Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Simona Sacchini | + 4639 word(s) | 4639 | 2022-01-28 03:04:52 | | | |
| 2 | Bruce Ren | Meta information modification | 4639 | 2022-02-07 01:11:13 | | | | |
| 3 | Bruce Ren | + 2 word(s) | 4641 | 2022-02-09 01:45:16 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Sacchini, S. Methodology and Neuromarkers for Cetaceans’ Brains. Encyclopedia. Available online: https://encyclopedia.pub/entry/19063 (accessed on 07 February 2026).
Sacchini S. Methodology and Neuromarkers for Cetaceans’ Brains. Encyclopedia. Available at: https://encyclopedia.pub/entry/19063. Accessed February 07, 2026.
Sacchini, Simona. "Methodology and Neuromarkers for Cetaceans’ Brains" Encyclopedia, https://encyclopedia.pub/entry/19063 (accessed February 07, 2026).
Sacchini, S. (2022, February 01). Methodology and Neuromarkers for Cetaceans’ Brains. In Encyclopedia. https://encyclopedia.pub/entry/19063
Sacchini, Simona. "Methodology and Neuromarkers for Cetaceans’ Brains." Encyclopedia. Web. 01 February, 2022.
Copy Citation
Cetacean brain sampling may be an arduous task due to the difficulty of collecting and histologically preparing such rare and large specimens. Thus, one of the main challenges of working with cetaceans’ brains is to establish a valid methodology for an optimal manipulation and fixation of the brain tissue, which allows the samples to be viable for neuroanatomical and neuropathological studies.
cetaceans
dolphins
beaked whales
neuroanatomy
neuropathology
methodology
immunohistochemistry
neuromarkers
1. Introduction
In the course of evolution, cetaceans have undergone modifications with respect to their ancestral terrestrial status. Throughout the phylogenesis, the cetaceans’ sphlancnocraniums lengthened in order to obtain a greater hydrodynamic body shape, the upper airways (the blowhole) migrated dorsally, while the neurocranium shortened (Figure 1). The skull acquired a post-orbital compression and an antero-orbital elongation, called “cranial telescoping” [1]. However, the brain does not diminish its size; it folds over itself and thus acquires a “boxing glove” shape, also due to the lack of most of the olfactory structures in the frontal lobes and the pronounced width of the temporal lobe (Figure 2, right). One of the most eminent transformations occurred in the toothed whales (odontocetes) in their size, structure, and neuroanatomical organization. A unique characteristic of the toothed whales is the exceptionally large size of the brain, in some species both in absolute and in relative terms [2], and the extremely dense folding of the neocortex [3] (Figure 2, right). The size of the dolphin’s brain compared to body size varies between apes and humans [4]. Toothed whale brains may range from about 200–2000 g [5] to 9300 and 9200 g, the maximum sizes found in the killer whale (Orcinus orca) [6] and the sperm whale (Physeter macrocephalus) [7], respectively. In addition, female sperm and killer whales evolved a brain larger than expected for their body mass (encephalization quotient > 1), with values near the ranges of primates, dolphins, and elephants [8].
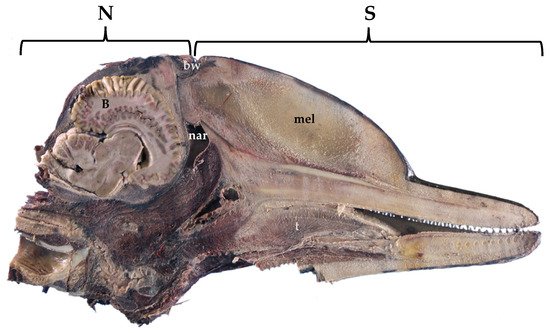
Figure 1. Sagittal section of a fixed dolphin head, showing the lengthening of the sphlancnocranium (S), the shortening of the neurocranium (N), and the position of the brain (B). bw, blowhole; mel, melon; nar, nares; t, tongue. Atlantic spotted dolphin. Stenella frontalis.
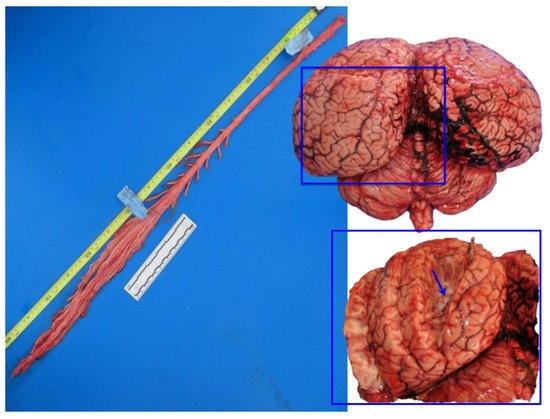
Figure 2. Fresh spinal cord (left) and fresh brain (right). In the inset, the lateral ventricle is exposed (arrow), in the proximity of the sagittal cleft. Striped dolphin, Stenella coeruleoalba.
The animal cerebral tissue is fragile due to the general lack of connective tissue and the high percentage of lipid content and the post mortem period negatively affects the conservation, quality, and stiffness of brain tissue. Undisputedly, where the best possible morphology is required, animals should be anesthetized and subjected to cardiac perfusion with saline, followed by a formalin flush [9][10]. In any event, the study of cetaceans provides valuable information on the conditions of the researchers seas. Cetaceans are homeotherms, long-lived species, and are located at the top of the marine food chain. Thus, they are considered bioindicator species and sentinels of the health of the sea. The lifespan of these animals can be compared, in many species, to the one of humans, and makes cetaceans, particularly odontocetes, a new and more authentic comparative natural model for the study of certain neurodegenerative diseases in humans [11][12][13][14][15]. Although immersion fixation has been considered inadequate, especially in large brains, where the fixative is slow to penetrate [16] and different protocols have been proposed for cetaceans’ large brains [2][17], it is possible to obtain viable material for the microscopic study of cortical [18][19][20][21], subcortical [20][22][23], and brainstem regions [14][20][24][25].
