Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tabassum Khan | + 3792 word(s) | 3792 | 2022-01-17 06:39:22 | | | |
| 2 | Rita Xu | Meta information modification | 3792 | 2022-01-27 07:41:41 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Khan, T. Antimicrobial Essential Oils. Encyclopedia. Available online: https://encyclopedia.pub/entry/18833 (accessed on 07 February 2026).
Khan T. Antimicrobial Essential Oils. Encyclopedia. Available at: https://encyclopedia.pub/entry/18833. Accessed February 07, 2026.
Khan, Tabassum. "Antimicrobial Essential Oils" Encyclopedia, https://encyclopedia.pub/entry/18833 (accessed February 07, 2026).
Khan, T. (2022, January 26). Antimicrobial Essential Oils. In Encyclopedia. https://encyclopedia.pub/entry/18833
Khan, Tabassum. "Antimicrobial Essential Oils." Encyclopedia. Web. 26 January, 2022.
Copy Citation
Microbial pathogens are the most prevalent cause of chronic infections and fatalities around the world. Antimicrobial agents including antibiotics have been frequently utilized in the treatment of infections due to their exceptional outcomes. However, their widespread use has resulted in the emergence of multidrug-resistant strains of bacteria, fungi, viruses, and parasites.
essential oils
nanoparticles
antimicrobials
1. Introduction
Microorganism-caused infections are a source of concern for public health. Overuse or underuse of antimicrobials has resulted in the global rise of multidrug-resistance in microorganisms, including bacteria, fungi, viruses, parasites, and protozoans. Every year, more than two million people suffer from infections with antimicrobial resistance and by the year 2050, the annual global mortality rate of these infections is expected to reach 10 million [1]. Antimicrobial resistance develops and continues to transmit across different species of bacteria due to various factors such as conjugation, transformation, and transduction processes of the gene transfer cycle. Therefore, new and unique alternative antimicrobials are needed to combat multidrug resistance [2][3][4].
Essential oils are aromatic liquids produced through a series of complex metabolic pathways in plants with the goal of defending them from a wide range of pathogens and are commonly extracted by steam distillation [4][5]. Different factors influencing the chemical compositions of EOs include the species, climatic conditions, soil condition, fertilization, genotype, mode of production, harvest seasons, and extraction procedure. Two major groups of chemical compounds present in EOs are (i) aromatic and aliphatic compounds, and (ii) hydrocarbon terpenes (isoprenes) and terpenoids (isoprenoids). Terpenes are five-carbon isoprene units (C5H8) that constitute the largest class of chemical compounds present in essential oils. Terpenes are categorized as mono-, sesqui-, di-, ses-, tri-, and tetra-terpenes or alternate hemi-terpenes based on the number of carbon atoms present in the structure. However, the monoterpenes and sesquiterpenes are the most important constituents of essential oils responsible for their characteristic aroma of EOs. Monoterpenes are composed of two isoprene units and exist in monocyclic, bicyclic, and acyclic forms, whereas sesquiterpenes are made up of three isoprene units and occur in acyclic, monocyclic, bicyclic, and tricyclic forms. Chemical modification of a terpene or sesquiterpene, through oxidation or structural rearrangement, produces different terpenoids. Thus, EOs with diverse chemical compositions exhibit a wide range of therapeutic effects [6][7][8].
1.1. Mechanism of Action and Bacterial Spectrum
Essential oils have been widely explored on a large scale as potential sources of novel antimicrobial agents, food preservatives, and alternative treatments for infectious diseases due to their antifungal, antiparasitic, antibacterial, and antiviral properties (Table 1) [4]. The antimicrobial mechanism of action varies with the type of EO or the strain of the microorganism used. Gram-negative bacteria have a thick lipopolysaccharide layer which reduces the susceptibility of microorganisms towards EOs but gram-positive bacteria will lack this lipopolysaccharide. Hence, EOs can enter gram-positive bacteria easily as compared to gram-negative bacteria. Due to the presence of lipoteichoic acid, the entry of EOs into gram-positive microbial cells is eased. Various research investigations have demonstrated that the bioactive components contained in EOs attach to the cell surface and penetrate the phospholipid bilayer of the cell membrane, followed by membrane damage, which causes negative impacts on cell metabolic activities and cell death. Alteration of the cell membrane integrity results in the loss of important intracellular components such as proteins, reducing sugars, ATP, and DNA, and also blocks ATP synthesis and associated enzymes, resulting in electrolyte leakage and cell death. At the minimum inhibitory concentration (MIC), it was found that the EOs damaged the bacterial cell membrane. However, at the minimum bactericidal concentration (MBC), the EOs destroyed the bacterial cells [3][4].
