartłomiej Gaida; Alina Brzęczek-Szafran; Insights into the Properties and Potential Applications of Renewable Carbohydrate-Based Ionic Liquids: A Review. Molecules 2020, 25, 3285, 10.3390/molecules25143285.
- Nirmaljeet Kaur; Avtar Singh; Harish Kumar Chopra; Exploring Low-Cost Natural Precursors as Chiral Building Blocks in Synthesis: Chiral Carbohydrate-Ionic Liquids. Mini-Reviews in Organic Chemistry 2018, 15, 208-219, 10.2174/1570193x15666171218161135.
- Katarzyna Krukiewicz; Dominika Kobus; Roman Turczyn; Karol Erfurt; Anna Chrobok; Manus J.P. Biggs; Low resistance, highly corrugated structures based on poly(3,4-ethylenedioxythiophene) doped with a d-glucopyranoside-derived ionic liquid. Electrochemistry Communications 2020, 110, 106616, 10.1016/j.elecom.2019.106616.
- Floriana Billeci; H. Q. Nimal Gunaratne; Francesca D'anna; Grace G. Morgan; Kenneth R. Seddon; Natalia V. Plechkova; A magnetic self-contained thermochromic system with convenient temperature range. Green Chemistry 2019, 21, 1412-1416, 10.1039/c8gc03906b.
- Alina Brzeczek; Karol Erfurt; Agata Blacha-Grzechnik; Maciej Krzywiecki; Sławomir Boncel; Anna Chrobok; Carbohydrate Ionic Liquids and Salts as All-in-One Precursors for N-Doped Carbon. ACS Sustainable Chemistry & Engineering 2019, 7, 19880-19888, 10.1021/acssuschemeng.9b05297.
- Mei Hong; Ziyue Miao; Xiao-Ling Xu; Qiang Zhang; Magnetic Iron Oxide Nanoparticles Immobilized with Sugar-Containing Poly(ionic liquid) Brushes for Efficient Trapping and Killing of Bacteria. ACS Applied Bio Materials 2020, 3, 3664-3672, 10.1021/acsabm.0c00298.
- Jing Chen; Die Li; Chunyang Bao; Qiang Zhang; Controlled synthesis of sugar-containing poly(ionic liquid)s. Chemical Communications 2020, 56, 3665-3668, 10.1039/c9cc09858e.
- Juliusz Pernak; Kamil Czerniak; Agnieszka Biedziak; Katarzyna Marcinkowska; Tadeusz Praczyk; Karol Erfurt; Anna Chrobok; Herbicidal ionic liquids derived from renewable sources. RSC Advances 2016, 6, 52781-52789, 10.1039/C6RA06703D.
- Fatima Javed; Faheem Ullah; Hazizan Md Akil; Synthesis, characterization and cellulose dissolution capabilities of ammonium-based room temperature ionic liquids (RTILs). Pure and Applied Chemistry 2018, 90, 1019-1034, 10.1515/pac-2017-0315.
- Floriana Billeci; Francesca D'anna; Marta Feroci; Patrizia Cancemi; Salvatore Feo; Antonella Forlino; Francesca Tonnelli; Kenneth Richard Seddon; H. Q. Nimal Gunaratne; Natalia V. Plechkova; et al. When Functionalization Becomes Useful: Ionic Liquids with a “Sweet” Appended Moiety Demonstrate Drastically Reduced Toxicological Effects. ACS Sustainable Chemistry & Engineering 2019, 8, 926-938, 10.1021/acssuschemeng.9b05507.
- Rui Yuan; Yuan-Jiang Wang; Yue Fang; Wen-Hui Ge; Wei Lin; Ming-Qi Li; Jiang-Biao Xu; Yu Wan; Yun Liu; Hui Wu; et al. The first direct synthesis of chiral Tröger’s bases catalyzed by chiral glucose-containing pyridinium ionic liquids. Chemical Engineering Journal 2017, 316, 1026-1034, 10.1016/j.cej.2017.02.026.
- Nirmaljeet Kaur; Harish K. Chopra; Synthesis and applications of carbohydrate based chiral ionic liquids as chiral recognition agents and organocatalysts. Journal of Molecular Liquids 2020, 298, 111994, 10.1016/j.molliq.2019.111994.
- Karol Erfurt; Ilona Wandzik; Krzysztof Walczak; Karolina Matuszek; Anna Chrobok; Hydrogen-bond-rich ionic liquids as effective organocatalysts for Diels–Alder reactions. Green Chemistry 2014, 16, 3508-3514, 10.1039/c4gc00380b.
- Alina Brzeczek; Przemysław Więcek; Maciej Guzik; Anna Chrobok; Combining amino acids and carbohydrates into readily biodegradable, task specific ionic liquids. RSC Advances 2020, 10, 18355-18359, 10.1039/d0ra03664a.
- Karol Erfurt; Marta Markiewicz; Agnieszka Siewniak; Dawid Lisicki; Mariusz Zalewski; Stefan Stolte; Anna Chrobok; Biodegradable Surface Active D-Glucose Based Quaternary Ammonium Ionic Liquids in the Solventless Synthesis of Chloroprene. ACS Sustainable Chemistry & Engineering 2020, 8, 10911–10919, 10.1021/acssuschemeng.0c03239.
- Floriana Billeci; Francesca D'anna; H. Q. Nimal Gunaratne; Natalia V. Plechkova; Kenneth R. Seddon; “Sweet” ionic liquid gels: materials for sweetening of fuels. Green Chemistry 2018, 20, 4260-4276, 10.1039/c8gc01615a.