2. Materials and Methods
2.1. The Workflow for Cetaceans’ Brain Examination: From Fresh to Fixed Samples
From 2009 to 2016, 281 cetaceans stranded and died in the Canary Islands (Spain). Systematic pathological studies were performed on the carcasses to determine the cause of death and/or stranding. Brains were obtained from 51 specimens of six different species of the suborder Odontoceti: striped dolphin (Stenella coeruleoalba) (n = 10), Atlantic spotted dolphin (Stenella frontalis) (n = 7), common dolphin (Delphinus delphis) (n = 3), bottlenose dolphin (Tursiops truncatus) (n = 3); short-finned pilot whale (Globicephala macrorhynchus) (n = 3), Risso’s dolphin (Grampus griseus) (n = 5), pygmy sperm whale (Kogia breviceps) (n = 2), Blainville’s beaked whale (Mesoplodon densirostris) (n = 5), Cuvier’s beaked whale (Ziphius cavirostris) (n = 8), Gervais’ beaked whale (Mesoplodon europaeus) (n = 4), and True’s beaked whale (Mesoplodon mirus) (n = 1). The bottlenose dolphins, one newborn and two adults, came from a controlled environment and died of natural causes. The postmortem times were different, due to the intrinsic logistic aspects of each stranding, but never exceeded 48 h (decomposition code 1 and 2, very fresh and fresh).
2.2. Opening of the Skull
The necropsy protocol was carried out as standardized and published by Thijs Kuiken and Manuel García Hartmann in 1991 [26], with some modifications and added contributions. The opening of the cranial cavity was performed with a swinging saw. Four lines were drawn in the skull and a window was created in order to offer the best access to the brain, as follows:
-
one dorsal parallel to the nuchal ridge (crista occipitalis externa) at 1 cm from it (slightly more caudally in some species such as beaked whales or BW and pilot whales),
-
another ventral and parallel to (a), bypassing the occipital condyles; and
-
two lateral and perpendicular to (a) and (b), passing through the parietal and the squamosal bones, in the temporal fossa.
In the biggest size animals (i.e., sperm-, baleen-, and beaked- whales), the aforesaid window should be drawn more caudally. In fact, the thickness of the skull, and sometimes the ossification of the falx cerebri and tentoria cerebelli [27], cripples any effort to achieve the brain and force to draw the window just around the occipital condyles.
2.3. Cautious Sampling of Fresh Brain at Necropsy: A Key Step
Brains were removed, coded for freshness, and usually dissected within 24–48 h after death. A superficial sampling of fresh unfixed brain was usually done, in order to complement investigations with microbiological, virological, and toxicological (included biotoxins) studies. The following samples were taken: the cerebral cortex (rostrally and caudally), the pons, the cerebellum, and the medulla oblongata. Then, in order to expose the lateral ventricles, a cut was made in each hemisphere (as shown in the inset of Figure 2).
At this time, a biopsy punch was inserted in the lateral ventricles, first rostral-ward, and then caudal-ward, ensuring in this way to take samples of the caudate nucleus (rostral) and the thalamus (caudal). A sample of the choroid plexus was also taken.
2.4. Fixation of Cetaceans’ Brains: A Challenge
Cetaceans’ brains are usually obtained after different post-mortem periods, making it difficult to control autolysis times. The post-mortem times are different but should never exceed 24 h, in particular for those brains used for neuroanatomical studies. Brains were immersion-fixed at the time of necropsy in 10% neutral-buffered formalin (4% formaldehyde solution, pH 7.4).
Due to the great encephalic volume and the slow rate of diffusion of the formalin (0.5 cm per hour), some longitudinal cuts (2 to 4) were made in both cerebral and cerebellar hemispheres, before immersion. Cuts were mainly superficial (Figure 2, right) but in each cerebral hemisphere, at least one of them entered deep in order to expose the lateral ventricles and to allow the fixative to go inside the ventricular system (the same cut as Section 2.2). The opening of the ventricular system has been proposed by other authors [9][10] and has been adapted here to the characteristic short ventricular system of these marine mammals [28]. To respect the optimum volume ratio of fixative tissue, which is 10–20:1 [9], brains were dipped in a container with a minimum volume of 20 L of fixative. Absorbent paper was placed at the bottom of the container to avoid sticking of the brain to the plastic surface. Brains were left in the fixative for at least 72 h, and the correct fixation was monitored throughout the days.
2.5. Sectioning of the Whole Brain and Postfixation
Brains can then be sectioned in sagittal, transverse (cross) or dorsal sections. However, cross sections were usually chosen because they offer a better evaluation of neuropathological lesions (i.e., uni- or bilateral distribution of the lesions, affected areas, etc.) and a finer study of the neuroanatomy [9]. A dual sided edged knife for precision brain sectioning or a slicing machine were used for cross-cutting. Sections (1–1.5 cm thick) were returned to the fixative for at least other 48 h before proceeding to the final sampling of the brain. Indeed, sections were then stored in a 4 or 10-L volume container with fresh fixative, separated from each other by filter paper.
2.6. Routinary Neuropathologic Investigations (FFPE)
For neuropathological purposes, we used the routine Formalin-Fixed Paraffin-Embedded (FFPE) procedure. Regrettably, unlike domestic mammals, the researchers greatest guide for sampling is directed only by gross findings, since we do not usually have any information on neurological or diagnostic examination.
