In organic viticulture, copper-based fungicides are commonly used to suppress Downy Mildew infection, caused by the oomycete Plasmopara viticola. However, the frequent and intensive use of such fungicides leads to accumulation of the heavy metal in soil and nearby waters with adverse effects on the ecosystem. Therefore, alternative, organic fungicides against Downy Mildew are urgently needed to reduce the copper load in vineyards. In this entry, the use of Warburgia ugandensis Sprague (Family Canellacea) leaf and bark extracts as potential fungicides against Downy Mildew were evaluated. In vitro (microtiter) and in vivo (leaf discs, seedlings) tests were conducted, as well as field trials to determine the efficacy of the extracts against Downy Mildew. The results revealed an MIC100 of 500 µg/mL for the leaf extract and 5 µg/mL for the bark extract. Furthermore, experiments with leaf discs and seedlings demonstrated a strong protective effect of the extracts for up to 48 h under (semi-) controlled conditions. However, in field trials the efficacy of the extracts distinctly declined, regardless of the extracts’ origin and concentration.
1. Introduction
Downy Mildew (DM) of grapevine, caused by the oomycete
Plasmopara viticola, is one of the most threatening and harmful diseases in viticulture. Ever since it was introduced to European vineyards in 1876, probably by shipping of American grape cuttings, this pathogen caused massive yield losses in vine-growing regions all over the world
[1][2]. Therefore, protective measures were urgently needed in the second half of the 19th century to control this disease, and it was a coincidence that led to the first plant protection product (PPP) against DM, the Bordeaux mixture
[3]. Copper cations (Cu
2+), which were the actual active substance against
P. viticola in the mixture, then served as a basis for PPP to combat DM all over the world, mainly as Cu oxychloride, Cu sulfate and Cu hydroxide
[4]. Furthermore, even almost 150 years after its discovery and after the development of other, more effective PPPs against DM, copper-based fungicides are still widely used in vineyards, especially in organic viticulture
[2][5][6].
Although Cu is of considerable importance for crop protection, due to its high efficacy and reduced resistance risk, it has become a growing public concern over the last decades
[6]. The continuous and intensive application of Cu-based fungicides leads to an accumulation of the heavy metal in agricultural soils and nearby waters and as a result negatively affects the ecosystems
[2][7][8][9][10]. In organic viticulture, this problem is even more severe since alternatives with comparable efficacies to copper are rare presently or even non-existing since the ban of phosphonates
[11]. Therefore, more PPPs are strongly needed to extend the “toolbox” for protection against DM and with that, reducing the cooper input in the environment
[12].
One possible source of PPPs for organic agriculture is extracts derived from plant parts (e.g., leaves, bark, roots, fruits) or whole plants, so-called botanicals
[13][14][15][16][17][18][19]. The interest in botanicals, not only as pesticides in agriculture but also as pharmaceuticals, massively increased in the last decades mostly due to public concerns regarding off-target effects by synthetic pesticides/drugs
[20][21][22][23]. Also in viticulture, several plant extracts were evaluated regarding their efficacy against DM, with promising results:
Salvia officinalis [24],
Vitis vinifera [25],
Juncus effusus [26],
Larix decidua [27],
Verbesina lanata [28],
Magnolia officinalis [29],
Yucca schidigera,
Glycyrrhiza glabra [30]. However, despite strong efforts, so far, no PPP based on plant extracts could fulfill all criteria for a highly effective fungicide against DM in viticulture.
