Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Daniele Veclani | + 4296 word(s) | 4296 | 2021-11-02 04:43:55 | | | |
| 2 | Dean Liu | Meta information modification | 4296 | 2022-01-27 09:28:30 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Veclani, D. Platinum Based Cytostatic Drugs. Encyclopedia. Available online: https://encyclopedia.pub/entry/18882 (accessed on 08 February 2026).
Veclani D. Platinum Based Cytostatic Drugs. Encyclopedia. Available at: https://encyclopedia.pub/entry/18882. Accessed February 08, 2026.
Veclani, Daniele. "Platinum Based Cytostatic Drugs" Encyclopedia, https://encyclopedia.pub/entry/18882 (accessed February 08, 2026).
Veclani, D. (2022, January 27). Platinum Based Cytostatic Drugs. In Encyclopedia. https://encyclopedia.pub/entry/18882
Veclani, Daniele. "Platinum Based Cytostatic Drugs." Encyclopedia. Web. 27 January, 2022.
Copy Citation
Platinum based cytostatic drugs (Pt CDs) are among the most used drugs in cancer treatments which are administered via intravenous infusion and released partially intact or as transformation products.
cytostatic drugs
1. Introduction
Cancer, considered the second cause of death in the world, is a primary global public health issue [1]. The International Agency for Research on Cancer (IARC) estimates that by 2040, 30.2 million new cases of cancer will be diagnosed worldwide, with more than 16 million expected deaths [2]. The increment in cancer frequency will consequently be associated with an increase in the use of cytostatic drugs (CDs) to treat this disease [3]. CDs are cytotoxic molecules designed and applied to cause cellular dysfunction. These compounds inhibit the growth of cancer cells by altering their metabolism, blocking cell division and reproduction [4]. Despite all the advantages, cytostatic damage is not exclusively specific to tumor cells, but has an impact on all body cells, causing adverse side effects such as renal, digestive, hematopoietic, liver, and dermal [4]. Once administered, these compounds are excreted from the body unchanged or as metabolites that pass into the effluents of hospitals and homes, until they arrive in the sewage system and so the wastewater treatment plant (WWTP) [3]. The removal efficiency in the WWTP depends on the treatment process and the substance, but in general CDs are poorly biodegradable and are released into the terrestrial and aquatic systems contaminating rivers, and even into the sea, at trace levels [5]. Another source to be considered is the effluents from pharmaceutical manufacturing plants that have been unrestrictedly discharged into the environment [6][7][8][9].
These water bodies contaminated by CDs, in many cases, can harm the lives of humans and other aquatic organisms exposed to them, causing long-term damage to those eukaryotic organisms, even at trace level. For this reason, the EU Commission Decision 2000/532/EC251 classified the cytotoxic and cytostatic medicinal products as hazardous wastes [10].
Platinum-based CD are the most employed drugs in cancer treatments, and it is estimated that their usage will continue to increase in the next years. Cisplatin is the most relevant anticancer drug ever discovered, and as of today about 50% of all cancer patients are cured with this metal drug [11]. Over the last 30 years numerous platinum compounds were tested [12][13][14][15], but only a few obtained international marketing approval: carboplatin, oxaliplatin, nedaplatin, heptaplatin, and lobaplatin (Figure 1) [14].
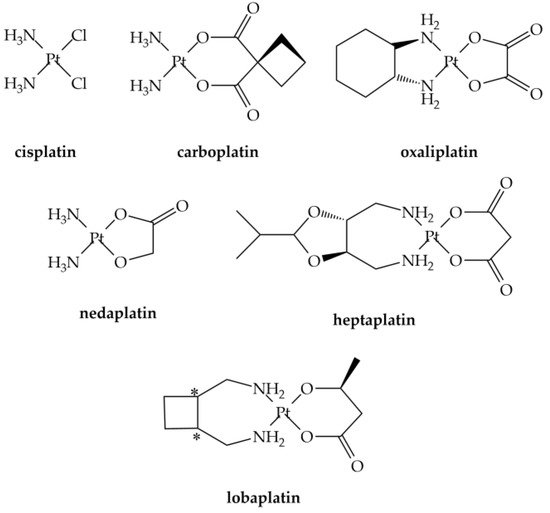
Figure 1. Clinically approved platinum anticancer drugs. *: chiral centres.
Studies addressing their occurrence in different environments, as well as the proper elimination or degradation methods from the wastewaters (WW), are numerous [6][16][17][18][19]. Despite their concentrations, hospital wastewaters (HWW), sewage, and natural waters are very low (typically in the ng L−1 range or lower) [6][20]. Numerous studies have measured the cytostatic concentrations in surface waters. Most of these studies have reported that, in general, these drug residues are safe for the aquatic biota, with few exceptions [21][22].
The presence of cisplatin and cisplatin-based cytostatics has been found to display an apparently low environmental risk [23]; however, recent evidence suggests that predictions of cytostatic concentrations in the water bodies have been underestimated [24].
This evidence, together with the expected increase of the use of cytostatics in the coming years and the lack of research on potential chronic damage [25], could put the health of entire ecosystems at risk.
2. Action Mechanism, Speciation and Determinations Analysis of Pt-Based CDs
2.1. Reactivity
Since Rosenberg and co-workers [26][27] unexpectedly discovered the antiproliferative action of the cis-diamminedichloroplatinum(II) (Pt(NH3)2Cl2) (Figure 1), cisplatin, many studies have been carried out to develop compounds with less severe side effects and improved efficacy [14][15][28].
Experimental evidence revealed that the mechanism of action (Figure 2) of classical platinum CDs is characterized by four steps process: (1) cellular uptake, (2) activation by the chloride substitution reactions, (3) DNA binding, and (4) cellular processing of DNA lesions leading to cell death [28].
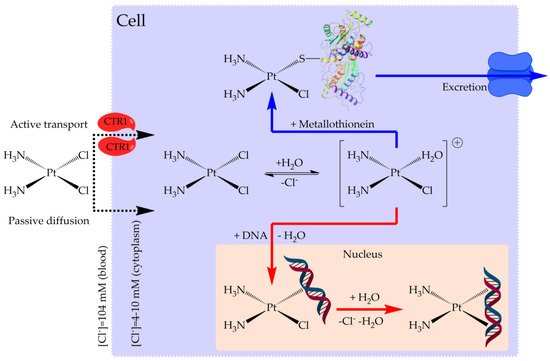
Figure 2. Mechanism of action of cisplatin.
Passive diffusion and active transport, facilitated by membrane proteins, are both involved in cellular uptake [29]. In the blood stream, where the chloride concentration is relatively high, the chloride release is suppressed, while, in the cell, they are replaced by water in solution to produce positively charged “aquated” molecules (Figure 3) [30]. These aqua-complexes (and in particular Pt(NH3)2(OH2)Cl+) are very reactive and can form covalent bonds with DNA bases. Numerous experimental and theoretical studies were devoted to clarify these reactions [31][32][33][34][35][36][37][38][39][40].