Table 1. Major chemical components of essential oils and their antimicrobial activities.
| Biological Source of Essential Oils | Part | Antimicrobial Activities | Major Chemical Components | Mechanism of Action | References |
|---|---|---|---|---|---|
| Bunium persicum | Seeds | L. monocytogenes, Listeria grayi andAspergillusflavus | γ-Terpinene, 1-phellandrene, γ-terpene, cuminaldehyde |
Cell membrane disruption and cytolytic leakage Swelling and reduction in membrane function |
[9][10][11][12] |
| Cananga odorata | Flower | Hepatitis B virus (HBV), Bacillus. subtilis, E. coli, S. typhi, Shigella shiga, Streptococcus-β-haemolyticus and A. flavus | Linalool, β-caryophyllene |
Disruption of cell membrane integrity Induces apoptosis via nuclear condensation and fragmentation pathways including disruption of mitochondrial membrane potential |
[13][14][15][16] |
| Carum copticum | Seeds | S. aureus, Staphylococcus epidermidis, Bacillus cereus, E. coli, S. typhimurium, Proteus vulgaris | Thymol, γ-Terpinene, ρ-cymene |
Depolarization of the cytoplasmic membrane and disruption of cell membrane integrity and decrease intracellular ATP levels | [17][18][19] |
| Cinnamomum zeylanicum | Bark | Borrelia burgdorferi, E. coli., S. aureus, and P. aeruginosa | Carvacrol | Depolarization of the cytoplasmic membrane and disruption of cell membrane integrity | [17][20][21] |
| Citrus bergamia | Peel | Campylobacter jejuni, E. coli, L. monocytogenes, B. cereus, and S. aureus | Linalool, Citral, Linalyl acetate |
Disruption of cell membrane integrity Induction of changes in ATP concentration, cell membrane hyperpolarization, and reduction in cytoplasmic pH |
[22][23] |
| Citrus reticulata | Peel | S. aureus, E. coli, Penicillium italicum and Penicillium. digitatum | Limonene and γ-Terpinene | Cell membrane disruption and cytolytic leakage | [24] |
| Cymbopogon citratus | Leaves | HSV-1, HSV-2, S. aureus, E. coli and Gaeumannomyces graminis | Citral | Induction of changes in ATP concentration, cell membrane hyperpolarization, and reduction in cytoplasmic pH | [25][26] |
| Eugenia caryophyllata | Flower buds | B. cereus, S. typhimurium and E. coli | Eugenol, β-caryophyllene |
Cell membrane disruption and cytolytic leakage Induces apoptosis via nuclear condensation and fragmentation pathways including disruption of mitochondrial membrane potential |
[13][12][27] |
| Eucalyptus globulus | Leaves | S. aureus and S. pyogenes | 1,8-cineol α-pinene |
Disruption of cell membrane integrity and cytolytic leakage | [28] |
| Foeniculum vulgare | Seeds and Leaves | S. aureus, E. coli, and A. flavus | Anethole | Disruption of cell membrane integrity | [29][30] |
| Homalomena pineodora | Leaves | B. cereus, B. subtilis, S. aureus, MRSA, E. coli, Proteus mirabilis, Yersinia sp., K. pneumoniae, Shigella boydii, S. typhimurium, Acinetobacter anitratus, P. aeruginosa, Candida albicans and Candida utilis | 2-octylcyclopentanone | Cell membrane disruption and cytolytic leakage | [31] |
| Lavandula angustifolia Sevastopolis | Whole plant | MRSA, S. aureus and E. coli | Linalool, Borneol, Camphor |
Disruption of cell membrane integrity and cytolytic leakage | [32][33] |
| Lippia sidoides | Leaves | Stegomyia aegypti larvae | Thymol | Depolarization of the cytoplasmic membrane and disruption of cell membrane integrity | [17][34] |
| Matricaria chamomilla | Fresh or dried flower heads | Leishmania amazonensis,E. coli, P. aeruginosa, B. subtilis, S. aureus, S. pyogenes, Schizosaccharomyces pombe, C. albicans and Candida tropicalis | α-Bisabolol | Cell membrane disruption and cytolytic leakage | [35] |
| Melaleuca alternifolia | Leaves | S. aureus, E. coli, L. monocytogenes, C. albicans, P. aeruginosa and A. niger | Terpinen-4-ol | Cell membrane disruption and cytolytic leakage | [36][37] |