Warburgia ugandensis Sprague (Family Canellaceae; WU), commonly known as “Uganda Green Heart Tree”, is an evergreen plant, which is mainly distributed in East and South Africa
[31][32][33]. For generations, traditional healers were using WU extracts made of bark, roots or leaves to treat different kinds of diseases/ailments like malaria, tuberculosis, skin diseases, ulcers, lung problems or intestinal worms, to name a few
[31][34][35][36][37]. The reason for its high and diverse therapeutic potential is linked to a broad arsenal of phytochemicals belonging to the groups of drimane sesquiterpenes, tannins and mannitol
[33][38][39]. Three of these phytochemicals are polygodial, warburganal and muzigadial, all showing antifungal activity against
Saccharomyces cerevisiae,
Candida albicans,
C. utilis, and
Sclerotinia libertiana [40][41]. Also, against the soil pathogens
Fusarium oxysporum,
Alternaria passiflorae, and
Aspergillus niger, WU extracts showed inhibitory effects
[42]. Besides antifungal properties, extracts made of WU exhibit further activities, e.g., antibacterial, antimycobacterial and antiplasmodial activities
[31][34][43][44][45][46]. Concerning toxicity, a study conducted on mice classified WU extracts as non-cytotoxic and showed a lethal dose of (LD
50) > 5000 mg/kg body weight
[47]. Although highly concentrated WU extract was toxic to VERO cells, no toxicity was found by mammalian macrophage cells
[35][48][49]. As a conclusion, extracts made of WU may be used as powerful active compounds in future PPPs against a wide variety of pathogens with minimal health risks. These properties are ideal requirements for an application of the extracts as fungicide in organic agriculture.
2. Microtiter Assay
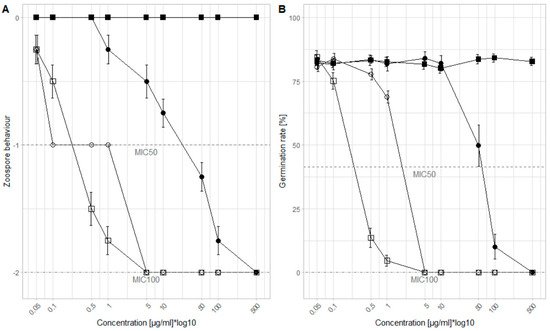
We tested the concentration range in which the WU extracts show an inhibitory effect against P. viticola. The MIC50 and MIC100 values for each extract were determined related to the zoospore behavior and the germination rate of the sporangia (Figure 1). Regarding the zoospore behavior, for the WLD, no germination (MIC100) appeared at 500 µg/mL. However, the zoospore mobility was still negatively affected (MIC50) at 25 µg/mL. The bark extract was more effective than the leaf extract with an MIC100 of 5 µg/mL and an MIC50 of 0.1 µg/mL. Compared to the Cu-fungicide, with an MIC100 of 5 µg/mL and an MIC50 of 0.25 µg/mL, the WBD had the same efficacy. Also in relation to the germination rate, the Cu-fungicide and the WBD share the same MIC100 of 5 µg/mL. However, the MIC50 of the Cu-fungicide was lower (0.25 µg/mL) compared to the WBD (2.5 µg/mL). For WLD, the efficacy was reduced when compared to the other two test products: at a concentration of 500 µg/mL all sporangia stopped germinating (MIC100) and at a concentration of 50 µg/mL the germination rate could nearly be halved (MIC50).
Figure 1. Results of the microtiter assays, (A) zoospore behavior and (B) germination rate of sporangia (n = 16): Control (■), Cu-fungicide (□), WLD (●), WBD (○). Three behavior categories were defined to measure the zoospore behavior: (0) normal zoospore motion; (−1) no or unusual zoospore motion; (−2) no zoospore release. Nine concentrations of each extract were selected to examine the inhibition efficacy: 0.05; 0.1; 0.5; 1; 5; 10; 50; 100; and 500 µg/mL. Y-axis is log10 transformed to generate a more coherent plot. MIC50 is indicated as a dashed line, MIC100 as a dotted-dashed line.