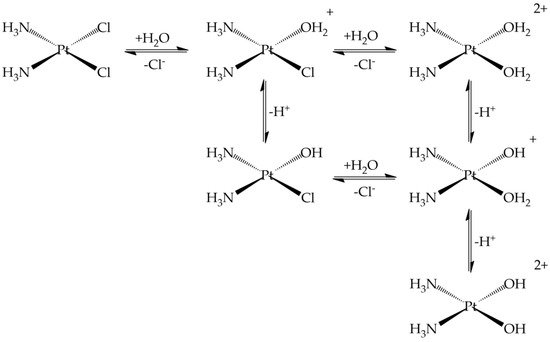
Figure 3. Ligand substitution (chloride) and protonation equilibria for cisplatin.
The final cisplatin-DNA adducts present coordinated intra- and inter-strand cross-links which activate a series of processes ultimately leading to cell death. It is interesting to note that these reactive aqua-derivatives of cisplatin are also detected in the urine with a speciation dependent on the temperature, chloride concentration, and pH (Figure 3) [41].
Carboplatin or cis-diammine-(1,1-cyclobutanecarboxylato)platinum(II) (Figure 1) is an antineoplastic drug widely used against cancer of head, neck, lung, and ovaries [42][43]. Carboplatin is an early example of attempt to develop less toxic platinum alkylating agents, and was introduced in clinical use in the mid-1980s [44]. The principal chemical structure difference between carboplatin and cisplatin was the presence of bidentate dicarboxylate (CBDCA) ligand instead of the two chloride ligands of cisplatin molecule [12]. It shows lower reactivity and slower DNA binding kinetics [12] which makes it less toxic than cisplatin [45]. Oxaliplatin (trans-L-diaminocyclohexane oxalatoplatinum(II)) emerged as the prominent third-generation platinum based anticancer drugs [46]. In the late 1970s, oxaliplatin was suggested as a possible anticancer drug but the FDA approval was obtained only in 2002 [46]. It was employed in the treatment of several kinds of cancers, including colon cancer, which had not responded to cisplatin treatment [46], gastrointestinal, and gynecologic cancers [47]. It is interesting to note that carboplatin and oxaliplatin present a quite different chemical reactivity profile, which affects the speciation in excreted samples (urine): in the first case the intact drug is excreted, while for oxaliplatin up to 17 metabolites were determined [48].
2.2. Prediction of Platinum-Based CDs Release into the Environment
The evacuation of urine and feces from patients treated with anticancer agent is regarded as the primary source of platinum-based anticancer drugs and their related metabolites. These compounds have been unrestrictedly discharged into the environment, via hospital effluents and/or municipal WW, and possibly also effluents from pharmaceutical manufacturing plants [9][48][49][50][51][52].
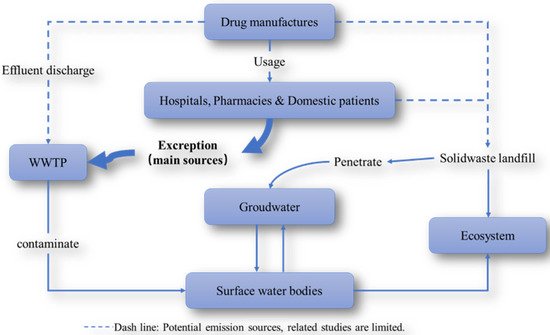
Figure 4. Routes through which platinum-based anticancer drugs are input and transported in the environment.
As early as more than 20 years ago, researchers have been keenly aware that Pt-containing pharmaceutical residues frequently appeared in HWW. The calculation of the PEC (predicted environmental concentrations) of platinum-based cytostatic compounds, as well as their quantification through total Pt determination methods [48][53][54][55] are the methods employed to predict and determine the environmental occurrence of these compounds.
In one interesting study [17] it was attempted to predict the possible concentrations range of four cytostatics in the sewage effluent and surface waters of various states in the European Union: 5-fluorouracil (5FU), capecitabine (CB), cyclophosphamide (CP), and carboplatin. The prediction of the range of cytostatics concentrations was made by using literature data on human excretion values, publicly available consumption data, and sewage removal rates of every country. The results predicted mean effluent concentrations of carboplatin were 0.8–2.5 ng L−1, which are similar to 5-FU, and lower than CP and CB. Several studies have estimated the environmental concentrations of platinum-based CDs through the calculation of the PEC [9][16][56]. The PECs [57] of cisplatin, carboplatin, and oxaliplatin established on their consumption for the years 2004 and 2008 in France were determined. Interestingly, an increase in the PECs of the three drugs was observed in the year 2008 with respect to the year 2004. However, the same study [57] concluded that hospital effluents are not the principal source of anticancer drugs into the aquatic environment as also observed in ref. [54].
In ref. [56] a PEC and analytical study on HWW of the city of Qom indicated a Pt concentration lower than that of the EMEA guidelines and that the investigation and monitoring of the residual of cytotoxic anticancer drugs should be taken into account considering the low efficiency of conventional WWTP in removing platinum cytotoxic pharmaceutical compounds.
One point which has to be taken into account when considering data on platinum CDs occurrence is that the CDs-derived metal found in the environment is 1–2 orders of magnitudes lower than other manufacturing/industrial sources [58].
A recent risk assessment study has been conducted in Brazil [59] on several CDs which indicated that the highest PEC was associated with CP, 5-FU, and cytarabine while for cisplatin and carboplatin the value was significantly lower. However, authors underline the fact that the analysis was conducted in an urban area with a WWTP and that other, less developed, areas could have higher concentrations.
2.3. Analytical Techniques
Given the low concentrations involved in the analysis of biological and environmental samples, different techniques have been developed in recent years to try to decrease the limit of detection (LOD) of Pt-CDs in biological (urine, plasma) and especially environmental samples where the dilution factor is higher [60]. Additionally, it emerged that speciation is important to determine the amounts of intact drug and transformation products. In order to obtain these objectives, liquid chromatography protocols (mostly coupled with MS detection) or electroanalytical sensors have been developed in the last decades.
2.3.1. Biological Samples
High Performance Liquid Chromatography (HPLC) analysis of antitumor Pt-CDs based on UV-Vis detection exploiting the reaction of Pt complexes with sodium bisulfite to enhance the absorptivity was realized to detect cisplatin, carboplatin, and oxaliplatin in plasma and urine [61]. LOD for cisplatin, oxaliplatin, and carboplatin were 20, 40, and 60 nM, respectively.
An HPLC method was also proposed for cisplatin analysis in ultrafiltrate plasma in the presence of nickel chloride as internal standard. Cisplatin and the internal standard were chelated by exchange with diethyldithiocarbamate (DDTC) for UV-Vis detection. The limit of quantification was 0.03 μg mL−1 using only 0.5 mL of ultrafiltrate.