| Mentha piperita | Leaves | C. albicans, C. tropicalis, Pichia anomala andSaccharomycescerevisiae | Menthol, Menthone | Depolarization of the cytoplasmic membrane and disruption of cell membrane integrity | [38] |
| Nigella sativa | Seeds | S. aureus and Vibrio harveyii | Thymoquinone | Apoptosis by production of reactive oxygen species | [39][40] |
| Ocimum basilicum | Whole plant | C. albicans, S. aureus | Linalool | Disruption of cell membrane integrity and cytolytic leakage | [16][23] |
| Origanum vulgare | Leaves | Trichophyton tonsurans, Trichophyton violaceum, Trichophyton floccosum, T mentagrophytes | Carvacrol, Thymol | Depolarization of the cytoplasmic membrane and disruption of cell membrane integrity | [17][41] |
| Pistacia atlantica | Gum | S. aureus, S. enterica, E. coli and L. monocytogenes | α-Thujene, α-Pinene, Camphorene, Sabinene, β-Pinene, ∆3-Carene, Limonene |
Disruption of cell membrane integrity and cytolytic leakage | [42][43] |
| Pistacia lentiscus | Resin | E. coli and B. subtilis | α-Pinene, β-Pinene, β-myrcene, Linalool, trans-Caryophyllene and Camphene |
Disruption of cell membrane integrity and cytolytic leakage | [43][44] |
| Psidium guajava | Leaves | S. aureus, Salmonella spp. and E. coli | β- caryophyllene | Induction of apoptosis via nuclear condensation and fragmentation pathways including disruption of mitochondrial membrane | [13][45] |
| Punica granatum | Seeds | S. epidermidis | Punicalagin, punicalin | Cell membrane disruption and cytolytic leakage | [46][47][48] |
| Rosmarinus officinalis | Leaves | C. albicans, C. tropicalis | 1,8-Cineole, camphor | Disruption of cell membrane integrity and cytolytic leakage | [49][50] |
| Satureja hortensis | Leaves | S. aureus, Corynebacterium glutamicum, P. aeruginosa and E. coli, and C. albicans | Carvacrol, Thymol | Depolarization of the cytoplasmic membrane and disruption of cell membrane integrity | [17][51] |
| Syzygium aromaticum | Floral bud | E. coli, S. aureus, S. typhi, P. aeruginosa, B. cereus, L. monocytogenes | Eugenol, eugenyl acetate | Cell membrane disruption and cytolytic leakage | [12][52] |
| Thymus vulgaris | Leaves | M. furfur, C. albican, C. tropicalis, Candida glabrata, Candida kefyr and Candida guillermondii, S. aureus, S. pyogenes and E. coli | Thymol, p-cymene, Carvacrol |
Depolarization of the cytoplasmic membrane and disruption of cell membrane integrity | [17][53][54] |
| Zataria multiflora | Aerial parts | S. aureus, MRSA, S. epidermidis and P. aeruginosa | Carvacrol, Thymol, p-cymene |
Depolarization of the cytoplasmic membrane and disruption of cell membrane integrity | [17][55] |
The primary methods of action of antimicrobial drugs are categorised. Interference with cell wall biosynthesis (β-lactams and glycopeptides agents), inhibition of bacterial protein synthesis (macrolides and tetracyclines), interference with nucleic acid synthesis (fluroquinolones and rifampin), inhibition of a metabolic pathway (trimethoprim-sulfamethoxazole), and disruption of bacterial membrane structure (polymyxins and daptomycin) are all examples of these mechanisms [56]. The biosynthesis of cell walls, proteins, and nucleic acids are three of the principal targets for antibiotics. Bacteria have developed diverse resistances to antibiotics over the years in order to survive the flood of antibiotics. The processes differ, making the task of preventing resistance spread more difficult. As the threat of medication resistance grows, researchers are turning their attention to natural materials with antimicrobial capabilities, such as plants, as a potential supply of antimicrobial medicines in the future. Various mechanisms of the antimicrobial action of essential oils is depicted in Figure 1.