3. Leaf Disc Assay
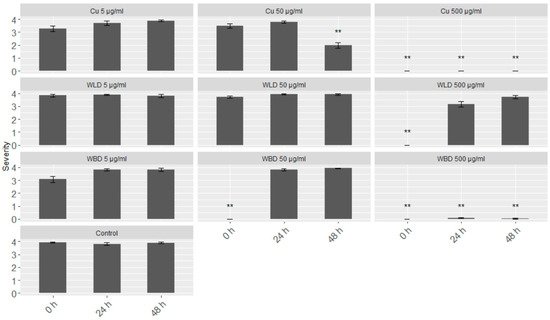
A leaf disc assay was chosen to test the efficacy of the WU extracts in an in vivo system. With three tested concentrations (5, 50 and 500 µg/mL) and three different infection time points (0, 24 and 48 h after treatment), the WLD had a protective effect only at the highest concentration, i.e., 500 µg/mL, and only if the inoculation took place directly after the treatment (0 h; Figure 2). When leaf discs were inoculated after 24 or 48 h, respectively, the leaf discs showed full sporulation (infection severity 4). WBD treatment resulted in a full protection or no sporulation (infection severity 0), respectively, when using 50 and 500 µg/mL, at 0 h. However, long-term protection, i.e., 24 and 48 h, only could obtained with 500 µg/mL WBD. Also, the Cu-fungicide at 500 µg/mL could maintain its protective effect up to 48 h. With 5 µg/mL, an infection could not be impeded, but with 50 µg/mL at 48 h the severity could significantly be reduced by half.
Figure 2. Merged (cv. ‘Müller–Thurgau’ and ‘Pinot Noir’) results of the leaf disc assays based on infection severity: (0) no sporulation, (1) minimal sporulation, (2) low sporulation, (3) medium sporulation, (4) full sporulation. For each treatment, three concentrations were tested (5, 50 and 500 µg/mL) and three different time points of sporangia inoculation (0, 24, and 48 h after spraying). Treatments were Cu-fungicide (Cu), WLD, WBD and control. Welch’s anova and Games–Howell test for multiple comparison: Significant differences of the treatments compared to control; ** p < 0.001; n = 48.
4. Seedlings under Semi-Controlled Conditions
A preliminary test with seedling plants, cv. ‘Müller–Thurgau’ and ‘Pinot Noir’, was performed to study the protection efficacy of the WU extracts under field conditions. Seedlings were grown in greenhouse, treated in the lab, but then placed in the field in order to simulate a natural exposure to abiotic factors (e.g., sunlight, humidity/aridity). After 24 and 48 h, respectively, the seedlings were brought to the lab and a leaf disc assay was performed (Figure 3). While under field conditions, there was no rain, the mean temperature was 16.5 °C (max. = 25.6 °C, min = 8.3 °C) and the mean relative humidity was 59.0% (max. = 88%, min = 32%).
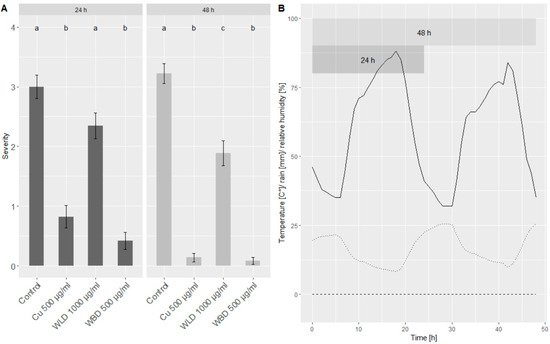
Figure 3. (A) Merged (cv. ‘Müller–Thurgau’ and ‘Pinot Noir’) results of the leaf disc assay conducted with seedling plants under semi-controlled conditions. Shown is the infection severity of leaf discs treated either with water (control), Cu-fungicide 500 µg/mL, WLD 1000 µg/mL or WBD 500 µg/mL: (0) no sporulation, (1) minimal sporulation, (2) low sporulation, (3) medium sporulation, (4) full sporulation. Welch’s anova and Games–Howell test for multiple comparison: Different letters indicate significant (p < 0.05; n = 72) differences between groups. (B) Weather conditions during the time of the experiment, when seedlings were placed in the field: Dashed line = rain, dotted line = temperature, solid line = relative humidity.
