Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Qinglan Zhao | + 2056 word(s) | 2056 | 2022-01-24 07:14:20 | | | |
| 2 | Qinglan Zhao | Meta information modification | 2056 | 2022-02-03 12:41:24 | | | | |
| 3 | Vicky Zhou | Meta information modification | 2056 | 2022-02-03 14:49:43 | | | | |
| 4 | Vicky Zhou | -55 word(s) | 2001 | 2022-02-07 04:08:35 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zhao, Q. Seawater Electrolysis. Encyclopedia. Available online: https://encyclopedia.pub/entry/19089 (accessed on 12 January 2026).
Zhao Q. Seawater Electrolysis. Encyclopedia. Available at: https://encyclopedia.pub/entry/19089. Accessed January 12, 2026.
Zhao, Qinglan. "Seawater Electrolysis" Encyclopedia, https://encyclopedia.pub/entry/19089 (accessed January 12, 2026).
Zhao, Q. (2022, February 03). Seawater Electrolysis. In Encyclopedia. https://encyclopedia.pub/entry/19089
Zhao, Qinglan. "Seawater Electrolysis." Encyclopedia. Web. 03 February, 2022.
Copy Citation
Hydrogen energy, as a clean and renewable energy, has attracted much attention in recent years. Water electrolysis via the hydrogen evolution reaction at the cathode coupled with the oxygen evolution reaction at the anode is a promising method to produce hydrogen. Given the shortage of freshwater resources on the planet, the direct use of seawater as an electrolyte for hydrogen production has become a hot research topic. Direct use of seawater as the electrolyte for water electrolysis can reduce the cost of hydrogen production due to the great abundance and wide availability. Various high-efficiency electrocatalysts have made great progress in seawater splitting and have shown great potential.
seawater splitting
metal oxides
metal phosphides
metal nitrides
electrocatalysts
1. Introduction
Due to the ever-increasing energy demand and the exponential growth of energy consumption, people are facing a great challenge caused by the shortage of fossil energy storage together with the related pollution. The energy conversion system, to generate green energy as an alternative to fossil fuels, becomes an essential part to build a roadmap towards sustainable energy future [1][2]. Among the green and sustainable energy, hydrogen is a clean energy resource with great abundance and significant energy density, since it can be produced from water without release of carbon dioxide or other toxic products in the process of conversion to other forms of energy. Fossil fuels (such as natural gas, coal, or biomass gasification) are still the main source for producing hydrogen in the current industrial production, occupying 96% of hydrogen production. Only 4% is produced through electrolytic water [3][4][5]. Theoretically, only a potential of 1.23 V is required for water splitting. However, a high voltage, typically larger than 1.8 V, is needed to trigger the water splitting reaction. Water electrolysis consists of a two-electron transfer cathodic hydrogen evolution reaction (HER) and a four-electron coupled anodic oxygen evolution reaction (OER). The large activation barriers in HER and OER pathways result in large overpotentials, thus leading to slow kinetics of water electrolysis [6][7][8]. Therefore, people must use electrocatalysts with high activity to reduce the activation energy barrier of HER and OER, lower the overpotential and ultimately achieve a more efficient energy conversion.
At this stage, research of electrolysis for hydrogen production using freshwater has yielded good results; however, research of seawater electrolysis for hydrogen production is still at the early stage [9][10]. The freshwater resource is scarce, most of which remains frozen or in the form of chemosynthetic water. On the contrary, seawater, accounting for 96.5% of the earth’s resources, is world-widely abundant. It is promising to utilize seawater as electrolyte for water electrolysis to produce hydrogen. However, there are up to 3.5 wt% salts in seawater in presence of different metal ions, which may participate in various competing electrochemical reactions to HER at the cathode, limiting the efficiency of water electrolysis [9][10][11][12]. Moreover, the presence of bacteria and microorganisms in natural seawater can lead to the formation of insoluble precipitates on the active site of the catalyst surface, which may affect the HER performance (Figure 1) [13][14]. On the other hand, high concentration of chloride ions (ca. 0.5 M) may block the active center of catalysts [15]. Furthermore, the chlorine evolution reaction (CER) may also occur as a competitive reaction to OER at the anode [16]. Under acidic conditions at pH = 0, the theoretical overpotential of four-electron transfer OER (1.23 V vs. SHE) is 130 mV lower than that of two-electron transfer CER, and thus, CER has faster kinetics [17]. Given that the overpotential of CER does not vary with pH as OER does, the inhibition of CER can be achieved by increasing the alkalinity of the electrolyte. Nevertheless, another reaction occurs in an alkaline environment, namely, the hypochlorite production reaction [18]. Therefore, in order to achieve high-efficiency hydrogen production and realize industrial seawater electrolysis, it is necessary to have a systematic summary and in-depth understanding of the reaction mechanism as well as the related parameters affecting the performance.