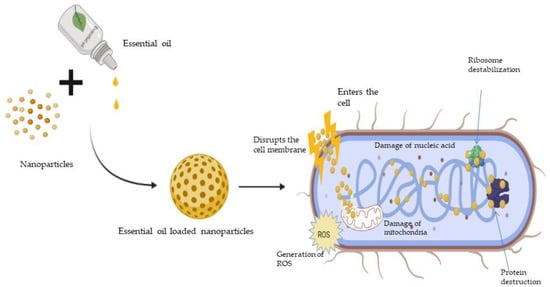
Figure 1. Mechanism of action of antimicrobial activity of essential oils.
Bunium persicum and Homalomena pineodora oil and its constituents such as γ-Terpinene, 1-phellandrene, γ-terpene, and cuminaldehyde exert antimicrobial action by cell membrane disruption, cytolytic leakage and swelling, and the reduction in membrane function [9]. Cananga odorata, Citrus bergamia, Cymbopogon citratus, Citrus reticulata, Lavandula angustifolia Sevastopolis, Rosmarinus officinalis, and Ocimum basilicum essential oils contain linalool, citral, borneol, camphor, and linalyl acetate that exhibit antimicrobial activity through the disruption of cell membrane integrity and induce changes in ATP concentration and cell membrane hyperpolarization, as well as reducing cytoplasmic pH [32]. Carum copticum, Cinnamomum zeylanicum, Lippia sidoides, Mentha piperita, Origanum vulgare, Thymus vulgaris, and Zataria multiflora essential oils are reported to consist of thymol, carvacrol, p-cymene, menthol, and menthone, and display antimicrobial effects through depolarization of the cytoplasmic membrane and disruption of the cell membrane integrity as well as the decreasing of intracellular ATP levels [17]. Eugenia caryophyllata, Eucalyptus globulus, Pistacia atlantica, Pistacia lentiscus, and Punica granatum essential oil and their constituents such as β-caryophyllene, Eugenol, α-Pinene, β-Pinene, β-myrcene, and 1,8-cineole exhibit antimicrobial activity by apoptosis via nuclear condensation and fragmentation pathways, including the disruption of mitochondrial membrane potential [13].