In another work, HPLC coupled with UV-Vis detection (direct or with post-column bisulfite derivatization) was also used for the quantitation of carboplatin in human plasma ultrafiltrate provided a LOD of 0.025 μg mL−1. After validation, this method was used to study the pharmacokinetic analysis of blood samples drawn from a patient that received a 400 mg m−2 dose of carboplatin [62].
Fast oxaliplatin determination in urine was developed by Hann et al. with an LOD of 0.05 µg L−1 oxaliplatin [63]. In this case, it was found that samples needed to be rapidly stored at −80 °C to detect the intact drug.
Carboplatin was measured in urine of a chemotherapy patient by using IDMS (isotope dilution mass spectrometry) technique both with LC-ICP-QMS (HPLC coupled with inductively coupled plasma source and elemental quadrupole based mass spectrometer) and LC-ESI-TOFMS (HPLC with a electrospray ionization source and time-of-flight MS) [64]. The procedural LOD for the two techniques were 0.1 and 15 ng g–1 respectively. However, it should be noted that 194Pt-enriched compound has to be prepared.
HPLC coupled with tandem mass spectrometry detection (HPLC–ESI-MS/MS) has been also used for the quantitative determination of Pt after derivatizing with DDTC [65]. The quantification was obtained using a triple quadrupole with electrospray ionization and detection was achieved using multiple reaction monitoring. The authors reported a LOD of 1 ng mL−1, and the quantifiable range was 3–3000 ng mL−1 in urine and rat plasma [65].
Atomic absorption spectrometry (AAS) was employed to determine cisplatin and carboplatin in human urine [66]. Samples from healthy individuals not subjected to treatment with platinum drugs were collected in polypropylene tubes (50 mL) and stored at 4 °C until analysis. Urine samples from a treated patient collected within 48 h was collected and stored at −4 °C. The obtained LOD resulted 0.004 mg L−1 of platinum.
Conjoint liquid chromatography (CLC) coupled on-line to UV and ICP-MS was employed for the speciation analysis of Pt in human serum spiked with cisplatin, oxaliplatin, and carboplatin [67]. The limit of quantitation (LOQ) was lower than 2.4 ng Pt mL−1. This method allowed for study the interaction of Pt-CDs with serum proteins and showed they were bound preferentially to human serum albumin (HSA).
A validated ICP-MS method for quantitative determination of platinum levels in rat urine, plasma, and tissues (rat liver, brain, lungs, kidney, muscle, heart, spleen, bladder, and lymph nodes) was also proposed [68] with a limit of quantitation of 5 ppb. In that case, the samples were treated by microwave digestion.
Two non-suppressed ion chromatography (IC) methods, one with an anion and one with a cation separation column, were used for determinations of cisplatin and carboplatin [69]. In this study, an inductively coupled plasma-atomic emission spectrometry (ICP-AES) was used as detector and the obtained LOD was 0.1 mg L−1 for both Pt-CDs.
A high throughput analytical method based on ICP-MS for the determination of total Pt in plasma, plasma ultrafiltrate, urine, and peritoneal fluid was proposed [70]. The claimed advantage of this protocol resides in the high sensitivity (LOD = 1.76 ng mL−1 Pt in plasma, 0.39 ng mL−1 in ultrafiltrate, 0.29 ng mL−1 in urine, and 0.30 ng mL−1 in peritoneal fluid) and fast and simple sample preparation.
Folens et al. [71] determined the release of platinum in the urine of patients by means of ICP-MS to obtain a pharmacokinetic model which suggested that retaining the Pt in the first 24 h after the treatment would be convenient for the recovery, as after the concentrations are very small (see also Section 5). For the analytical part they collected urine samples, which were subjected to a microwave digestion step followed by ICP-MS analysis with a LOD of 0.005 µg L−1.
As far as electroanalytical methods are concerned, several works have been made, mostly with the objective to develop sensitive sensors for fast analyses on biological and/or environmental samples.
An electrochemical cisplatin and carboplatin specific sensor was made with a thiolated and methylene blue-modified oligo-adenine (A)-guanine (G) DNA probe [72]. The obtained LOD was 500 nM, with linearity between 0.5 and 5 μM. This sensor was tested on simulated urine and saliva samples and, interestingly, it was insensitive to Pt(IV) compound or commonly prescribed antibiotics.
In 2006 Petrlova and coworkers developed a sensor for cisplatin by modifying a mercury drop electrode with metallothionine, a protein that reacts readily with platinum complexes [73]. Both modifications of the electrode and the identification of cisplatin were obtained by adsorptive transfer stripping technique and differential pulse voltammetry applied to the analysis of cisplatin in human blood serum. Results showed that the LOD was about 2.5 pmol in 5 μL (0.5 μM) with an interaction time of 400 s; this limit was calculated from the decrease of the highest observed signal (CdT) peak.
Carbon-based nanomaterials (CNM), such as carbon nanotubes (CNTs) and graphene, played a great role in the electronic and sensor field thanks to their peculiar proprieties [32][74][75]. A graphene-based electrochemical sensor made by nano-porous glassy carbon electrode (npGCE) and modified with graphene quantum dots (GQDs) functionalized with thionine groups showed sensitive and selective determination of cisplatin [76]. The determinations of cisplatin in different fluids, such as urine and blood serum samples, were obtained by cyclic voltammetry. The results showed that the linear range was 0.2–110 μM with the LOD of 90 nM. The authors stated that this was the lowest LOD obtained for cisplatin.
Another interesting sensor [77] for electrochemical determination of platinum complexes was obtained from the functionalization of screen printed electrodes with multi-walled carbon nanotubes and factory modified with carboxyl groups. The LOD and LOQ reported were 4.6 and 1.4 μmol L−1. These results were further compared with those obtained by HPLC, and the average error % (sensor/HPLC) was 3.4, indicating that the developed sensor was an appropriate alternative to the use of HPLC for cisplatin determination in biological samples.
Recently also a fluorescent sensor array has been used to detect platinum in clinical human blood samples [78]. These sensors were able also to distinguish between different compounds in the 0.5–5.0 μM range.
2.3.2. Environmental Samples
One of the first articles where the presence of cisplatin and carboplatin in sewage of five European hospitals was determined by Kummerer et al. using adsorptive voltammetry technique [50]. Their results showed that 70% of the drugs were excreted and reached the hospital effluents. The hospital effluents average daily concentrations were approximately 10–601 ng L−1 of Pt; these data were then compared with an estimation of the Pt emitted by cars. The authors observed that Pt emitted by hospitals was 3.3 to 1.3% of the estimated amount emitted by cars, indicating that the effluents of hospitals have a limited influence on municipal WW; however, the Pt species emitted by hospital should not be disregarded [50].