A representative sampling of the main areas of the brain has been made as follows (Figure 3):
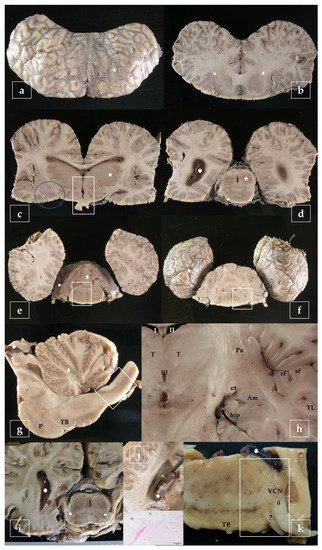
Figure 3. Representative brain sampling in cross sections. Details are given in the main text (Section 2.6). Striations are due to the dentated blade of the slicing machine (i.e., in b). Blainville’s beaked whale, Mesoplodon densirostris (a–f). Sagittal section of the brainstem. P, pons; TB, trapezoid body. Atlantic spotted dolphin, Stenella frontalis (g). Dorsal section of the brain, obtained at the level of the ventral limit of the telencephalon. The amygdaloid complex (Am), is located rostral to the tiny hippocampus (hip) and lateral to the optic tract (ot). Pu, putamen; sf, sylvian fissure; T, thalamus; TL, temporal lobe; I-II-III, 1st-2nd-3rd ventricle. Atlantic spotted dolphin, Stenella frontalis (h). Cross section made as in (d) intended to show the substantia nigra (arrowheads). Gervais’ beaked whale, Mesoplodon europaeus (i). Cross section of the brain exposing the lateral ventricle. The choroid plexus presents cystic lesions (arrowheads). The animal was positive to herpesvirus and presented lesions in the brain [29]. Striped dolphin, Stenella coeruleoalba (j). Sample of the brainstem including the trapezoid body (TB), exposing de ventral cochlear nucleus (VCN), the facial nerve (7), and the vestibulocochlear nerve (8). Risso’s dolphin, Grampus griseus (k).
- - telencephalon: cortex (2 to 4 samples, at least frontopolar and occipital cortex) (a and f, stars), corpus striatum (b, arrowheads), amygdala (c, blue circle; h, Am), and hippocampus (h, hip);
- - diencephalon: thalamus and hypothalamus (c, star and rectangle);
- - mesencephalon: tectum (colliculus rostralis and caudalis) (d, star) and tegmentum (with the substantia nigra) (d and i, arrowheads);
- - rhombencephalon: at least pons (with the locus ceruleus) (e, rectangle), trapezoid body (with the cochlear nuclei) (f and k, rectangle), medulla oblongata (g, rectangle) and at least two samples of the cerebellum (included a sagittal section of the vermis) (e and g, arrowhead and star);
- - choroid plexus (d, g, i, j, and k, circle);
- - spinal cord (Figure 2, left): at least pars cervicalis and pars thoracica.
Hippocampus is a complex brain structure embedded deep into the temporal lobe. In these animals, it may be a hard issue to detect it, due to its particular small size [23]. However, it can easily be found out just behind the large amygdaloid complex (Figure 3h, hip).
The selected samples were then placed into standard tissue cassettes measuring 30 × 25 × 4 mm and postfixed for at least other 24 h. Larger size processing cassettes (Super Mega-Cassette System, size 70 × 50 × 15 mm) could be a better option. These types of cassettes allow the processing and/or embedding of large sections of brain but processing schedules must be adjusted accordingly.
Fixed brain samples were dehydrated through a series of graded ethanol baths and then infiltrated with paraffin wax. Tissues were embedded in paraffin wax blocks and processed for routinary haematoxylin eosin staining and, when necessary, histochemical procedures were used to identify microorganisms, intra/extracellular material (i.e., Periodic acid–Schiff, PAS) as well as to distinguish neurons, glial cells, neurofibrils, and myelin (i.e., Nissl, Bielschowsky, Luxol Fast Blue, etc.).
2.7. Immunoperoxidase Staining: Paraffin Embedded Tissues (p-IHC)
Immunohistochemistry has been established as a reliable methodology for both routine diagnostic and research activities in veterinary medicine. Paraffin embedded samples were deparaffinated with xylene and rehydrated in graded ethanol. Immunohistochemistry was carried out with standard immunohistochemical Avidin-Biotin Complex (ABC) protocol [30]. Endogenous peroxidase activity was blocked by incubation with 3% H2O2 in methanol for 30 min at room temperature (RT). Antigen retrieval was performed depending on the primary antibody (Table 1). Primary antibodies were selected after reading up in the literature. Some of them were already used in other animal species (i.e., tyrosine hydroxylase or calbindin) [31][32] while others were picked up from human investigations (i.e., α-synuclein or β-amyloid) [33][34]. While polyclonal antibodies usually recognize many epitopes and give immunohistochemical positive results, we also obtained promising results employing monoclonal antibodies as well (Table 1). Any biotin blocking reagent was used to reduce the background from endogenous biotin. Sections were incubated overnight at +4 °C with the primary antibody. Negative controls, consisting of the omission of the primary antibody and incubation with only 10% normal serum in phosphate-buffered saline (PBS), were performed for all the immunohistochemical assays.
Table 1. The table shows 20 primary antibodies used as neuromarkers in the cetaceans’ brains both for ff-IHC and/or p-IHC. monoclonal (M); polyclonal (P); free-floating immunohistochemistry (ff-IHC); formalin-fixed paraffin-embedded samples (FFPE); formalin fixed cryosectioned samples (FFCS); Mouse (Mo) Goat (G); Rabbit (R); Horse (H); Not applicable (N/A); Normal Serum (NS); Secondary Antibody (SA); Biotinylated anti-mouse IgG (BaMo); Biotinylated anti-rabbit IgG (BaR); Biotinylated anti-goat IgG (BaG); minutes (min).