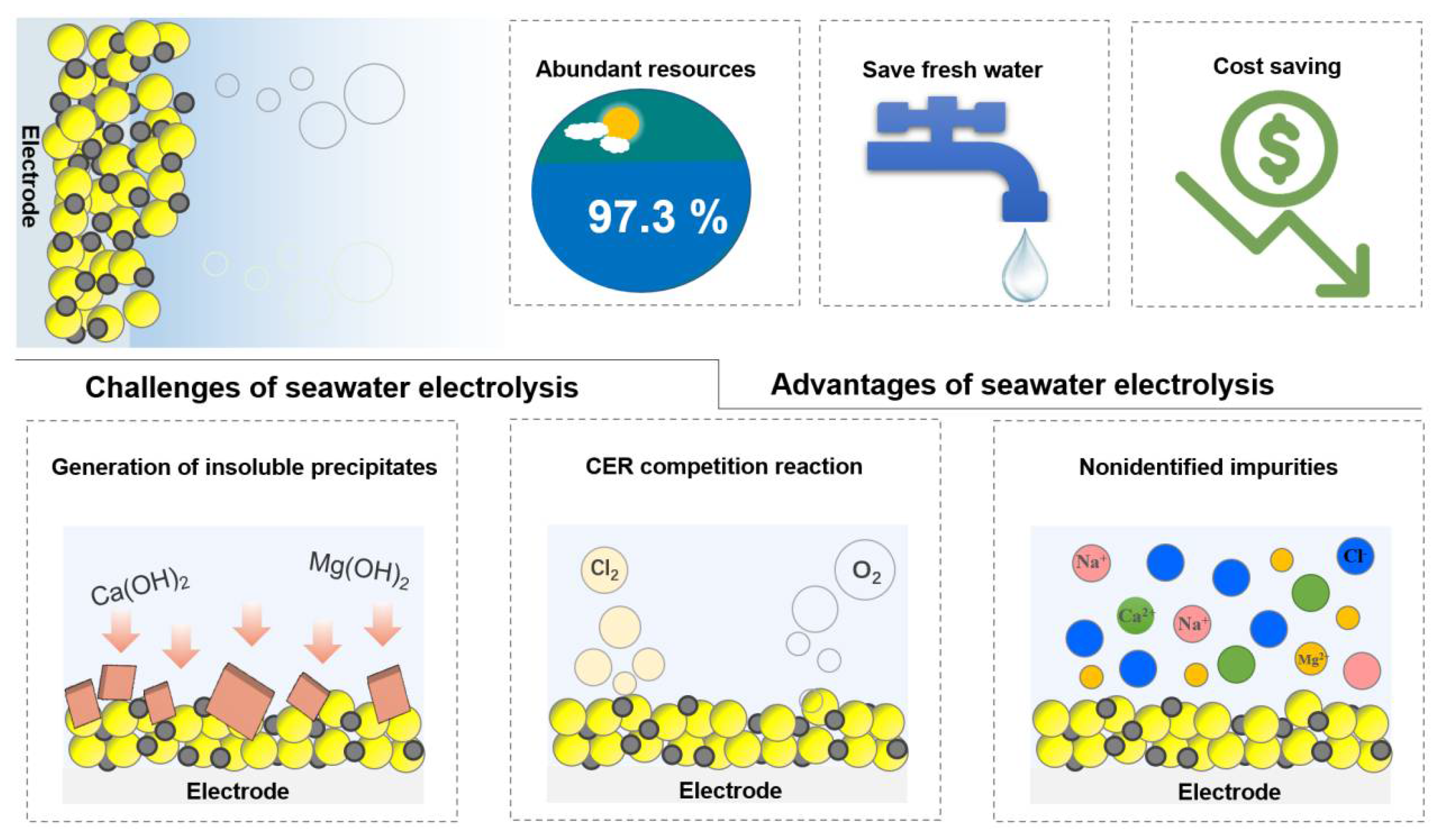
Figure 1. Advantages and challenges of seawater electrolysis.
Up to now, various catalysts have been studied into the seawater electrolysis [9][10][19][20][21][22][23][24][25][26][27][28][29][30][31][32]. Successful examples, such as metal (hydrogen) oxides, nitrides, phosphides, borides, and hybrid catalysts have been applied as catalysts for OER in seawater. In addition to what have been studied for OER, noble metal alloy, carbon-supported noble metals, MXene-based complexes as well as the hybrid materials with different composites or structures have been utilized as catalysts for HER in seawater. All these catalysts have their own merits and demerits in the seawater electrolysis.
2. Electrocatalysts for Driving Seawater Electrolysis
2.1. Electrocatalysts for OER
As discussed above, the competitive oxidation of chloride ions in seawater not only reduces the efficiency of the OER but also produces chlorine-containing species damaging the electrolyzer and causing environmental issues [33][34][35]. Consequently, excellent catalysts are needed to enable the selective OER. Up to now, a large number of materials, such transition metal oxides and hydroxides, metal phosphides, metal nitrides, metal borides, and hybrid electrocatalysts, have been explored as selective OER catalysts in seawater electrolysis (Table 1).