In other works, the presence of the CDs as total Pt in the matrix under study was determined. In 2005, Lenz et al. [48] measured the excreted CPC in HWW sampled in Vienna in a period of 28 days. As a result, it was found that the Pt concentrations were ranging from 4.7–145 μg L−1. Two years later, in 2007, the same group [79] measured the total Pt in the influents and effluents of a pilot membrane bioreactor (MBR) plant of the same hospital, and the concentrations oscillated from 3–250 μg L−1 and 2–144 μg L−1 in the influents and effluents, respectively. In this study, the consumption of Pt-CDs in the oncological ward was also recorded, and it was observed that (1) only the 27–34% of the administered Pt is found in the WW of the oncological ward; (2) around 51–64% of platinum is removed in the activated sludge MBR.
Another study performed in a hospital in UK [54] reported Pt concentrations ranging from 20.02 to 140 μg L−1 in the effluents and hospital’s main drain.
The presence of antitumor drugs in the hospital effluents and in WWTP influents and effluents form Slovenia and Spain and their metabolites was studied [55]. Cisplatin was determined as total Pt using ICP-MS. In Slovenia the Pt concentrations in hospital effluents was around 352 ng L−1. The Pt concentrations in the WWTP influents were around 27 ng L−1, while the Pt concentrations in effluents were below the LOD. In contrast, all the samples taken in Spain had Pt concentrations below the LOD of the method employed. The hospitals under study were different in every region, in Slovenia; the sample collection was made from the oncological ward, while in Spain samples were collected at a general hospital. The general hospital from Spain was approximately 4-times bigger, which means that the concentration of the platinum drugs in the influents and effluents was highly diluted, which may be a reason why the method could not detect them.
When platinum-based anticancer drugs repeatedly appear in hospital sewage, municipal WW receive a considerable contribution of excreted antineoplastic compounds as the result of outpatient treatment [80]. It is worth noting that the increasing number of outpatients nowadays results in the fact that domestic discharge will become another important source of Pt contamination as also suggested in ref. [17][56].
Santana et al. also sampled from a WWTP of Gran Canarias Island (Spain) for the determination of cytostatic platinum compounds and found that concentrations in the range 1.94–13,913 ng L−1 in HWW and 3.97 and 75.79 ng L−1 in WWTP by ICP-MS analysis. Authors presented an optimized method for the extraction and preconcentration of Pt-CDs in WW samples based on ion exchange solid phase extraction which allowed to reach a very low LOQ [81].
To solve many fundamental and important problems with a high degree of accuracy, precision, sensitivity, selectivity, and reproducibility, electroanalytical techniques can be easily employed [82].
Several platinum complexes, such as cisplatin, carboplatin, oxaliplatin, PtCl42− and PtCl2, was also coupled with flow injection analysis with electrochemical detection (FIA-ED) method [83]. The flow injection instrument was made with a solvent delivery pump and an electrochemical detector consisting in a: (i) working electrode: glassy carbon electrode; (ii) reference: hydrogen-palladium electrode; (iii) auxiliary electrode. A sample of water from the Ponavka river, where platinum complexes were added in concentrations of 10 and 40 μg mL−1, was used as a reference solutions to verified this method. Interestingly, the proposed method could discriminate between the Pt-CDs and the chloride complexes.
The health risk assessment of platinum CDs in drinking water of Qom province in Iran has been evaluated [84]. HPLC-MS was employed for the quantification of the components. The results of this study showed cytostatics concentrations above 100 ng L−1 in every case and in the case of carboplatin above 900 ng L−1 in the WWTP influents.
Determinations of carboplatin and oxaliplatin in WW samples was carried out also by HPLC-MS/MS [85]. The LODs obtained for carboplatin and oxaliplatin were 0.013 and 0.090 ng L−1, while LOQ was 0.4 and 0.027 ng L−1, respectively. Recovery (%) was 0.78 and 0.74 and the RSD% was between 6.0 to 8.9% and 7.5 to 7.9% for carboplatin and oxaliplatin respectively, while the correlation coefficients were 0.9998 and 0.9990. The environmental risk assessment was carried out and the risk quotients (RQ) obtained were 0.51 and 0.038 for carboplatin and oxaliplatin respectively, indicating that these compounds had low environmental exposure risk. RQ value is the ratio between the predicted environmental concentration and the prognosticated no-effect concentration. A value of RQ < 1.0 shows no significant risk; RQ between 1.0 and 10 indicates small possible adverse effects; a value higher than 10 indicates significant potential for adverse effects and value ≥ 100 indicates that potential side effects can be predictable.
In 2015, the PEC/analytical study at the hospitals of Qom [57] correlated the calculate calculated RQhww was 1.19. ICP–OES, and limit of detection was determined (LODs = 1 µg L−1).
In addition to the methods for determining the concentration of the platinum-based drugs, the separation methods for these compounds are also important. For example, pentafluorophenylpropyl- functionalized silica gel was employed to separate platinum-based drugs, and HPLC-ICP-MS was used to the determinations of cisplatin and its hydrolysis products, carboplatin, and oxaliplatin in urine sample and in HWW samples [86]. The limits of detection determined were 0.09, 0.10, and 0.15 μg L−1 for cisplatin, carboplatin, and oxaliplatin, respectively. Moreover, the stability of carboplatin and oxaliplatin, at different chloride concentrations to simulate the conditions of WW and surface water, was obtained using this method. The results indicated that carboplatin was stable in pure water and in 1.5 mol L−1 Cl− solutions, while oxaliplatin degradation was improved by increasing the concentration of the chloride.
The determinations of cisplatin, its hydroxo complexes, and OH-dimers were obtained using HPLC with a naphthylethyl (NAP) group bonded with silica gel column. The mobile phase was constituted by 0.1 M sodium perchlorate, acetonitrile, and perchloric acid (290:10:3) [87]. The measurable range was 1 × 10−5 to 4 × 10−3 M for cisplatin the calibration curves correlation coefficient was 0.999 (p < 0.01) and the time of retentions time was 3.2, 3.4, 3.6, and 4.3–6.6 min for cisplatin, mono-chloride, OH-dimer, and none-chloride respectively. Moreover, the authors state that the determinations of cisplatin could be done by means of a μNAP column instead of an aminopropyl silyl silica gel column or C-18 column.
The results showed that separation was completed in approximately 2 min with column temperature at 30 °C. In cationic separation, the cisplatin elutes first, the opposite behavior was exhibited by the anionic one, and in both cases a second peak was detected and was attributed to a hydrolysis product of the drug. However, better results were obtained with the use of cationic chromatographic column where the detection limits were 0.1 mg L−1 of Pt for both compounds, whereas the repeatability oscillated from 3.1% to 5.9%.
The authors also observed that this method does not require special treatment and has been characterized by low cost and low time analysis, therefore making it a suitable method for the analysis of urine from cancer patients after clinical treatment with cisplatin and carboplatin.
2.3.3. Working Environments
The Pt-CDs (and other CDs) contamination in working environments has been carried out in several studies where biological and/or environmental (surfaces, air) samples were considered.