| Primary Antibody | Type | Specificity [Published Manuscript] | Diluition ff-IHC p-IHC |
Antigen Retrieval (Only for FFPE) |
NS | SA |
|---|---|---|---|---|---|---|
| c-Fos (4) Santa Cruz Biotechnology sc-52 |
P/R | c-Fos Protein | 1/100 (both) | Pronase, 7 min | G | BaR |
| c-Jun (D) Santa Cruz Biotechnology sc-44 |
P/R | c-Jun Protein | 1/100 (both) |
Citrate Buffer, 90–95 °C, 10 min (pH 6) |
G | BaR |
| HSP70 Abcam Ab6535 |
M/Mo | Heat Shock Protein 70 kD [35] | 1/100 (both) |
None | H | BaMo |
| Ubiquitin Dako Z045801 |
P/R | Human Ubiquitin | 1/100 (p-IHC) |
None | G | BaR |
| Neuroglobin Abcam Ab37258 |
M/Mo | Neuroglobin | 1/100 (p-IHC) |
None | H | BaMo |
| Calretinin Swant 6B3 |
M/Mo | Calretinin calcium-binding protein [35] | 1/500 (p-IHC) |
Wet autoclave method of Shin 118° C, 5 min | H | BaMo |
| Calbindin D-28k Swant 300 |
M/Mo | Calbindin calcium-binding protein | 1/500 (both) |
Wet autoclave method of Shin, 118° C, 5 min | H | BaMo |
| Parvalbumin Swant 235 |
M/Mo | Parvalbumin calcium-binding protein | 1/500 (p-IHC) |
Wet autoclave method of Shin, 118° C, 5 min | H | BaMo |
| GFAP DakoCytomation |
P/R | Glial Fibrillary Acidic Protein | 1/120 (p-IHC) |
None | G | BaR |
| nNOS Millipore Ab5380 |
P/R | Nitric Oxide Synthase | 1/300 (p-IHC) |
Wet autoclave method of Shin, 118° C, 5 min | G | BaR |
| TH Monosan MONX10786 |
M/Mo | Tyrosine Hydroxylase [14] | 1/200 (ff-IHC) 1/50 (p-IHQ) |
Wet autoclave method of Shin, 118° C, 5 min | H | BaMo |
| CRF Abcam Ab59023 |
P/G | Corticotropin Releasing Factor | 1/100 (ff-IHC) |
N/A | R | BaG |
| Vasopressin Abcam Ab39363 |
P/R | Vasopressin | 1/500 (ff-IHC) |
N/A | G | BaR |
| HSV1 Abcam Ab9533 |
P/R | Herpesvirus type I [29] | 1/50 (p-IHC) |
Pronase 10 min | G | BaR |
| CDV VMRD CDV-NP |
M/Mo | Nucleoprotein of Canine Distemper Virus [36] | 1/100 (p-IHC) |
Wet autoclave method of Shin, 118° C, 5 min | R | BaMo (1/20) |
| Laforin Novus Biologicals NBP2-24474 |
P/R | Human Laforin (EPM2A) | 1/100 (p-IHC) |
Wet autoclave method of Shin, 118° C, 5 min | G | BaR |
| B-Amyloid Invitrogen 700254 |
M/R | Beta Amyloid (H31L21) [15] | 1/100 (ff-IHC) |
N/A | G | BaR |
| NFT AHB0161 |
P/R | Neurofibrillary Tangles [15] | 1/100 (ff-IHC) |
N/A | G | BaR |
| α-Synuclein Abcam Ab27766 |
M/Mo | Alpha-synuclein (LB 509) | 1/100 (ff-IHC) |
N/A | H | BaMo |
| α-Synuclein Invitrogen 35-8300 |
M/Mo | Alpha-synuclein (Syn 505) | 1/100 (p-IHC) |
Wet autoclave method of Shin, 118° C, 5 min | H | BaMo |
The day after, sections were rinsed in PBS (three times, 5 min each) and incubated for 90 min at RT with a secondary antibody (diluted 1:200) in a solution containing 10% normal serum in PBS (Table 1). Sections were rinsed in PBS and incubated with an avidin-biotin complex (ABC, Vector Laboratories; PK-4000) for 1 h at RT and developed using the 3,3′-diaminobenzidine (DAB) peroxidase kit (Vector Laboratories, SK-4100) or the 3-amino-9-ethylcarbazole (AEC) peroxidase kit (Vector Laboratories, SK-4200). DAB sections were then dehydrated in ethanol, cleared in xylene and coverslipped with DPX (Sigma). In addition, after rinsing, some DAB immunolabeled sections were counterstained using thionine prior to dehydration and coverslipping. AEC sections were counterstained with a non-commercial Mayer’s hematoxylin and coverslipped with an aqueous-based mounting medium (Vector).
2.8. Cryoprotection and Preparation of the Sample (FFCS)
For neuroanatomical purposes, a selective sampling of the brain was made. Cryoprotection, as well as cryosectioning, ff-IHC, and Nissl staining (see next sections), were adapted to previous published protocols for rat brains [37][38]. It is important to leave the samples for a further period of 48 h in 10% neutral-buffered formalin or 4% paraformaldehyde (preferably under agitation). In this way, we can ensure an optimal fixation and stiffness of the samples. Later, in order to remove the excess of fixative, the samples were washed for 48 h in PBS, under agitation and changing the buffer at least 2–4 times. After rinsing in PBS, the samples should be cryoprotected by immersing them in a 30% sucrose and 0.1% sodium azide solution in PBS (pH 7.4) at +4° C, in order to avoid freezing artifacts [39]. The solution has the properties of protecting and nourishing the piece of tissue (sucrose) by preserving it in a buffered liquid (PBS), free of bacteria and fungi (sodium azide). When the sample gets soaked with the solution, it sinks to the bottom of the container, and it is considered fully cryoprotected and ready for the next step. When using a cryostat, samples were immersed in a mixture of PBS-sucrose and Optimum Cutting Temperature formulation (OCT) (1:1) overnight. The day after, the samples were included in a mold using the same mixture and were rapidly frozen, and 40–50 μm-thick serial sections were obtained with a cryostat [(Leica CM1950, Nussloch, Germany), University of Las Palmas de Gran Canaria]. Sections were stored in PBS (pH 7.4) solution with sodium azide (0.01%). On the other hand, when using a freezing sliding microtome [(Leica SM 2000 R), University of Bologna], samples were directed removed from the cryoprotecting solution, fixed to the sample holder stage, and rapidly frozen. Working temperatures ranged between −20 °C and −35 °C, depending on the external temperature. The sucrose crystallizes creating the sample’s adherence to the stage. The samples were then sectioned at 50–60 μm.