Table 1. Summary of OER performance of reported electrocatalysts.
| Catalyst | Electrolytes | Electrodes | Overpotential@10mA cm−2 (mV) | Tafel Slope (mV dec−1) | Mass Loading (mg cm−2) | Ref. |
|---|---|---|---|---|---|---|
| FTO/NiO | 1.0 M KOH + 0.5 M NaCl |
FTO | 401 | [36] | ||
| Pb2Ru2O7−x | 0.6 M NaCl | GCE | 500 | 48 | 0.2 | [37] |
| Pb2Ru2O7−x | 0.6 M NaCl + 0.1 M NaOH | GCE | 200 | 45 | 0.2 | [37] |
| Co(OH)2 | 0.25 M Mg(ClO4)2 | FTO | 125 | [38] | ||
| Co(OH)2 | MgCl2 | FTO | 63 | [38] | ||
| Mg|Co-MnO2/Co(OH)2 | 0.25 M Mg(ClO4)2 | FTO | 151 | [38] | ||
| Mg|Co-MnO2/Co(OH)2 | 0.25 M MgCl2 | FTO | 144 | [38] | ||
| NiFe-LDH | 1 M KOH + 0.5 M NaCl | NF | 227 (100 mA/cm2) | 0.32 | [39] | |
| S-(Ni,Fe)OOH | 1 M KOH + Seawater | NF | 300 (100 mA/cm2) | [19] | ||
| S-(Ni,Fe)OOH | 1 M KOH | NF | 281 (100 mA/cm2) | 48.9 | [19] | |
| Ni2P-Fe2P | 1 M KOH | NF | 452 (100 mA/cm2) | 58 | 15.0 | [40] |
| Ni2P-Fe2P | 1 M KOH + Seawater | NF | 581 (100 mA/cm2) | 15.0 | [40] | |
| Co-Fe2P | 1 M KOH | NF | 274 (100 mA/cm2) | 45 | 2.0 | [41] |
| Co-Fe2P | 1 M KOH + 0.5 M NaCl | NF | 460 (100 mA/cm2) | 2.0 | [41] | |
| Ti@NiB | 1 M KOH + 0.5 M NaCl | Ti plate | 397 (50 mA/cm2) | 34.2 | 3.2 | [42] |
| Co-Fe-O-B | 1 M KOH + 0.5 M NaCl | GCE | 294 | 0.1 | [23] | |
| multilayered NiFeBx | 1 M KOH + 0.5 M NaCl | 263 ± 14 | [43] | |||
| NiMoN@NiFeN | 1 M KOH + Seawater | NF | 369 (500 mA/cm2) | [18] | ||
| Fe-Ni(OH)2/Ni3S2 | 1 M KOH + 0.5 M NaCl | NF | 269 | 46 | [44] | |
| CoPx@FeOOH | 1 M KOH Seawater | NF | 283 (100 mA/cm2) | 1.82 | [24] |
FTO, fluorine-doped tin oxide; GCE, glass carbon electrode; NF, Ni foam.
2.2. Electrocatalysts for HER
Previous studies have shown few competitive reactions to the HER in seawater electrolytes, unlike those in the anode compartment. However, the main problem of cathodic HER is the presence of impurities in seawater, which leads to blockage and corrosion of the active sites, resulting in low efficiency and poor stability [45]. At this stage, mostly reported electrocatalysts for HER in seawater electrolysis include noble metal alloys, carbon-supported noble metals, MXene based complexes, metal phosphides, metal oxides, metal hydroxides, metal nitrides, hybrid electrocatalysts, and so on (Table 2).
Table 2. Summary of HER performance of reported electrocatalysts.