Components of essential oils (mostly with phenolic structures) were able to display a broad spectrum of antibacterial activity, indicating that the chemical structures of the components have a significant impact on their effectiveness and manner of antibacterial action [56]. To understand the efficiency of EOs in comparison to antibiotics Gavanji et al. evaluated the antibacterial activity of Artemisia kermanensis, Lavandula officinalis, and Zataria multiflora Boiss essential oils against Staphylococcus aureus, Pseudomonas aeruginosa, and Klebsiella pneumoniae with ampicillin, penicillin, and tetracycline as positive control antibiotics. Different concentrations of essential oils (0.08–100 µg/disk) were used and the results showed that the concentration of 100 µg/disk of each of the three essential oils was more efficient compared with lower concentrations on the bacteria. A comparison between the three plant essential oils (at a concentration of 100 µg/disk) and positive control antibiotics (ampicillin, penicillin, and tetracycline of 10 µg, 10 µg and 30 µg) demonstrated that Z. multiflora Boiss essential oil (at 24 h, 48 h and 72 h) exhibited a stronger antibacterial effect (bigger inhibition zone) against S. aureus (27.80 ± 0.20, 28.67 ± 0.33, 28.67 ± 0.33), K. pneumonia (27.83 ± 0.12, 28.10 ± 0.21, 28.10 ± 0.21) and P. aeruginosa (19.90 ± 0.27, 20.40 ± 0.23, 20.53 ± 0.18) respectively. Since bacteria are becoming increasingly resistant to antibiotics, employing these essential oils as natural and alternative antibacterial compounds may be beneficial. Some other examples of essential oils or plant extracts commonly used for their antimicrobial properties are tea tree oil, ylang-ylang, betel pepper, manuka, eucalyptus, arnica, lemon verbena, rosemary, green tea extract, and calendula. Although extensively practiced since ancient times, the use of natural extracts from plants as antimicrobial compounds declined after the development of synthetic antibiotics [57]. Duarte et al. demonstrated that EOs with MIC values of up to 0.5 mg/mL have strong antibacterial action, EOs with MIC values between 0.6 and 1.5 mg/mL have moderate antimicrobial activity, and EOs with MIC values over 1.6 mg/mL have weak antimicrobial activity [58]. Essential oils showed activity against Helicobacter pylori in the MIC range of 20–589 µg/mL and demonstrated activity against bacteria most frequently isolated from the respiratory tract including Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, and Streptococcus pyogenes at the MIC range of 1.56–6.25 µg/mL. Monoterpene alcohols and aldehydes, as well as phenols and cinnamaldehyde, were the most active ingredients, with MIC-values of 160–300 µg/mL against both S. pneumoniae and H. influenzae. In vitro cytotoxicity studies of various EOs such as lavender oil, lemon oil, clove oil, thyme oil, and mentha oil on different cell lines such as HMEC-1 (microvascular endothelial cells), HNDF (dermal fibroblasts), 153BR (fibroblasts), and RC-37 demonstrated an effective concentration (cytotoxic to 50% of the tested cells) range of 5–1950 µg/mL [59].
The antimicrobial potential of EOs is determined by the spectrum of microbial targets it affects. Essential oils obtained from clove, cinnamon, oregano, pimento, rosemary, and thyme demonstrated strong antibacterial activity against Staphylococcus aureus, Salmonella typhi, and Pseudomonas aeruginosa.
-
Clove essential oil demonstrated in vitro inhibitory and bactericidal activity at a concentration of 0.304 mg/mL against S. aureus, Escherichia coli, Listeria monocytogenes, and Salmonella typhimurium [60]. The antiviral activity of eugenol, the primary component of clove essential oil, was investigated in vitro against the Herpes simplex virus (HSV)-1 and HSV-2 viruses. The replication of these viruses was inhibited with IC50 values of 25.6 µg/mL and 16.2 µg/mL against HSV-1 and HSV-2, respectively [59]. The MIC value of clove oil against L. monocytogenes was found to be 0.5 mg/mL [61].
-
Lavender EO obtained from L. angustifolia Mill. has a strong antiseptic effect against antibiotic-resistant strains, e.g., Staphylococcus aureus, that are resistant to methicillin (MRSA) or vancomycin-resistant strains of Enterococcus sp. (VRE). The antimicrobial activity of Lavender EO was evaluated against L. monocytogenes (24 strains) and Salmonella enterica (10 food strains). MIC ≥ 10.0 μL/mL inhibited Salmonella; MIC of 0.3 μL/mL inhibited L. monocytogenes, revealing noticeable activity, especially on clinical strains. This activity appears to be related to EOs composition. The highest antimicrobial activities were demonstrated in the specific constituents such as linalool (38.17 and 61.98%), camphor (8.97 and 10.30%), and 1,8-cineole (6.89 and 8.11%, respectively) [62].
-
Thyme EO was found to have antiviral action against Herpes simplex virus (HSV1, DNA virus) with IC50 values of 11 µg/mL [62]. Thyme EO was also tested for its ability to fight strains that cause acute bacterial pharyngitis and throat irritation. β-haemolytic Streptococci strains, such as S. pyogenes, cause this infection. T. vulgaris EO was found to be effective against S. pyogenes strains obtained from throat of patients [63]. At a concentration of 0.06%, thyme EO that was rich in γ-terpinene (68.415%) and p-thymol (24.721%) totally inhibited the growth of Fusarium graminearum Fg 06–17 [64].