2.9. Immunoperoxidase Staining: Free-Floating Immunohistochemistry (ff-IHC)
Sections proceeding from formalin fixed free-floating sections were treated with 3% H2O2 in PBS for 30 min at RT, in order to eliminate endogenous peroxidase activity, and rinsed in PBS (three times, 10 min each). To block non-specific binding, sections were incubated in a solution containing 10% normal serum, and 0.5% Triton X-100 (Merck, Darmstadt) to permeabilize the tissue in PBS for 2 h at RT. Thereafter, a first set of sections were incubated in the primary antibodies (Table 1), during 18 h, at +4 °C. Negative controls consisting of the omission of the primary antibody and incubation with only 10% normal serum in PBS were performed for all the immunohistochemistry experiments. After 18 h, sections were rinsed in PBS (three times, 10 min each) and incubated for 45 min at RT with a secondary antibody (diluted 1:200) in a solution containing 1% normal serum in PBS (Table 1). Some primary antibodies may require a 36 h period of incubation, in refrigeration at + 4 °C. Keeping the sections in immersion permits their conservation and prevents them from drying out. The sections were successively rinsed in PBS and incubated with an avidin-biotin complex (ABC, Vector Laboratories; PK-4000) for 1 h at RT. Finally, the sections were developed using a 3,3′-diaminobenzidine (DAB) peroxidase kit (Vector Laboratories, SK-4100) and mounted on coated slides to dry overnight. DAB Slides were then dehydrated in ethanol, cleared in xylene and coverslipped with DPX (Sigma). AEC sections were coverslipped with an aqueous-based mounting medium (Vector). In addition, some DAB immunolabeled sections were counterstained using thionine (Section 2.10) prior to dehydration and coverslipping.
2.10. Nissl Staining
For Nissl staining, thionine (Lauth’s violet) was the election dye. Thionine is a strongly metachromatic dye, useful for the staining of acid mucopolysaccharides. It is a common nuclear stain and can be used for the demonstration of Nissl substance in neurons. Sections were taken out of the first 24-well plate containing PBS with sodium azide, mounted on gelatin-coated slides, and dried. The sections were defatted and soaked for 1 h in a mixture of chloroform and 100% ethanol (1:1), rehydrated through a graded series of 100%, 96%, 80%, 70%, and 50% ethanol and distilled water (2 min each), stained 30 min in a 0.125% thionine (Fisher Scientific, Waltham, MA, USA) solution, rapidly dehydrated (few dips each step), left 2 min in 100% ethanol, cleared 10 min in xylene, and coverslipped with Entellan (Merck, Darmstaldt, Germany).
2.11. TUNEL Staining
In order to provide in situ detection of apoptosis in brain tissues, using a TUNEL (TdT-mediated dUTP-biotin Nick End-Labeling) based assay, the two following kits were used: TACS® 2 TdT-DAB In Situ Apoptosis Detection Kit Reagent (Trevigen, 4810-30-K) and NeuroTACS™ II In Situ Apoptosis Detection Kit (Trevigen, 4823-30-K). The TACS® 2 TdT-DAB Kits utilize a cation optimization system to enhance the in situ detection of apoptosis in a TUNEL based assay. Enzymatic incorporation of biotinylated nucleotides in fragmented DNA is performed by the TdT enzyme. Biotin labeling is detected using streptavidin-horseradish peroxidase and DAB. The manufacturer step-by-step protocol was used, with the only exception that samples were then counterstained with thionine instead of methyl green, due to the better results given by thionine. Two positive controls were used in each experiment: a sample of a cetacean prescapular lymphatic ganglion and a brain sample incubated with TACS-Nuclease.
An OLYMPUS BX41 light microscope was used for the histological and histopathological observations with the support of the Digital software Imaging Solutions, CellA.
3. Results
3.1. Evaluation of Brain Quality
In order to achieve an optimal fixation of the brain, it was fundamental to:
- - Provide longitudinal cuts to expose the lateral ventricles and allow the entry of the fixative;
- - Make cross-sections of the brain after at least 72 h of immersion in the fixative. Once a great percentage of the fixation process was achieved, serial cuts of the brain allowed a greater fixation;
- - Finally, the post-fixation of the selected samples permitted to complete the fixation of the tissues and provide the necessary firmness for their next processing;
- - Reposition of new fixative was made after cross sectioning the brain (immersion in a smaller container during 48 h) and then after sampling (postfixed during 24 h).
Sections proceeding from brains, which have not been processed as indicated in the protocol of manipulation and fixation, generated drawbacks and artifacts like:
- - Tissue rupture and wearing, dark, shrunken and pycnotic neurons of that brains incorrectly handled during necropsy;
- - Poor fixation of the deepest subcortical structures of those brains, which did not receive cross-sections. This resulted in poor tissue quality, predisposing to easy rupture of the sections, especially during the continuous manipulation in ff-IHC, poor immunoreactivity to the neuromarkers, and a very low affinity to thionine;
- - The prolonged permanence in the fixative resulted in the loss of tissue quality, which predisposed it to an easy rupture of the sections, loss of antigenicity, and a very low affinity to thionine. In addition, formalin pigment accumulation was observed, as a background deposition and occasionally within the neurons mimicking the neuromelanin pigment;
Finally, frozen unfixed and uncryoprotected brains were shrunken, very breakable during ff-IHC, and presenting vacuolations.