| Catalyst | Eectrode | Electrolytes | Onset Potential (mV) |
Overpotential @10mA cm−2 (mV) |
Tafel Slope (mV dec−1) |
Exchange Current Density (mA cm−2) | Mass Loading (mg cm−2) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Pt | Ti mesh | seawater | 151.80 | 285 | 45.8 | 7.336 × 10−5 | [25] | |
| Pt-Ru-Cr | Ti mesh | seawater | 129.89 | 256 | 45.7 | 9.280 × 10−5 | [25] | |
| Pt-Ru-Fe | Ti mesh | seawater | 125.92 | 248 | 45.2 | 9.337 × 10−5 | [25] | |
| Pt-Ru-Co | Ti mesh | seawater | 112.79 | 222 | 44.8 | 9.339 × 10−5 | [25] | |
| Pt-Ru-Ni | Ti mesh | seawater | 103.25 | 206 | 44.5 | 1.006 × 10−4 | [25] | |
| Pt-Ru-Mo | Ti mesh | seawater | 96.22 | 196 | 44.0 | 1.080 × 10−4 | [25] | |
| Pt/C | GCE | seawater | 185 | 59 | 1.05 × 10−4 | 0.199 | [46] | |
| PtNi5 | GCE | seawater | 380 | 119 | 8.51 × 10−5 | 0.199 | [46] | |
| PtCr0.1 | Ti mesh | seawater | 166.03 | 283.8 | 3.90 × 10−5 | [26] | ||
| PtFe0.1 | Ti mesh | seawater | 157.69 | 275.2 | 4.55 × 10−5 | [26] | ||
| PtCo0.1 | Ti mesh | seawater | 149.93 | 266.5 | 5.18 × 10−5 | [26] | ||
| PtNi0.1 | Ti mesh | seawater | 147.75 | 263.3 | 5.27 × 10−5 | [26] | ||
| PtMo0.1 | Ti mesh | seawater | 142.50 | 254.6 | 5.35 × 10−5 | [26] | ||
| Ti/NiPt | Ti foil | seawater | 230 | 111 | 9.59 × 10−3 | [47] | ||
| Ti/NiAu | Ti foil | seawater | 410 | 170 | 3.35 × 10−3 | [47] | ||
| RuCo | Ti foil | seawater | 253 | 107 | 4.71 × 10-3 | [48] | ||
| RuCoMo1 | Ti foil | seawater | 354 | 137 | 2.69 × 10−3 | [48] | ||
| NiRuIr_G | seawater | 80 | 48 | [49] | ||||
| 0.5Rh-G1000 | GCE | 1 M PBS | 250 | 1.0 | [50] | |||
| 0.5Rh-G1000 | GCE | seawater | 340 | 1.0 | [50] | |||
| 0.5Rh-GS1000 | GCE | 1 M PBS | 19 | 1.0 | [50] | |||
| 0.5Rh-GS1000 | GCE | seawater | 320 | 1.0 | [50] | |||
| 2.4% Pt@mh-3D MXene | GCE | seawater | 280 | 0.2 | [51] | |||
| VS2@V2C | GCE | seawater (PH = 0) | 148 (20 mA cm−2) | 37 | 27.55 | [27] | ||
| h-MoN@ BNCNT |
GCE | seawater | 128 | 0.254 | [52] | |||
| NiCoP/NF | NF | seawater | 287 mV | 2.0 | [53] | |||
| PSS-PPy/Ni-Co-P | CF | artificial seawater | 144 | [54] | ||||
| C-Co2P | GCE | 1 M KOH | 30 | 2.18 | [28] | |||
| C-Co2P | GCE | 1 M KOH, 0.5 M NaCl, 41.2 × 10−3 M MgCl2 and 12.5 × 10−3 M CaCl2 | 192 (1000 mA cm−2) | 2.18 | [28] | |||
| Ru-CoOx | NF | 1 M KOH + seawater | 630 (100 mA cm−2) | [55] | ||||
| Mo5N6 | GCE | 1 M KOH | 94 | 0.41 | [12] | |||
| Ni-SN@C | GCE | 1 M KOH | 28 | 0.255 | [56] | |||
| Ni-SN@C | GCE | 1 M KOH seawater | 23 | 0.255 | [56] | |||
| NiCoN|NixP| NiCoN | NF | seawater | 165 | 1.26 | [57] | |||
| Ni5P4/ Ni2+δOδ(OH)2-δ | CC | seawater | 144 | [58] |
PBS, phosphate buffer solution; GCE, glass carbon electrode; NF, Ni foam; CC, carbon cloth; NiRuIr_G, graphene-supported NiRuIr; 0.5Rh-G1000, Rh supported by N-doped carbon nanosheets; 0.5Rh-GS1000, Rh supported by N/S-codoped carbon nanosheets; mh-3D MXene, multilevel hollow MXene; BNCNT, boron, nitrogen codoped CNT; C-Co2P, carbon doped Co2P.
3. Conclusions and Outlook
Seawater is an inexhaustible resource on the planet; thus, the hydrogen production through the electrolysis of seawater can be effective in alleviating the energy crisis to some extent. Despite many efforts devoted into the field of seawater electrolysis in recent years, there is large space for the improvement of seawater electrolysis for high-performance production of hydrogen. Obviously, seawater electrolysis is more complex than freshwater electrolysis due to the presence of multiple cations and anions. Herein concluded the recent progress and summarize in detail the current status of research on HER and OER catalysts for seawater electrolysis, from fundamental mechanisms to the performance of catalysts.