A mean severity of around three (medium sporulation) was observed for both time points (24 and 48 h) of the control treatment. Seedlings treated with 500 µg/mL of the Cu-fungicide showed a minimum sporulation (1) at 24 h and almost no sporulation (0) at 48 h. Compared to the control, when treated with 1000 µg/mL WLD, the DM severity could be slightly reduced: at 24 h by 22% and at 48 h by 40%. Using 500 µg/mL WBD, the severity, compared to the control treatment, could be reduced by 86% (24 h) and 98% (48 h), respectively, resulting in almost no sporulation.
5. Field Trials
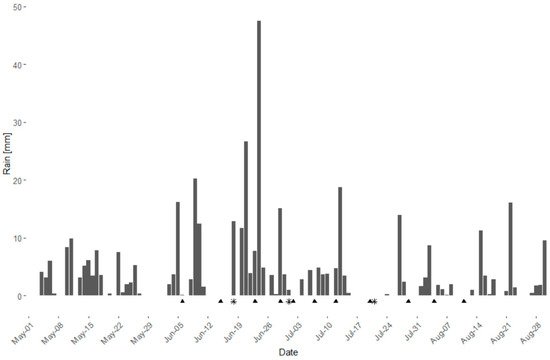
In the season 2021 a field trial in two vineyards (cv. ‘Riesling’ and ‘Dornfelder’) was conducted to test the efficacy of the WU extracts under field conditions. The season 2021 was characterized by multiple and partially heavy rainfalls (Figure 4); from May to August, 57 out of 123 days showed rainfall, with a total amount of 411.8 mm and a mean precipitation of 7.2 mm per rain day.
Figure 4. Daily rainfall (mm) during the season 2021 from May to August at the Julius Kühn-Institute facilities in Siebel-dingen, Rhineland-Palatinate, Germany. Triangles indicate date of plant protection application (∑ = 11). Asterisks indicate date of DM assessment (∑ = 3).
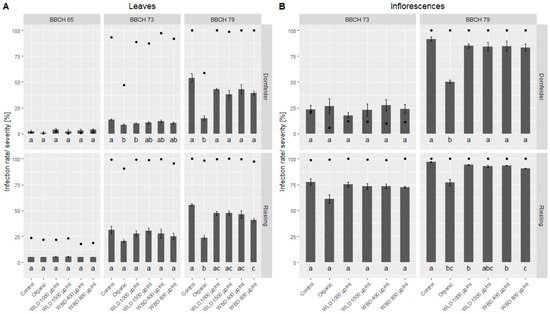
Due to massive and frequent rain events, DM primary infection and consequently multiple secondary infections occurred in the trial fields (Figure 5). In the untreated control blocks, the infection rate on leaves reached 100% in the last monitoring (at BBCH 79) with a severity of around 55%. This was true for both trial fields, ‘Dornfelder’ and ‘Riesling’. Also on inflorescences, the infection rate in both fields was 100% in the last assessment, with a severity of 91% and 97%, respectively.
Figure 5. Mean DM infection rate (dots) and severity (bars) in the two trial fields, ‘Dornfelder’ and ‘Riesling’. (A) Leaves were assessed on three phenological stages (BBCH 65, BBCH 73, BBCH 79) and (B) inflorescences on two phenological stages (BBCH 73, BBCH 79). Welch’s anova and Games–Howell test for multiple comparison: Different letters indicate significant differences in mean infection severity; p < 0.05; ‘Dornfelder’: n = 5, ‘Riesling’: n = 4.
Regarding leaf infection, on the first assessment (full flowering, BBCH 65), in both vineyards no differences in efficacy (based on infection severity) could be found between the different treatments (Table 1); however, DM infection severity (1.0–5.5%) in general was low at this time. Two weeks later, when berries had groat-size (BBCH 73) and the infection rate and severity increased, a slight protective effect of the organic treatment could be noticed with an efficacy of 36.3% for ‘Dornfelder’ and 33.3% for ‘Riesling’. Among the WU extracts, WLD 1000 µg/mL reached the highest efficacy at this stage with 26.6%. When majority of the berries were touching (BBCH 79), the efficacy of the organic treatment increased up to 71.7% in the ‘Dornfelder’ field and 57.2% in the ‘Riesling’ field. Here, the efficacy of the WU extracts varied between 13.8% (WLD 1500 µg/mL, ‘Riesling’) and 30.3% (WLD 1400 µg/mL, ‘Dornfelder’).