3.2. Neuromarkers for Neuroanatomical Studies
Histochemical thionine Nissl staining has been a key dye to explore most of the brain areas and nuclei of the examined species. Nissl staining offered a first evaluation of the brain quality. Alternatively to thionine, cresyl violet may be used; however, thionine provided better coloration of neurons in cetacean brain samples. Some of the investigated brain areas were the red nucleus and the nucleus ellipticus, the motor nucleus of the oculomotor (III) nerve, the motor nucleus of the trigeminal (V) nerve, the cochlear nuclei (the nucleus cochlearis ventralis, VCN, and the nucleus cochlearis dorsalis, DCN; Figure 4a), the motor nucleus of the facial nerve, the olivary nuclei, the hippocampus (Figure 4b), the claustrum, the amygdaloid complex (corpus amygdaloideum), the corpus striatum, the hypothalamic nuclei (Figure 4c), the locus ceruleus, the substantia nigra (Figure 4d), as well as other areas of the prosencephalon and the rhombencephalon. VCN and DCN, whose locations vary between the medulla oblongata and the pons according to mammalian species, have been localized near the trapezoid body in all the examined cetacean species.
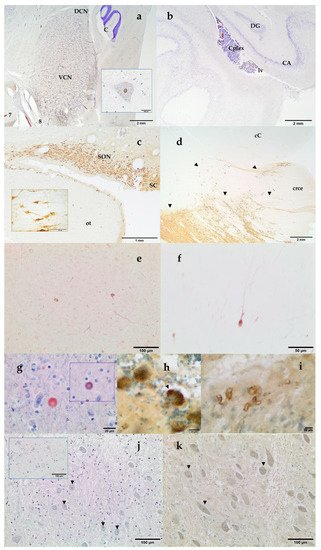
Figure 4. VCN and Dorsal Cochlear Nucleus (DCN). 7, facial nerve; 8 vestibulocochlear nerve; C, cerebellum. Thionine. Striped dolphin, Stenella coeruleoalba. Inset: intranuclear strong c-fos positivity in a giant neuron of the VCN. Paraffin embedded brain sample. DAB counterstained with thionine. Atlantic spotted dolphin, Stenella frontalis (a) Left Hippocampus. Archicortex: DG, Gyrus dentatus and CA, cornu Ammonis. Cplex, choroid plexus, lv, lateral ventricle. Thionine. Atlantic spotted dolphin, Stenella frontalis (b); Hypothalamic tuberal area: supraoptic nucleus (SON) and suprachiasmatic (SC) nucleus. ot, optic tract. Inset: neurons of the SON. Free-floating, not embedded brain sample. DAB. Vasopressin. Blainville’s beaked whale, Mesoplodon densirostris (c); Substantia nigra and crus cerebri. Distribution of the Tyrosine Hydroxylase immunoreactive neurons (arrowheads), which make up the mesencephalic substantia nigra. crce, crus cerebri; cC, caudal colliculus. Free-floating, not embedded brain sample. DAB. Atlantic spotted dolphin, Stenella frontalis (d); nNOS immunoreaction in the cortical neurons. Paraffin embedded brain sample. DAB not counterstained. Striped dolphin, Stenella coeruleoalba (e) Parvalbumin immunoreaction in the cortical neurons. Paraffin embedded brain sample. DAB not counterstained. Striped dolphin, Stenella coeruleoalba (f) Alpha-synuclein immunopositive round body in the neuropil of the mesencephalon. Inset: Ubiquitin immunopositivity in the same round body. Paraffin embedded brain sample. AEC counterstained with Mayer’s hematoxylin. Short-finned pilot whale, Globicephala macrorhynchus (g) Purkinje cells show diffuse granular and vacuolar (arrowhead) cytoplasmic NFT-positive labeling. Free-floating immunolabeling. DAB counterstained with thionine. Blainville’s beaked whale, Mesoplodon densirostris (h) Vascular amyloid deposition in the amygdaloid complex. Free-floating immunolabeling. DAB counterstained with thionine. Atlantic spotted dolphin, Stenella frontalis (i); Mild intranuclear TUNEL positivity in some multipolar small spherical and multipolar globular neurons (arrowheads) of the VCN. Counterstained with thionine. Cuvier’s beaked whale, Ziphius cavirostris. Inset: Positive control, VCN incubated with TACS-Nuclease. Neuronal and glial nuclear positivity. Counterstained with thionine. Newborn bottlenose dolphin, Tursiops truncatus. (j); Ubiquitin nuclear staining (arrowheads) in the anterior part of the VCN. Paraffin embedded brain sample. DAB counterstained with thionine. Atlantic spotted dolphin, Stenella frontalis (k).
In addition, neuromarkers as Tyrosine Hydroxylase (TH), Vasopressin, Corticotropin Releasing Factor (CRF), Glial Fibrillary Acidic Protein (GFAP), Calretinin, Calbindin, and Parvalbumin (Table 1) have been used to identify and deeply characterize different structures of the toothed whale brain. Key areas of the stress circuitry have been investigated: the amygdaloid complex (calbindin), the paraventricular, supraoptic (Figure 4c), and suprachiasmatic (Figure 4c) nuclei of the hypothalamus (vasopressin and CRF), the locus ceruleus and the substantia nigra (Figure 4d) (TH). TH-immunoreactive neurons have also been identified in the hypothalamus. In addition to thionine, calbindin D-28k allowed a complete description of the 12 deep, superficial, and other nuclei which make up the amygdaloid complex. Special interest has been given to the central nucleus of the amygdala, a key protagonist of the stress system (unpublished data). The use of the immunohistochemical technique against the antibody calbindin D-28k permitted a better definition and identification of the limits of the central nucleus of the amygdala. This nucleus was identified as a triangular area, presenting an intense immunoreactivity in the neuropil. Cortical neurons have been explored through parvalbumin, calbindin, calretinin, and nNOS (Figure 4e,f). Finally, GFAP has been used to detect normal, reactive, or neoplastic astrocytes.
3.3. Neuromarkers for Neuropathological Studies
Some neuromarkers for infectious (Distemper Virus, Herpesvirus type 1), neoplasic (GFAP), and neurodegenerative diseases (neurofibrillary tangles, β -amyloid, α-synuclein, ubiquitin, and laforin) have also been tested (Table 1). Vascular Aβ deposits (Figure 4i) as well as intranuclear Aβ immunoreactivity and amyloid plaques were also found. NFT are composed of insoluble paired helical filaments of a highly phosphorylated form of the microtubule-associated protein τ (tau) and associated lipids. NFT positivity has been observed both as uniform cytoplasmic or as granular to vacuolar cytoplasmic inclusions (Figure 4h). Alpha-synuclein was detected as round PAS-negative bodies both intraneuronal and/or in the neuropil (Figure 4g). At the same time, these structures presented positivity to ubiquitin antibody (Figure 4g, inset).