Extensive research has been carried out on various materials, such as metal oxides (hydroxides), metal nitrides, metal phosphides, metal borides, noble metal alloy catalysts, and so on, for seawater electrolysis. Still, the catalytic activity and stability of most of the reported catalysts are not satisfactory to meet the requirement for practical application. As can be found from Table 1 and Table 2, the overpotential of these catalysts is not low enough. Furthermore, most of the electrolytes for current research are artificial saline water rather than real seawater, which increases the complexity of seawater electrolysis for hydrogen production. Therefore, in order to achieve high-performance seawater electrolysis, it is believed that more efforts are needed in the following directions:
- (1) Combining experimental and theoretical analyses to further confirm the reaction pathways and active sites of catalysts for HER and OER in seawater electrolysis: in addition to monometallic compounds, various polymetallic compounds and heterostructured catalysts have been extensively investigated as catalysts in seawater electrolysis. It is a trend to design catalysts composed of more than a single metal component by taking advantage of the synergistic effect of multimetal components. As catalyst components become more complex, it is more difficult to identify the electrocatalytic reaction pathways as well as the active sites. Therefore, systematic research based on theoretical analyses is necessary, which can provide guidance for designing materials with desirable structures and properties.
- (2) Employing in situ characterization methods to unravel the true active sites of catalysts: many current electrocatalysts, such as metal oxides, phosphides, and nitrides, undergo surface oxidation or reconstitution during seawater electrolysis, which means that the true active sites of the catalyst may be altered during the reaction. As each step in the catalytic reaction process changes rapidly, scientists need in situ characterization techniques to track changes in the intermediates during the catalytic reaction process. It will provide clear principle and guidance to design high-efficiency catalysts if more in situ techniques such as in situ XAS, Raman, Fourier transform infrared spectroscopy, and other novel techniques are involved for the mechanistic studies.
- (3) Exploring and developing electrocatalysts with high activity and stability in seawater: Not only multiple cations but also chloride anions in seawater interfere with the water splitting reactions. It is highly desirable to synthesize catalysts with higher selectivity to HER and OER than other competitive reactions. To obtain highly efficient electrocatalysts for seawater electrolysis, modulating the electronic structure of active sites is of great significance and plays a major role in the improvement of catalytic performance. To optimize the electronic structure of catalysts, alloying, vacancy engineering, heteroelement doping, and interface engineering are common methods. Integration of different active materials into a hybrid catalyst is also a good solution for developing high-performance catalysts.
- (4) Designing advanced reactors specific for seawater electrolysis: The current research of seawater electrolysis for hydrogen production is mostly focused on the catalysts. To realize the electrocatalytic production of hydrogen, herein need to consider the entire reactor rather than the catalysts only. It is necessary to reasonably design reactors which are adaptable to specific seawater electrolysis. For example, the design of asymmetric reactors is considered to be more promising [31][59], which consists of alkaline water in the anode chamber and seawater in the cathode chamber. Such design not only facilitates the diffusion of Cl− to the anode but also protects the anode catalyst, which is of significance in seawater electrolysis.
References
- Chu, S.; Cui, Y.; Liu, N. The path towards sustainable energy. Nat. Mater. 2017, 16, 16–22.
- Jacobson, M.Z.; Colella, W.; Golden, D. Cleaning the air and improving health with hydrogen fuel-cell vehicles. Science 2005, 308, 1901–1905.
- Fukuzumi, S.; Lee, Y.M.; Nam, W. Fuel production from seawater and fuel cells using seawater. ChemSusChem 2017, 10, 4264–4276.
- Kumaravel, V.; Abdel-Wahab, A. A short review on hydrogen, biofuel, and electricity production using seawater as a medium. Energy Fuels 2018, 32, 6423–6437.
- Zhang, J.; Hu, W.; Cao, S.; Piao, L. Recent progress for hydrogen production by photocatalytic natural or simulated seawater splitting. Nano Res. 2020, 13, 2313–2322.
- Tian, L.; Zhai, X.; Wang, X.; Pang, X.; Li, J.; Li, Z. Morphology and phase transformation of α-MnO2/MnOOH modulated by N-CDs for efficient electrocatalytic oxygen evolution reaction in alkaline medium. Electrochim. Acta 2020, 337, 135823.
- Li, Z.; Song, M.; Zhu, W.; Zhuang, W.; Du, X.; Tian, L. MOF-derived hollow heterostructures for advanced electrocatalysis. Coord. Chem. Rev. 2021, 439, 213946.
- Xu, S.; Zhao, H.; Li, T.; Liang, J.; Lu, S.; Chen, G.; Gao, S.; Asiri, A.M.; Wu, Q.; Sun, X. Iron-based phosphides as electrocatalysts for the hydrogen evolution reaction: Recent advances and future prospects. J. Mater. Chem. A 2020, 8, 19729–19745.
- Dresp, S.R.; Dionigi, F.; Klingenhof, M.; Strasser, P. Direct electrolytic splitting of seawater: Opportunities and challenges. ACS Energy Lett. 2019, 4, 933–942.
- Tong, W.; Forster, M.; Dionigi, F.; Dresp, S.; Erami, R.S.; Strasser, P.; Cowan, A.J.; Farràs, P. Electrolysis of low-grade and saline surface water. Nat. Energy 2020, 5, 367–377.