-
Essential Oil of Cinnamomum zeylanicum demonstrated 100% inhibition effect at 3.1 µL/mL concentration against influenza virus A1/Denver/1/57 (H1N1) with 30 min exposure. In both liquid and vapour phases, Eugenol, the main component of Cinnamomum zeylanicum EO, exhibited the most significant anti-influenza activity [65]. Cinnamon essential oil was recently used to improve zein film for food packaging, which now contains an extra 4% concentration of chitosan nanoparticles (CNP). The combined antibacterial capabilities of EO and nanoparticles not only inhibited the development of Escherichia coli (PTCC 1163) and Staphylococcus aureus (PTTC 25923), but also increased the tensile strength and decreased the elongation of the composite zein film [66].
-
Tea tree EO has been used in products for oral hygiene and dermatological uses due to its antibacterial characteristics. Porphyromonas gingivalis (MIC and MBC = 0.007%) and Porphyromonas endodontalis (MIC = 0.007% and MBC = 0.5%) bacteria that cause halitosis are both inhibited by tea tree EO [67]. The antibacterial activities of tea tree essential oils (EOs) that are commercially accessible were examined. Five out of the ten EOs were active. Components identified in tea tree essential oil inhibited bacterium viability in Pseudomonas aeruginosa biofilm and caused oxidative damage in Candida glabrata [68]. Essential oil of Melaleuca alternifolia, on the other hand, displayed only minimal antifungal activity against Aspergillus niger (MIC = 625 µg/mL), which was attributed to the active components terpinen-4-ol and α-terpineol [69].
1.2. Stability and Bioavailability of Essential Oils
Essential Oils are susceptible to degradation due to external factors such as light, temperature, oxidation, or hydrolysis. The final composition of EOs depends on chemical composition of EOs, plant material processing and storage, distillation methods, and subsequent storage of EOs. The chemical constituents of EOs have a significant impact on its stability. Double bonds present in the constituents undergo autoxidation as hydrogen atom abstraction leads to resonance-stabilized radicals. Conjugated double-bonds can stabilize radicals formed by polyunsaturated terpene hydrocarbons. Simultaneously, isomerization to tertiary radicals might occur, resulting in oxidative degradation. The presence of aerial oxygen triggers spontaneous free radical chain reactions, which result in the formation of unstable hydroperoxides that breakdown in the presence of light, heat, or rising acidity. Stable secondary oxidation products include monovalent to polyvalent alcohols, aldehydes, ketones, epoxides, peroxides, acids, or oxygen-bearing polymers. Some terpenoids transform into oxidized secondary products without the creation of hydroperoxides. Since headspace oxygen diffuses into the sample over time, the EOs should be maintained in completely filled containers or, if possible, treated with inert gas to remove any leftover air and prevent oxidative reactions. Light and temperature are the other two elements that are strongly linked to EO oxidative degradation. Light enhances autoxidation and the generation of alkyl radicals in monoterpenes, catalyzes intramolecular isomerization events or trans–cis conversions, and boosts monoterpene degradation. Heat speeds up chemical reactions and aids in the development of primary auto-oxidation products, such as hydroperoxides, which are then degraded when the temperature rises, yielding final oxidation products. At high temperatures, volatiles are thermolabile and vulnerable to rearrangement processes. Cleavage of double bonds, epoxidation, dehydrogenation into aromatic systems, and allylic oxidation into alcohols, ketones, and aldehydes are the four types of oxidative processes that occur during the thermal breakdown of terpenes. The production of alkyl or hydroxyl radicals is more apparent at higher temperatures because oxygen solubility is lower whereas storing EOs at low temperatures promotes oxygen solubility in liquids, resulting in the formation of peroxide. Compounds, primarily isoprenoids, easily oxidize in complex mixtures such as EOs, causing rearrangement and breakdown processes in more stable structures. In exchange, phenylpropanoids found in essential oils work as antioxidants, scavenging free radicals and protecting other molecules from degradation. Essential Oils are losing their quality as a result of the decomposition mechanisms detailed above. Changes in colour, consistency, and odour are the most visible indications of age, with the latter being particularly unpleasant and smelly. The biological activity of EOs is significantly influenced by their general physicochemical characteristics (complexity and interactions of individual compounds) and constituents (low molecular weight, presence of diverse functional groups in the molecule, reactivity, and hydrophobicity) [70].