In 1997 Nygren and Lundgren [88] reported the results of a study in which the platinum in blood and urine and air samples was determined by adsorptive voltammetry. No different levels of airborne Pt were found in oncological wards with respect to empty rooms. Authors reported that staff nurses had the highest Pt levels, possibly due to the closer contacts with the patients, and that improved facilities and procedures could decrease contamination.
Occupational exposure of pharmacy technicians and pharmacists of 14 different hospitals in Germany to several CDs (including Pt-CDs) was also monitored and the samples were analyzed by GC-MS (gas chromatography MS), HPLC, and voltammetry (for Pt LOD: 1 ng L−1 urine) [89]. They found that in some cases Pt was detected in the urine of the screened personnel. This suggested that improved CD manipulation and storage protocols should have been implemented. Additionally, in this work it was concluded that the probability of airborne contamination was very small (as also in ref. [88]).
An interesting study concerned the determination of residual cisplatin present in rinse water used to clean surfaces exposed during manufacturing [90]. In this study the samples were obtained by swabbing the surfaces with a derivatizing solution and then analyzing with HPLC with UV detection. Additionally, in this case, detection was made using DDTC to complex Pt(II) to enhance the sensitivity of UV-Vis measurements.
Hori et al. [91] studied the Pt present in hair and surface wipe samples obtained from hospital workers who had or had not manipulated Pt-CDs, patients treated with Pt-CDs, and non-medical staff. Samples were collected then, and at 5-year distance. Pt content was determined by ICP sector field mass spectrometry (ICP-SFMS) with a LOQ of 0.001411 ng mL−1 and 0.001271 ng mL−1 in the two sets of samples. This interesting study suggested that trace level Pt from exposure to Pt-CDs was associated with the frequency of handling of such compounds and a significant decrease of contamination after the revision of a safety procedures was observed.
A rapid LC-MS/MS method was developed to analyze a group of six CDs including oxaliplatin on stainless steel [92]. The samples were analyzed by HPLC-MS/MS with a LOD of 1.36 ng mL−1. This method could be applied to accurately determine surface contamination at concentrations below the recommended levels [92] and therefore it is suitable for monitoring purposes.
A study on the exposition to CDs at patients’ homes has been carried out by Böhlandt et al. [93]. Even if it does not concern working environment, the sampling techniques are similar. Additionally, it is important as other works [54][56][79] concluded that the release of Pt-CDs in the environment does not occurs primarily from the hospitals. In ref. [93], wipe samples from several homes and urine samples were collected from patients and family members. Samples were analyzed for several CDs including platinum (as marker of Pt-CDs). Significant contamination was found on every surface type with highest concentrations in bathroom surfaces. While patients’ urinary drug concentrations often were elevated for more than 48 h after administration, no drug residues were detectable in the family members’ urine.
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30.
- WHO. IARC Cancer Tomorrow. Available online: https://gco.iarc.fr/tomorrow/en (accessed on 18 October 2021).
- Gouveia, T.I.A.; Alves, A.; Santos, M.S.F. New insights on cytostatic drug risk assessment in aquatic environments based on measured concentrations in surface waters. Environ. Int. 2019, 133, 105236.
- Lancharro, P.M.; De Castro-Acuña Iglesias, N.; González-Barcala, F.J.; González, J.D.M. Evidence of exposure to cytostatic drugs in healthcare staff: A review of recent literature. Farm. Hosp. 2016, 40, 604–621.
- Ioannou-Ttofa, L.; Fatta-Kassinos, D. Cytostatic Drug Residues in Wastewater Treatment Plants: Sources, Removal Efficiencies and Current Challenges. In Fate and Effects of Anticancer Drugs in the Environment; Heath, E., Isidori, M., Kosjek, T., Filipič, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 103–138.
- Zhang, J.; Chang, V.W.C.; Giannis, A.; Wang, J.-Y. Removal of cytostatic drugs from aquatic environment: A review. Sci. Total Environ. 2013, 445–446, 281–298.
- Toolaram, A.P.; Kümmerer, K.; Schneider, M. Environmental risk assessment of anti-cancer drugs and their transformation products: A focus on their genotoxicity characterization-state of knowledge and short comings. Mutat. Res. Mutat. Res. 2014, 760, 18–35.
- Kosjek, T.; Negreira, N.; Heath, E.; López de Alda, M.; Barceló, D. Aerobic activated sludge transformation of vincristine and identification of the transformation products. Sci. Total Environ. 2018, 610–611, 892–904.
- Booker, V.; Halsall, C.; Llewellyn, N.; Johnson, A.; Williams, R. Prioritising anticancer drugs for environmental monitoring and risk assessment purposes. Sci. Total Environ. 2014, 473–474, 159–170.
- Jureczko, M.; Kalka, J. Cytostatic pharmaceuticals as water contaminants. Eur. J. Pharmacol. 2020, 866, 172816.
- Mitra, R.; Goddard, R.; Pörschke, K.R. 9,9-Difluorobispidine Analogues of Cisplatin, Carboplatin, and Oxaliplatin. Inorg. Chem. 2017, 56, 6712–6724.
- Dasari, S.; Tchounwou, B.P. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378.
- Wheate, N.J.; Walker, S.; Craig, G.E.; Oun, R. The status of platinum anticancer drugs in the clinic and in clinical trials. Dalt. Trans. 2010, 39, 8113–8127.
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The Next Generation of Platinum Drugs: Targeted Pt (II) Agents, Nanoparticle Delivery, and Pt (IV) Prodrugs. Chem. Rev. 2016, 116, 3436–3486.
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. Third row transition metals for the treatment of cancer. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2015, 373.
- Franquet-Griell, H.; Gómez-Canela, C.; Ventura, F.; Lacorte, S. Predicting concentrations of cytostatic drugs in sewage effluents and surface waters of Catalonia (NE Spain). Environ. Res. 2015, 138, 161–172.
- Johnson, A.C.; Oldenkamp, R.; Dumont, E.; Sumpter, J.P. Predicting concentrations of the cytostatic drugs cyclophosphamide, carboplatin, 5-fluorouracil, and capecitabine throughout the sewage effluents and surface waters of europe. Environ. Toxicol. Chem. 2013, 32, 1954–1961.
- Valcárcel, Y.; González Alonso, S.; Rodríguez-Gil, J.L.; Gil, A.; Catalá, M. Detection of pharmaceutically active compounds in the rivers and tap water of the Madrid Region (Spain) and potential ecotoxicological risk. Chemosphere 2011, 84, 1336–1348.
- Queirós, V.; Azeiteiro, U.M.; Soares, A.M.V.M.; Freitas, R. The antineoplastic drugs cyclophosphamide and cisplatin in the aquatic environment—Review. J. Hazard. Mater. 2021, 412, 125028.