- Cheng, F.; Feng, X.; Chen, X.; Lin, W.; Rong, J.; Yang, W. Synergistic action of Co-Fe layered double hydroxide electrocatalyst and multiple ions of sea salt for efficient seawater oxidation at near-neutral pH. Electrochim. Acta 2017, 251, 336–343.
- Jin, H.; Liu, X.; Vasileff, A.; Jiao, Y.; Zhao, Y.; Zheng, Y.; Qiao, S.-Z. Single-crystal nitrogen-rich two-dimensional Mo5N6 nanosheets for efficient and stable seawater splitting. ACS Nano 2018, 12, 12761–12769.
- Ge, R.; Wang, S.; Su, J.; Dong, Y.; Lin, Y.; Zhang, Q.; Chen, L. Phase-selective synthesis of self-supported RuP films for efficient hydrogen evolution electrocatalysis in alkaline media. Nanoscale 2018, 10, 13930–13935.
- Gao, S.; Li, G.-D.; Liu, Y.; Chen, H.; Feng, L.-L.; Wang, Y.; Yang, M.; Wang, D.; Wang, S.; Zou, X. Electrocatalytic H2 production from seawater over Co, N-codoped nanocarbons. Nanoscale 2015, 7, 2306–2316.
- Zhao, Q.; Wang, Y.; Lai, W.-H.; Xiao, F.; Lyu, Y.; Liao, C.; Shao, M. Approaching a high-rate and sustainable production of hydrogen peroxide: Oxygen reduction on Co–N–C single-atom electrocatalysts in simulated seawater. Energy Environ. Sci. 2021, 14, 5444–5456.
- Kuang, Y.; Kenney, M.J.; Meng, Y.; Hung, W.-H.; Liu, Y.; Huang, J.E.; Prasanna, R.; Li, P.; Li, Y.; Wang, L. Solar-driven, highly sustained splitting of seawater into hydrogen and oxygen fuels. Proc. Natl. Acad. Sci. USA 2019, 116, 6624–6629.
- Van de Krol, R.; Grätzel, M. Photoelectrochemical Hydrogen Production; Springer: New York, NY, USA, 2012; Volume 90.
- Yu, L.; Zhu, Q.; Song, S.; McElhenny, B.; Wang, D.; Wu, C.; Qin, Z.; Bao, J.; Yu, Y.; Chen, S. Non-noble metal-nitride based electrocatalysts for high-performance alkaline seawater electrolysis. Nat. Commun. 2019, 10, 1–10.
- Yu, L.; Wu, L.; McElhenny, B.; Song, S.; Luo, D.; Zhang, F.; Yu, Y.; Chen, S.; Ren, Z. Ultrafast room-temperature synthesis of porous S-doped Ni/Fe (oxy)hydroxide electrodes for oxygen evolution catalysis in seawater splitting. Energy Environ. Sci. 2020, 13, 3439–3446.
- Wu, X.; Yang, Y.; Zhang, T.; Wang, B.; Xu, H.; Yan, X.; Tang, Y. CeOx-decorated hierarchical NiCo2S4 hollow nanotubes arrays for enhanced oxygen evolution reaction electrocatalysis. ACS Appl. Mater. Interfaces 2019, 11, 39841–39847.
- El-Moneim, A.A.; Kumagai, N.; Hashimoto, K. Mn-Mo-W oxide anodes for oxygen evolution in seawater electrolysis for hydrogen production. Mater. Trans. 2009, 50, 1969–1977.
- Vos, J.G.; Wezendonk, T.A.; Jeremiasse, A.W.; Koper, M.T. MnOx/IrOx as selective oxygen evolution electrocatalyst in acidic chloride solution. J. Am. Chem. Soc. 2018, 140, 10270–10281.
- Gupta, S.; Forster, M.; Yadav, A.; Cowan, A.J.; Patel, N.; Patel, M. Highly efficient and selective metal oxy-boride electrocatalysts for oxygen evolution from alkali and saline solutions. ACS Appl. Energy Mater. 2020, 3, 7619–7628.
- Wu, L.; Yu, L.; McElhenny, B.; Xing, X.; Luo, D.; Zhang, F.; Bao, J.; Chen, S.; Ren, Z. Rational design of core-shell-structured CoPx@ FeOOH for efficient seawater electrolysis. Appl. Catal. B Environ. 2021, 294, 120256.
- Li, H.; Tang, Q.; He, B.; Yang, P. Robust electrocatalysts from an alloyed Pt–Ru–M (M = Cr, Fe, Co, Ni, Mo)-decorated Ti mesh for hydrogen evolution by seawater splitting. J. Mater. Chem. A 2016, 4, 6513–6520.
- Zheng, J.; Zhao, Y.; Xi, H.; Li, C. Seawater splitting for hydrogen evolution by robust electrocatalysts from secondary M (M = Cr, Fe, Co, Ni, Mo) incorporated Pt. RSC Adv. 2018, 8, 9423–9429.