3.4. Neuromarkers for Acoustic Trauma Research
A mild nuclear staining with TUNEL assay was detected in the VCN, as in the case of the Cuvier’s BW shown in Figure 4j, mainly in the multipolar small spherical and multipolar globular neurons of the VCN. Immunohistochemistry revealed different patterns of expression of c-fos, c-jun, HSP70, ubiquitin, neuroglobin, and nNOS in the cochlear nuclei of the toothed whales. Well preserved brain tissues usually presented a clear c-fos and c-jun nuclear staining. Ubiquitin has been detected both intranuclear (Figure 4k) and in the cytoplasm. HSP70 immunostaining was observed both intranuclear and as cytoplasmic granular deposits. nNOS and neuroglobulin immunopositivity was localized as uniform cytoplasmic staining (not shown).
References
- Cozzi, B.; Huggenberger, S.; Oelschläger, H. Chapter 3. Locomotion (Including Osteology and Myology). In Anatomy of Dolphins; Academic Press: San Diego, CA, USA, 2017; pp. 33–89.
- Ridgway, S.H.; Carlin, K.P.; Van Alstyne, K.R.; Hanson, A.C.; Tarpley, R.J. Comparison of Dolphins’ Body and Brain Measurements with Four Other Groups of Cetaceans Reveals Great Diversity. Brain, Behav. Evol. 2016, 88, 235–257.
- Zilles, K.; Palomero-Gallagher, N.; Amunts, K. Development of cortical folding during evolution and ontogeny. Trends Neurosci. 2013, 36, 275–284.
- Huggenberger, S. The size and complexity of dolphin brains—a paradox? J. Mar. Biol. Assoc. 2008, 88, 1103–1108.
- Oelschläger, H.H.A.; Oelschläger, J.S. Brain. In Encyclopedia of Marine Mammals, 2nd ed.; Perrin, W.F., Würsig, B., Thewissen, J.G.M., Eds.; Academic Press: San Diego, CA, USA, 2002; pp. 134–149.
- Würsig, B.; Perrin, W.F.; Thewissen, J.G.M. History of Marine Mammal Research. In Encyclopedia of Marine Mammals, 2nd ed.; Academic Press: London, UK, 2009; pp. 565–569.
- Jacobs, M.S.; Jensen, A.V. Gross aspects of the brain and a fiber analysis of cranial nerves in the great whale. J. Comp. Neurol. 1964, 123, 55–71.
- Cozzi, B.; Mazzariol, S.; Podestà, M.; Zotti, A.; Huggenberger, S. An Unparalleled Sexual Dimorphism of Sperm Whale Encephalization. Int. J. Comp. Psychol. 2016, 29, 29.
- Fix, A.S.; Garman, R.H. Practical Aspects of Neuropathology: A Technical Guide for Working with the Nervous System. Toxicol. Pathol. 2000, 28, 122–131.
- Cammermeyer, J. Nonspecific Changes of the Central Nervous System in Normal and Experimental Material. In The Structure and Function of Nervous Tissue V6–Structure IV and Physiology IV, 1st ed.; Bourne, G., Ed.; Academic Press: Cambridge, MA, USA, 1972.
- Sarasa, M.; Pesini, P. Natural non-trasgenic animal models for research in Alzheimer’s disease. Curr. Alzheimer Res. 2009, 6, 171–178.
- Gunn-Moore, D.; Kaidanovich-Beilin, O.; Gallego Iradi, M.C.; Gunn-Moore, F.; Lovestone, S. Alzheimer’s disease in humans and other animals: A consequence of postreproductive life span and longevity rather than aging. Alzheimers Dement. J. Alzheimers Assoc. 2017, 14, 195–204.
- Di Guardo, G. Alzheimer’s disease, cellular prion protein, and dolphins. Alzheimer’s Dement. 2018, 14, 259–260.
- Sacchini, S.; Arbelo, M.; Bombardi, C.; Fernández, A.; Cozzi, B.; de Quirós, Y.B.; Herráez, P. Locus coeruleus complex of the family Delphinidae. Sci. Rep. 2018, 8, 5486.
- Sacchini, S.; Díaz-Delgado, J.; Espinosa de Los Monteros, A.; Paz, Y.; Bernaldo de Quirós, Y.; Sierra, E.; Arbelo, M.; Herráez, P.; Fernández, A. Amyloid-beta peptide and phosphorylated tau in the frontopolar cerebral cortex and in the cerebellum of toothed whales: Aging vs hypoxia. Biol. Open 2020, 9, bio054734.
- Morgane, P.J.; Jacobs, M.S. Comparative Anatomy of the Cetacean Nervous System. In Functional Anatomy of Marine, Mammals; Harrison, R.J., Ed.; Academic Press: New York, NY, USA, 1972; Volume 1, pp. 117–244.
- Knudsen, S.K.; Mørk, S.; Øen, E.O. A novel method for in situ fixation of whale brains. J. Neurosci. Methods 2002, 120, 35–44.
- Hof, P.R.; Van Der Gucht, E. Structure of the cerebral cortex of the humpback whale, Megaptera novaeangliae (Cetacea, Mysticeti, Balaenopteridae). Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2007, 290, 1–31.
- Butti, C.; Sherwood, C.C.; Hakeem, A.Y.; Allman, J.M.; Hof, P.R. Total number and volume of Von Economo neurons in the cerebral cortex of cetaceans. J. Comp. Neurol. 2009, 515, 243–259.