- Tripathi, A.K.; David, A.; Govil, T.; Rauniyar, S.; Rathinam, N.K.; Goh, K.M.; Sani, R.K. Environmental Remediation of Antineoplastic Drugs: Present Status, Challenges, and Future Directions. Processes 2020, 8, 747.
- Franquet-Griell, H.; Cornadó, D.; Caixach, J.; Ventura, F.; Lacorte, S. Determination of cytostatic drugs in Besòs River (NE Spain) and comparison with predicted environmental concentrations. Environ. Sci. Pollut. Res. 2017, 24, 6492–6503.
- Santos, M.S.F.; Franquet-Griell, H.; Lacorte, S.; Madeira, L.M.; Alves, A. Anticancer drugs in Portuguese surface waters—Estimation of concentrations and identification of potentially priority drugs. Chemosphere 2017, 184, 1250–1260.
- Martín, J.; Camacho-Muñoz, D.; Santos, J.L.; Aparicio, I.; Alonso, E. Occurrence and ecotoxicological risk assessment of 14 cytostatic drugs in wastewater. Water. Air. Soil Pollut. 2014, 225, 1896.
- Franquet-Griell, H.; Pueyo, V.; Silva, J.; Orera, V.M.; Lacorte, S. Development of a macroporous ceramic passive sampler for the monitoring of cytostatic drugs in water. Chemosphere 2017, 182, 681–690.
- Quadra, G.R.; Oliveira de Souza, H.; Costa, R.d.S.; Fernandez, M.A.d.S. Do pharmaceuticals reach and affect the aquatic ecosystems in Brazil? A critical review of current studies in a developing country. Environ. Sci. Pollut. Res. 2017, 24, 1200–1218.
- Rosenberg, B.; Van Camp, L.; Krigas, T. Inhibition of Cell Division in Escherichia Coli by Electrolysis Products from a Platimun Electrode. Nature 1965, 205, 698–699.
- Rosenberg, B.; Van Camp, L.; Trosko, J.E.; Mansout, V.H. Platinum Compounds: A New Class of Potente Antitumour Agents. Nature 1969, 222, 385–386.
- Kelland, L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584.
- Rottenberg, S.; Disler, C.; Perego, P. The rediscovery of platinum-based cancer therapy. Nat. Rev. Cancer 2021, 21, 37–50.
- Berners-Price, S.J.; Appleton, T.G. The Chemistry of Cisplatin in Aqueous Solution. In Platinum-Based Drugs in Cancer Therapy; Kelland, L.R., Farrell, N.P., Eds.; Humana Press: Totowa, NJ, USA, 2000; pp. 3–35.
- Melchior, A.; Sánchez Marcos, E.; Pappalardo, R.R.; Martínez, J.M. Comparative study of the hydrolysis of a third- and a first-generation platinum anticancer complexes. Theor. Chem. Acc. 2011, 128, 627–638.
- Veclani, D.; Tolazzi, M.; Melchior, A. Molecular interpretation of pharmaceuticals’ adsorption on carbon nanomaterials: Theory meets experiments. Processes 2020, 8, 642.
- Wang, D.; Lippard, S.J. Cellular processing of platinum anticancer drugs. Nat. Rev. 2005, 4, 307–320.
- Miller, S.E.; House, D.A. The hydrolysis products of cis-diamminedichloroplatinum(II). I. The kinetics of formation and anation of the cis-diammine(aqua)chloroplatinum(II) cation in acidic aqueous solution. Inorg. Chim. Acta 1989, 161, 131–137.
- Miller, S.E.; House, D.A. The hydrolysis products of cis-dichlorodiammineplatinum(II) 2. The kinetics of formation and anation of the cis-diamminedi(aqua)platinum(II) cation. Inorg. Chim. Acta 1989, 166, 189–197.
- Miller, S.E.; House, D.A. The hydrolysis products of cis-diamminedichloroplatinum(II) 5. The anation kinetics of cis-Pt(X)(NH3)2(OH2)+ (X=Cl, OH) with glycine, monohydrogen malonate and chloride. Inorg. Chim. Acta 1991, 187, 125–132.
- Ahmad, S. Kinetic aspects of platinum anticancer agents. Polyhedron 2017, 138, 109–124.
- Credendino, R.; Minenkov, Y.; Liguori, D.; Piemontesi, F.; Melchior, A.; Morini, G.; Tolazzi, M.; Cavallo, L. Accurate experimental and theoretical enthalpies of association of TiCl4 with typical Lewis bases used in heterogeneous Ziegler–Natta catalysis. Phys. Chem. Chem. Phys. 2017, 19, 26996–27006.
- Veclani, D.; Tolazzi, M.; Cerón-Carrasco, J.P.; Melchior, A. Intercalation Ability of Novel Monofunctional Platinum Anticancer Drugs: A Key Step in Their Biological Action. J. Chem. Inf. Model. 2021, 61, 4391–4399.
- Dell’Anna, M.M.; Censi, V.; Carrozzini, B.; Caliandro, R.; Denora, N.; Franco, M.; Veclani, D.; Melchior, A.; Tolazzi, M.; Mastrorilli, P. Triphenylphosphane Pt(II) complexes containing biologically active natural polyphenols: Synthesis, crystal structure, molecular modeling and cytotoxic studies. J. Inorg. Biochem. 2016, 163, 346–361.
- Hann, S.; Koellensperger, G.; Stefánka, Z.; Stingeder, G.; Fürhacker, M.; Buchberger, W.; Mader, R.M. Application of HPLC-ICP-MS to speciation of cisplatin and its degradation products in water containing different chloride concentrations and in human urine. J. Anal. At. Spectrom. 2003, 18, 1391–1395.
- Boyd, L.; Muggia, F. Carboplatin/Paclitaxel Induction in Ovarian Cancer: The Finer Points. Oncology 2018, 32, 422–424.
- Gridelli, C.; Chen, T.; Ko, A.; O’Brien, M.E.; Ong, T.J.; Socinski, M.A.; Postmus, P.E. Nab-paclitaxel/carboplatin in elderly patients with advanced squamous non-small cell lung cancer: A retrospective analysis of a phase iii trial. Drug Des. Devel. Ther. 2018, 12, 1445–1451.
- Narveson, L.; Kathol, E.; Rockey, M.; Henry, D.; Grauer, D.; Neupane, P. Evaluation of Weekly Paclitaxel, Carboplatin, and Cetuximab in Head and Neck Cancer Patients With Incurable Disease. Int. J. Radiat. Oncol. 2016, 94, 933–934.
- Alexander, C.; Nithyakumar, A.; Paul, M.W.B.; Arockia Samy, N. Platinum(II) complexes of imidazophenanthroline-based polypyridine ligands as potential anticancer agents: Synthesis, characterization, in vitro cytotoxicity studies and a comparative ab initio, and DFT studies with cisplatin, carboplatin, and oxaliplatin. J. Biol. Inorg. Chem. 2018, 23, 833–848.