Table 1. Efficacy (%) of the WU extracts based on the assessment data (only infection severity) from the trial field experiment conducted in 2021 in two vineyards (‘Dornfelder’ and ‘Riesling’). Shown is the mean efficacy (±sd) on three and two, respectively, different phenological stages of each tested treatment: Organic, WLD 1000 µg/mL, WLD 1500 µg/mL, WBD 400 µg/mL and WBD 800 µg/mL.
| |
|
|
|
Leaf |
|
Inflorescences |
| |
|
n |
BBCH 65 |
BBCH73 |
BBCH 79 |
BBCH 73 |
BBCH 79 |
| Organic |
‘Dornfelder’ |
5 |
40.0 ± 54.8 |
36.3 ± 12.5 |
71.7 ± 11.4 |
−33.7 ± 102.5 |
45.2 ± 2.2 |
| ‘Riesling’ |
4 |
0.0 ± 0.0 |
33.3 ± 10.1 |
57.2 ± 8.4 |
20.0 ± 17.4 |
20.6 ± 6.5 |
| WLD 1000 µg/mL |
‘Dornfelder’ |
5 |
16.0 ± 47.7 |
26.6 ± 4.3 |
18.0 ± 16.8 |
18.1 ± 34.0 |
7.0 ± 7.8 |
| ‘Riesling’ |
4 |
−10.0 ± 11.5 |
6.8 ± 34.2 |
14.2 ± 9.4 |
2.3 ± 11.9 |
2.8 ± 0.5 |
| WLD 1500 µg/mL |
‘Dornfelder’ |
5 |
40.0 ± 54.8 |
21.9 ± 14.9 |
30.3 ± 14.4 |
−3.4 ± 65.2 |
8.4 ± 8.1 |
| ‘Riesling’ |
4 |
−10.0 ± 11.5 |
−1.0 ± 28.0 |
13.8 ± 9.4 |
4.6 ± 11.6 |
4.4 ± 2.0 |
| WBD 400 µg/mL |
‘Dornfelder’ |
5 |
3.3 ± 7.5 |
9.6 ± 33.9 |
19.1 ± 22.1 |
−23.8 ± 66.4 |
7.6 ± 7.7 |
| ‘Riesling’ |
4 |
0.0 ± 0.0 |
8.2 ± 35.9 |
16.7 ± 13.1 |
4.2 ± 14.6 |
3.6 ± 1.0 |
| WBD 800 µg/mL |
‘Dornfelder’ |
5 |
3.3 ± 7.5 |
21.9 ± 20.9 |
25.2± 18.3 |
−3.1 ± 29.1 |
9.0 ± 5.2 |
| ‘Riesling’ |
4 |
0.0 ± 0.0 |
17.9 ± 28.0 |
26.5 ± 7.0 |
5.5 ± 10.1 |
6.4 ± 0.5 |
In the first assessment of the inflorescences (BBCH 73) the infection rate and severity differed between the two vineyards and so did the efficacy. No differences were found in the low infected ‘Dornfelder’ field between the five treatments, since infection severity was more or less the same for all treatments (17.8–27.4%). In the ‘Riesling’ field, the infection rate was almost around 100% in all treatments and according to the infection severity, the organic treatment revealed an efficacy of 20% while the WU extracts only showed an efficacy between 2.3% and 5.5% (Table 1). At BBCH 79 the infection rate of the inflorescences was 100% in all treatments of the ‘Dornfelder’ field. Here, the organic treatment had an efficacy of 45.2% and the WU extracts showed an efficacy between 7.0% and 9.0%. In the already highly infested ‘Riesling’ field, the infection severity of the inflorescences slightly increased, from BBCH 73 to BBCH 79, but the efficacy of the five treatments did not change over time; the organic treatment yielded 20.6% and the WU extracts reached 2.8% to 6.4%.