- Sacchini, S. Macroscopic and Microscopic, Histochemical and Immunohistochemical Characterization of the Central Nucleus of the Amygdala, Supraoptic and Paraventricular Nuclei of the Hypothalamus, and the Locus Coeruleus of the Brain of Toothed Whales. PhD Thesis, Universidad de Las Palmas de Gran Canaria, Las Palmas de Gran Canaria, Spain, 2015.
- Ridgway, S.H.; Brownson, R.H.; Van Alstyne, K.R.; Hauser, R.A. Higher neuron densities in the cerebral cortex and larger cerebellums may limit dive times of delphinids compared to deep-diving toothed whales. PLoS ONE 2019, 14, e0226206.
- Ecozzi, B.; Eroncon, G.; Egranato, A.; Egiurisato, M.; Ecastagna, M.; Eperuffo, A.; Epanin, M.; Eballarin, C.; Emontelli, S.; Epirone, A. The claustrum of the bottlenose dolphin Tursiops truncatus (Montagu 1821). Front. Syst. Neurosci. 2014, 8, 42.
- Patzke, N.; Spocter, M.A.; Karlsson, K.E.; Bertelsen, M.F.; Haagensen, M.; Chawana, R.; Streicher, S.; Kaswera, C.; Gilissen, E.; Alagaili, A.N.; et al. In contrast to many other mammals, cetaceans have relatively small hippocampi that appear to lack adult neurogenesis. Anat. Embryol. 2015, 220, 361–383.
- Manger, P.R.; Ridgway, S.H.; Siegel, J.M. The locus coeruleus complex of the bottlenose dolphin (Tursiops truncatus) as revealed by tyrosine hydroxylase immunohistochemistry. J. Sleep Res. 2003, 12, 149–155.
- Kern, A.; Seidel, K.; Oelschläger, H. The Central Vestibular Complex in Dolphins and Humans: Functional Implications of Deiters’ Nucleus. Brain Behav. Evol. 2009, 73, 102–110.
- Kuiken, T.; García-Hartmann, M. In Proceedings of the first European Cetacean Society Workshop on Cetacean Pathology: Dissection Techniques and Tissue Sampling, Leiden, The Netherlands, 13–14 September 1991.
- Nojima, T. Developmental pattern of the bony falx and bony tentorium of spotted dolphins (stenella attenuata) and the relationship between degree of development and age. Mar. Mammal. Sci. 1988, 4, 312–322.
- Cozzi, B.; Huggenberger, S.; Oelschläger, H. Chapter 6. Brain, spinal cord, and cranial nerves. In The Anatomy of dolphins. Insights into Body Structure and Function; Cozzi, B., Huggenberger, S., Oelschläger, H., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 197–304.
- Sierra, E.; Fernández, A.; Felipe-Jiménez, I.; Zucca, D.; Díaz-Delgado, J.; Puig-Lozano, R.; Câmara, N.; Consoli, F.; Díaz-Santana, P.; Suárez-Santana, C.; et al. Histopathological Differential Diagnosis of Meningoencephalitis in Cetaceans: Morbillivirus, Herpesvirus, Toxoplasma gondii, Brucella sp., and Nasitrema sp. Front. Veter Sci. 2020, 7, 650.
- Ramos-Vara, J.A. Technical Aspects of Immunohistochemistry. Veter Pathol. 2005, 42, 405–426.
- Lawson, A.V.; Furness, J.B.; Klemm, H.M.; Pontell, L.; Chan, E.; Hill, A.; Chiocchetti, R. The brain to gut pathway: A possible route of prion transmission. Gut 2010, 59, 1643–1651.
- Bombardi, C.; Grandis, A.; Chiocchetti, R.; Lucchi, M.L. Distribution of calbindin-D28k, neuronal nitric oxide synthase, and nicotinamide adenine dinucleotide phosphate diaphorase (NADPH-d) in the lateral nucleus of the sheep amygdaloid complex. Z. Für Anat. Und Entwickl. 2006, 211, 707–720.
- Yang, Y.; Keene, C.; Peskind, E.R.; Galasko, D.R.; Hu, S.-C.; Cudaback, E.; Wilson, A.M.; Li, G.; Yu, C.-E.; Montine, K.S.; et al. Cerebrospinal Fluid Particles in Alzheimer Disease and Parkinson Disease. J. Neuropathol. Exp. Neurol. 2015, 74, 672–687.
- Sparkman, D.R.; Hammon, K.M.; White, C.L. Production and characterization of a monospecific antiserum (A128) to disaggregated Alzheimer paired helical filaments. J. Histochem. Cytochem. 1990, 38, 703–715.
- Ramírez, T.; Sacchini, S.; Paz, Y.; Rosales, R.S.; Câmara, N.; Andrada, M.; Arbelo, M.; Fernández, A. Comparison of Methods for the Histological Evaluation of Odontocete Spiral Ganglion Cells. Animals 2020, 10, 683.
- Sierra, E.; Fernandez, A.; Suárez-Santana, C.M.; Xuriach, A.; Zucca, D.; de Quirós, Y.B.; García-Álvarez, N.; De La Fuente, J.; Sacchini, S.; Andrada, M.; et al. Morbillivirus and Pilot Whale Deaths, Canary Islands, Spain, 2015. Emerg. Infect. Dis. 2016, 22, 740–742.
- Bombardi, C. Distribution of 5-HT2A receptor immunoreactivity in the rat amygdaloid complex and colocalization with γ-aminobutyric acid. Brain Res. 2011, 1370, 112–128.
- Bombardi, C. Neuronal localization of 5-HT2A receptor immunoreactivity in the rat hippocampal region. Brain Res. Bull. 2012, 87, 259–273.
- Rosene, D.; Roy, N.J.; Davis, B.J. A cryoprotection method that facilitates cutting frozen sections of whole monkey brains for histological and histochemical processing without freezing artifact. J. Histochem. Cytochem. 1986, 34, 1301–1315.
More
Information
Subjects:
Anatomy & Morphology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Entry Collection:
Neurodegeneration
Revisions:
3 times
(View History)
Update Date:
09 Feb 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