- Wang, Z.; Xu, W.; Yu, K.; Feng, Y.; Zhu, Z. 2D heterogeneous vanadium compound interfacial modulation enhanced synergistic catalytic hydrogen evolution for full pH range seawater splitting. Nanoscale 2020, 12, 6176–6187.
- Xu, W.; Fan, G.; Zhu, S.; Liang, Y.; Cui, Z.; Li, Z.; Jiang, H.; Wu, S.; Cheng, F. Electronic structure modulation of nanoporous cobalt phosphide by carbon doping for alkaline hydrogen evolution reaction. Adv. Funct. Mater. 2021, 46, 2107333.
- Wang, C.; Shang, H.; Jin, L.; Xu, H.; Du, Y. Advances in hydrogen production from electrocatalytic seawater splitting. Nanoscale 2021, 13, 7897–7912.
- Yao, Y.; Gao, X.; Meng, X. Recent advances on electrocatalytic and photocatalytic seawater splitting for hydrogen evolution. Int. J. Hydrogen Energy 2021, 46, 9087–9100.
- Khatun, S.; Hirani, H.; Roy, P. Seawater electrocatalysis: Activity and selectivity. J. Mater. Chem. A 2021, 9, 74–86.
- Bolar, S.; Shit, S.; Murmu, N.C.; Kuila, T. Progress in theoretical and experimental investigation on seawater electrolysis: Opportunities and challenges. Sustain. Energy Fuels 2021, 5, 5915–5945.
- Sohrabnejad-Eskan, I.; Goryachev, A.; Exner, K.S.; Kibler, L.A.; Hensen, E.J.; Hofmann, J.P.; Over, H. Temperature-dependent kinetic studies of the chlorine evolution reaction over RuO2 (110) model electrodes. ACS Catal. 2017, 7, 2403–2411.
- Kraft, A.; Stadelmann, M.; Blaschke, M.; Kreysig, D.; Sandt, B.; Schröder, F.; Rennau, J. Electrochemical water disinfection Part I: Hypochlorite production from very dilute chloride solutions. J. Appl. Electrochem. 1999, 29, 859–866.
- Vos, J.; Koper, M. Measurement of competition between oxygen evolution and chlorine evolution using rotating ring-disk electrode voltammetry. J. Electroanal. Chem. 2018, 819, 260–268.
- Juodkazytė, J.; Šebeka, B.; Savickaja, I.; Petrulevičienė, M.; Butkutė, S.; Jasulaitienė, V.; Selskis, A.; Ramanauskas, R. Electrolytic splitting of saline water: Durable nickel oxide anode for selective oxygen evolution. Int. J. Hydrogen Energy 2019, 44, 5929–5939.
- Gayen, P.; Saha, S.; Ramani, V. Selective seawater splitting using pyrochlore electrocatalyst. ACS Appl. Energy Mater. 2020, 3, 3978–3983.
- Okada, T.; Abe, H.; Murakami, A.; Shimizu, T.; Fujii, K.; Wakabayashi, T.; Nakayama, M. A bilayer structure composed of Mg| Co-MnO2 deposited on a Co (OH)2 film to realize selective oxygen evolution from chloride-containing water. Langmuir 2020, 36, 5227–5235.
- Tu, Q.; Liu, W.; Jiang, M.; Wang, W.; Kang, Q.; Wang, P.; Zhou, W.; Zhou, F. Preferential adsorption of hydroxide ions onto partially crystalline NiFe-layered double hydroxides leads to efficient and selective OER in alkaline seawater. ACS Appl. Energy Mater. 2021, 4, 4630–4637.
- Wu, L.; Yu, L.; Zhang, F.; McElhenny, B.; Luo, D.; Karim, A.; Chen, S.; Ren, Z. Heterogeneous bimetallic phosphide Ni2P-Fe2P as an efficient bifunctional catalyst for water/seawater splitting. Adv. Funct. Mater. 2021, 31, 2006484.
- Wang, S.; Yang, P.; Sun, X.; Xing, H.; Hu, J.; Chen, P.; Cui, Z.; Zhu, W.; Ma, Z. Synthesis of 3D heterostructure Co-doped Fe2P electrocatalyst for overall seawater electrolysis. Appl. Catal. B Environ. 2021, 297, 120386.
- Zhang, Y.; Fu, C.; Fan, J.; Lv, H.; Hao, W. Preparation of NiB electrode via electroless plating toward high-efficient alkaline simulated seawater splitting. J. Electroanal. Chem. 2021, 901, 115761.
- Li, J.; Liu, Y.; Chen, H.; Zhang, Z.; Zou, X. Design of a multilayered oxygen-evolution electrode with high catalytic activity and corrosion resistance for saline water splitting. Adv. Funct. Mater. 2021, 2101820.