- Riddell, I.A. Cisplatin and Oxaliplatin: Our Current Understanding of Their Actions. Met. Ions Life Sci. 2018, 18.
- Rogers, B.B.; Cuddahy, T.; Briscella, C.; Ross, N.; Olszanski, A.J.; Denlinger, C.S. Oxaliplatin: Detection and management of hypersensitivity reactions. Clin. J. Oncol. Nurs. 2019, 23, 68–75.
- Lenz, K.; Hann, S.; Koellensperger, G.; Stefanka, Z.; Stingeder, G.; Weissenbacher, N.; Mahnik, S.N.; Fuerhacker, M. Presence of cancerostatic platinum compounds in hospital wastewater and possible elimination by adsorption to activated sludge. Sci. Total Environ. 2005, 345, 141–152.
- Liu, X.; Zhang, J.; Yin, J.; Duan, H.; Wu, Y.; Shao, B. Analysis of hormone antagonists in clinical and municipal wastewater by isotopic dilution liquid chromatography tandem mass spectrometry. Anal. Bioanal. Chem. 2010, 396, 2977–2985.
- Kümmerer, K.; Helmers, E.; Hubner, P.; Mascart, G.; Milandri, M.; Reinthaler, F.; Zwakenberg, M. European hospitals as a source for platinum in the environment in comparison with other sources. Sci. Total Environ. 1999, 225, 155–165.
- Kümmerer, K. Drugs in the environment: Emission of drugs, diagnostic aids and disinfectants into wastewater by hospitals in relation to other sources—A review. Chemosphere 2001, 45, 957–969.
- Johnson, A.C.; Jürgens, M.D.; Williams, R.J.; Kümmerer, K.; Kortenkamp, A.; Sumpter, J.P. Do cytotoxic chemotherapy drugs discharged into rivers pose a risk to the environment and human health? An overview and UK case study. J. Hydrol. 2008, 348, 167–175.
- Lenz, K.; Koellensperger, G.; Hann, S.; Weissenbacher, N.; Mahnik, S.N.; Fuerhacker, M. Fate of cancerostatic platinum compounds in biological wastewater treatment of hospital effluents. Chemosphere 2007, 69, 1765–1774.
- Vyas, N.; Turner, A.; Sewell, G. Platinum-based anticancer drugs in waste waters of a major UK hospital and predicted concentrations in recipient surface waters. Sci. Total Environ. 2014, 493, 324–329.
- Isidori, M.; Lavorgna, M.; Russo, C.; Kundi, M.; Žegura, B.; Novak, M.; Filipič, M.; Mišík, M.; Knasmueller, S.; de Alda, M.L.; et al. Chemical and toxicological characterisation of anticancer drugs in hospital and municipal wastewaters from Slovenia and Spain. Environ. Pollut. 2016, 219, 275–287.
- Besse, J.-P.; Latour, J.-F.; Garric, J. Anticancer drugs in surface waters: What can we say about the occurrence and environmental significance of cytotoxic, cytostatic and endocrine therapy drugs? Environ. Int. 2012, 39, 73–86.
- Ghafuria, Y.; Yunesian, M.; Nabizadeh, R.; Mesdaghinia, A.; Dehghani, M.H.; Alimohammadi, M. Environmental risk assessment of platinum cytotoxic drugs: A focus on toxicity characterization of hospital effluents. Int. J. Environ. Sci. Technol. 2018, 15, 1983–1990.
- Mukherjee, S.; Mehta, D.; Dhangar, K.; Kumar, M. Environmental fate, distribution and state-of-the-art removal of antineoplastic drugs: A comprehensive insight. Chem. Eng. J. 2021, 407, 127184.
- da Silva, R.F.; de Lima Moura, L.; Gavião, L.O.; Brito Alves Lima, G.; Dausacker Bidone, E. Local environmental risk assessment of anticancer drugs in a developing country. Hum. Ecol. Risk Assess. Int. J. 2020, 26, 2142–2161.
- Santana-Viera, S.; Montesdeoca-Esponda, S.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Analytical Methodologies for the Determination of Cytostatic Compounds in Environmental Matrices. In Fate and Effects of Anticancer Drugs in the Environment; Heath, E., Isidori, M., Kosjek, T., Filipič, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 165–195. ISBN 978-3-030-21048-9.
- Kizu, R.; Yamamoto, T.; Yokoyama, T.; Tanaka, M.; Miyazaki, M. A Sensitive Postcolumn Derivatization/UV Detection System for HPLC Determination of Antitumor Divalent and Quadrivalent Platinum Complexes. Chem. Pharm. Bull. 1995, 43, 108–114.
- Burns, R.B.; Embree, L. Validation of high-performance liquid chromatographic assay methods for the analysis of carboplatin in plasma ultrafiltrate. J. Chromatogr. B Biomed. Sci. Appl. 2000, 744, 367–376.
- Koellensperger, G.; Hann, S. Ultra-fast HPLC-ICP-MS analysis of oxaliplatin in patient urine. Anal. Bioanal. Chem. 2010, 397, 401–406.
- Koellensperger, G.; Stefanka, Z.; Meelich, K.; Galanski, M.; Keppler, B.K.; Stingeder, G.; Hann, S. Species specific IDMS for accurate quantification of carboplatin in urine by LC-ESI-TOFMS and LC-ICP-QMS. J. Anal. At. Spectrom. 2008, 23, 29–36.
- Shaik, A.N.; Altomare, D.A.; Lesko, L.J.; Trame, M.N. Development and validation of a LC–MS/MS assay for quantification of cisplatin in rat plasma and urine. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1046, 243–249.
- da Costa, A.C.; Vieira, M.A.; Luna, A.S.; de Campos, R.C. Determination of platinum originated from antitumoral drugs in human urine by atomic absorption spectrometric methods. Talanta 2010, 82, 1647–1653.
- Martinčič, A.; Cemazar, M.; Sersa, G.; Kovač, V.; Milačič, R.; Ščančar, J. A novel method for speciation of Pt in human serum incubated with cisplatin, oxaliplatin and carboplatin by conjoint liquid chromatography on monolithic disks with UV and ICP-MS detection. Talanta 2013, 116, 141–148.
- Zhang, T.; Cai, S.; Forrest, W.C.; Mohr, E.; Yang, Q.; Forrest, M.L. Development and Validation of an Inductively Coupled Plasma Mass Spectrometry (ICP-MS) Method for Quantitative Analysis of Platinum in Plasma, Urine, and Tissues. Appl. Spectrosc. 2016, 70, 1529–1536.
- Zachariadis, G.A.; Misopoulou, O.E. Determination of Cisplatin and Carboplatin Anticancer Drugs by Non-suppressed Ion Chromatography with an Inductively Coupled Plasma Atomic Emission Detector. Anal. Lett. 2018, 51, 1060–1070.