- Cui, B.; Hu, Z.; Liu, C.; Liu, S.; Chen, F.; Hu, S.; Zhang, J.; Zhou, W.; Deng, Y.; Qin, Z. Heterogeneous lamellar-edged Fe-Ni(OH)2/Ni3S2 nanoarray for efficient and stable seawater oxidation. Nano Res. 2021, 14, 1149–1155.
- Lu, X.; Pan, J.; Lovell, E.; Tan, T.H.; Ng, Y.H.; Amal, R. A sea-change: Manganese doped nickel/nickel oxide electrocatalysts for hydrogen generation from seawater. Energy Environ. Sci. 2018, 11, 1898–1910.
- Zheng, J. Seawater splitting for high-efficiency hydrogen evolution by alloyed PtNix electrocatalysts. Appl. Surf. Sci. 2017, 413, 360–365.
- Zhang, Y.; Li, P.; Yang, X.; Fa, W.; Ge, S. High-efficiency and stable alloyed nickel based electrodes for hydrogen evolution by seawater splitting. J. Alloys Compd. 2018, 732, 248–256.
- Niu, X.; Tang, Q.; He, B.; Yang, P. Robust and stable ruthenium alloy electrocatalysts for hydrogen evolution by seawater splitting. Electrochim. Acta 2016, 208, 180–187.
- Sarno, M.; Ponticorvo, E.; Scarpa, D. Active and stable graphene supporting trimetallic alloy-based electrocatalyst for hydrogen evolution by seawater splitting. Electrochem. Commun. 2020, 111, 106647.
- Liu, Y.; Hu, X.; Huang, B.; Xie, Z. Surface engineering of Rh catalysts with N/S-codoped carbon nanosheets toward high-Performance hydrogen evolution from seawater. ACS Sustain. Chem. Eng. 2019, 7, 18835–18843.
- Xiu, L.; Pei, W.; Zhou, S.; Wang, Z.; Yang, P.; Zhao, J.; Qiu, J. Multilevel Hollow MXene Tailored Low-Pt Catalyst for Efficient Hydrogen Evolution in Full-pH Range and Seawater. Adv. Funct. Mater. 2020, 30, 1910028.
- Miao, J.; Lang, Z.; Zhang, X.; Kong, W.; Peng, O.; Yang, Y.; Wang, S.; Cheng, J.; He, T.; Amini, A. Polyoxometalate-derived hexagonal molybdenum nitrides (MXenes) supported by boron, nitrogen codoped carbon nanotubes for efficient electrochemical hydrogen evolution from seawater. Adv. Funct. Mater. 2019, 29, 1805893.
- Lv, Q.; Han, J.; Tan, X.; Wang, W.; Cao, L.; Dong, B. Featherlike NiCoP holey nanoarrys for efficient and stable seawater splitting. ACS Appl. Energy Mater. 2019, 2, 3910–3917.
- Tian, F.; Geng, S.; He, L.; Huang, Y.; Fauzi, A.; Yang, W.; Liu, Y.; Yu, Y. Interface engineering: PSS-PPy wrapping amorphous Ni-Co-P for enhancing neutral-pH hydrogen evolution reaction performance. Chem. Eng. J. 2021, 417, 129232.
- Wu, D.; Chen, D.; Zhu, J.; Mu, S. Ultralow Ru Incorporated Amorphous Cobalt-Based Oxides for High-Current-Density Overall Water Splitting in Alkaline and Seawater Media. Small 2021, 17, 2102777.
- Jin, H.; Wang, X.; Tang, C.; Vasileff, A.; Li, L.; Slattery, A.; Qiao, S.Z. Stable and highly efficient hydrogen evolution from seawater enabled by an unsaturated nickel surface nitride. Adv. Mater. 2021, 33, 2007508.
- Yu, L.; Wu, L.; Song, S.; McElhenny, B.; Zhang, F.; Chen, S.; Ren, Z. Hydrogen Generation from Seawater Electrolysis over a Sandwich-like NiCoN|NixP|NiCoN Microsheet Array Catalyst. ACS Energy Lett. 2020, 5, 2681–2689.
- Huang, Y.; Hu, L.; Liu, R.; Hu, Y.; Xiong, T.; Qiu, W.; Balogun, M.-S.J.T.; Pan, A.; Tong, Y. Nitrogen treatment generates tunable nanohybridization of Ni5P4 nanosheets with nickel hydr (oxy) oxides for efficient hydrogen production in alkaline, seawater and acidic media. Appl. Catal. B Environ. 2019, 251, 181–194.
- Dresp, S.; Thanh, T.N.; Klingenhof, M.; Brückner, S.; Hauke, P.; Strasser, P. Efficient direct seawater electrolysers using selective alkaline NiFe-LDH as OER catalyst in asymmetric electrolyte feeds. Energy Environ. Sci. 2020, 13, 1725–1729.
More
Information
Subjects:
Green & Sustainable Science & Technology; Engineering, Chemical; Nanoscience & Nanotechnology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.2K
Revisions:
4 times
(View History)
Update Date:
07 Feb 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