- Lemoine, L.; Thijssen, E.; Noben, J.-P.; Adriaensens, P.; Carleer, R.; Speeten, K. Van der A validated inductively coupled plasma mass spectrometry (ICP-MS) method for the quantification of total platinum content in plasma, plasma ultrafiltrate, urine and peritoneal fluid. J. Pharm. Biomed. Anal. 2018, 152, 39–46.
- Folens, K.; Mortier, S.T.F.C.; Baeten, J.; Couvreur, K.; Michelet, R.; Gernaey, K.V.; De Beer, T.; Du Laing, G.; Nopens, I. Modelling and sensitivity analysis of urinary platinum excretion in anticancer chemotherapy for the recovery of platinum. Sustain. Chem. Pharm. 2016, 4, 46–56.
- Wu, Y.; Lai, R.Y. Tunable Signal-Off and Signal-On Electrochemical Cisplatin Sensor. Anal. Chem. 2017, 89, 9984–9989.
- Petrlova, J.; Potesil, D.; Zehnalek, J.; Sures, B.; Adam, V.; Trnkova, L.; Kizek, R. Cisplatin electrochemical biosensor. Electrochim. Acta 2006, 51, 5169–5173.
- Volder, M.F.L.D.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon Nanotubes: Present and Future Commercial Applications. Science 2013, 339, 535–539.
- Suvarnaphaet, P.; Pechprasarn, S. Graphene-Based Materials for Biosensors: A Review. Sensors 2017, 17, 2161.
- Gholivand, M.B.; Ahmadi, E.; Mavaei, M. A novel voltammetric sensor based on graphene quantum dots-thionine/nano-porous glassy carbon electrode for detection of cisplatin as an anti-cancer drug. Sens. Actuators B Chem. 2019, 299, 126975.
- Materon, E.M.; Wong, A.; Klein, S.I.; Liu, J.; Sotomayor, M.D.P.T. Multi-walled carbon nanotubes modified screen-printed electrodes for cisplatin detection. Electrochim. Acta 2015, 158, 271–276.
- Mitchell, L.; Shen, C.; Timmins, H.C.; Park, S.B.; New, E.J. A Versatile Fluorescent Sensor Array for Platinum Anticancer Drug Detection in Biological Fluids. ACS Sens. 2021, 6, 1261–1269.
- Lenz, K.; Mahnik, S.N.; Weissenbacher, N.; Mader, R.M.; Krenn, P.; Hann, S.; Koellensperger, G.; Uhl, M.; Knasmüller, S.; Ferk, F.; et al. Monitoring, removal and risk assessment of cytostatic drugs in hospital wastewater. Water Sci. Technol. 2007, 56, 141–149.
- Ferrando-Climent, L.; Rodriguez-Mozaz, S.; Barceló, D. Development of a UPLC-MS/MS method for the determination of ten anticancer drugs in hospital and urban wastewaters, and its application for the screening of human metabolites assisted by information-dependent acquisition tool (IDA) in sewage samples. Anal. Bioanal. Chem. 2013, 405, 5937–5952.
- Santana-Viera, S.; Padrón, M.E.T.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Quantification of cytostatic platinum compounds in wastewater by inductively coupled plasma mass spectrometry after ion exchange extraction. Microchem. J. 2020, 157, 104862.
- Jadon, N.; Jain, R.; Sharma, S.; Singh, K. Recent trends in electrochemical sensors for multianalyte detection—A review. Talanta 2016, 161, 894–916.
- Kominkova, M.; Heger, Z.; Zitka, O.; Kynicky, J.; Pohanka, M.; Beklova, M.; Adam, V.; Kizek, R. Flow injection analysis with electrochemical detection for rapid identification of platinum-based cytostatics and platinum chlorides in water. Int. J. Environ. Res. Public Health 2014, 11, 1715–1724.
- Ghafuria, Y.; Yunesian, M.; Nabizadeh, R.; Mesdaghinia, A.; Dehghani, M.H.; Alimohammadi, M. Platinum cytotoxic drugs in the municipal wastewater and drinking water, a validation method and health risk assessment. Hum. Ecol. Risk Assess. 2018, 24, 784–796.
- Alimohammadi, M.; Asadi-Ghalhari, M.; Ghafuri, Y. Development of an analytical method for determination of carboplatin and oxaliplatin in resource water, prediction and environmental risk assessment. J. Environ. Treat. Tech. 2020, 8, 1168–1175.
- Hann, S.; Stefánka, Z.; Lenz, K.; Stingeder, G. Novel separation method for highly sensitive speciation of cancerostatic platinum compounds by HPLC-ICP-MS. Anal. Bioanal. Chem. 2005, 381, 405–412.
- Kato, R.; Sato, T.; Kanamori, M.; Miyake, M.; Fujimoto, A.; Ogawa, K.; Kobata, D.; Fujikawa, T.; Wada, Y.; Mitsuishi, R.; et al. A novel analytical method of cisplatin using the HPLC with a naphthylethyl group bonded with silica gel (πNAP) column. Biol. Pharm. Bull. 2017, 40, 290–296.
- Nygren, O.; Lundgren, C. Determination of platinum in workroom air and in blood and urine from nursing staff attending patients receiving cisplatin chemotherapy. Int. Arch. Occup. Environ. Health 1997, 70, 209–214.
- Schreiber, C.; Radon, K.; Pethran, A.; Schierl, R.; Hauff, K.; Grimm, C.-H.; Boos, K.-S.; Nowak, D. Uptake of antineoplastic agents in pharmacy personnel. Part II: Study of work-related risk factors. Int. Arch. Occup. Environ. Health 2003, 76, 11–16.
- Raghavan, R.; Burchett, M.; Loffredo, D.; Mulligan, J.A. Low-Level (PPB)Determination of Cisplatin in Cleaning Validation (Rinse Water) Samples. II. A High-Performance Liquid Chromatogrphic Method. Drug Dev. Ind. Pharm. 2000, 26, 429–440.
- Hori, A.; Shimura, M.; Iida, Y.; Yamada, K.; Nohara, K.; Ichinose, T.; Yamashita, A.; Shirataki, J.; Hagiwara, S. Occupational exposure of platinum-based anti-cancer drugs: Five-year monitoring of hair and environmental samples in a single hospital. J. Occup. Med. Toxicol. 2020, 15, 29.
- Jeronimo, M.; Colombo, M.; Astrakianakis, G.; Hon, C.-Y. A surface wipe sampling and LC–MS/MS method for the simultaneous detection of six antineoplastic drugs commonly handled by healthcare workers. Anal. Bioanal. Chem. 2015, 407, 7083–7092.
- Böhlandt, A.; Sverdel, Y.; Schierl, R. Antineoplastic drug residues inside homes of chemotherapy patients. Int. J. Hyg. Environ. Health 2017, 220, 757–765.
More
Information
Subjects:
Chemistry, Inorganic & Nuclear
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
19 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