The bioavailability and bioaccessibility of plant metabolites, including EOs and their individual terpene compounds, are analyzed using various in vivo and in vitro methods. Bioaccessibility is often estimated using in vitro digestive models. The majority of these approaches work by altering the pH and introducing certain digestive enzymes to simulate the conditions of gastrointestinal (GI) system. In vivo animal and clinical research have also been used to investigate bioavailability. Physiochemical, biochemical, and physiological interactions all have an impact on the bioavailability of EOs. It is considered that intravenous administration of EOs has the highest bioavailability (100%) and that other administration routes have lower bioavailability. However, as observed for 1,8-cineole, which has a bioavailability rate of 95.6%, the bioavailability of EO components administered orally may be very high. Nonetheless, recent studies show that most EOs are readily absorbed when applied topically, orally, or through the lungs. Most EO compounds are known to penetrate from the surface of skin, through the stratum corneum, into the dermis, and finally into the bloodstream. The high percutaneous absorption rates of EOs should be included in systemic toxicity risk assessments due to their lipophilic properties. The beneficial effects of essential oils on the respiratory system when inhaled are well-known. For large systemic concentrations required for bioactivity in the colon, rectal suppositories are employed. However, due of the great sensitivity of the rectal mucosal membrane to EOs and the potential for irritation, dosages and concentrations should be carefully controlled [71].
2. Essential Oils in Combination with Antibiotics
Many approaches are investigated to resolve the antimicrobial resistance crisis. The creation of antibiotic alternatives, as well as the discovery or development of adjuvants, are among the potential options explored. The current state of knowledge on the modes of action of EO elements and their synergy with antibiotics is provided, as well as proposed pathways by which they interact (Table 2). To improve antibiotic efficacy, researchers must identify ways to improve drug diffusion through bacterial membranes and/or to inhibit efflux pumps, which are a common resistance mechanism in gram-negative bacteria. A proposed specific target for EO components is the inhibition of efflux pumps, responsible for antibiotic resistance. Hence, EOs can be used in combination with antibiotics. The checkerboard assay with Fractional Inhibitory Concentration Index (FICI) computation is the most commonly reported assay method [72].
Table 2. Antibiotics in combination with Essential oils and their interactions.
| Antibiotics | Essential Oils/Essential Oil Constituents | * FICI | Organisms | Interaction | Reference |
|---|---|---|---|---|---|
| Amoxicillin, Ciprofloxacin | Ajowan oil Thymol |
0.36–1 | P. aeruginosa, S. aureus and S. pneumoniae | Synergism—EO/thymol with amoxicillin against MRSA; EO with ciprofloxacin against P. aeruginosa, S. aureus and S. pneumoniae; Thymol with ciprofloxacin against P. aeruginosa and S. pneumoniae | [73] |
| Cefepime | Rosemary oil | - | P. aeruginosa | Synergism | [74] |
| Ciprofloxacin Fluconazole | Thymus atlanticus | 0.25–0.50 | Bacillus subtilis, Micrococcus luteus, Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, K. pneumoniae and Candida parapsilosis, Candida albicans, Candida glabrata, Candida krusei | Synergism | [75] |
| Ciprofloxacin Fluconazole | Linaria ventricosa | 0.26 to 0.50 | E. coli, C. albicans and C. glabrata | Synergism | [76] |
| Doxycycline | Carvacrol, eugenol and cinnamaldehyde | 0.7–1.3 | Acinetobacter baumannii K. pneumoniae E. coli P. aeruginosa |
Additive or indifferent inhibitory activity Synergistic bactericidal activity |
[77] |
| Fluconazole Amphotericin B |
T. satureioides T. pallidus A. leucotrichus T. leptobotrys O. compactum A. herba alba |
0.25–0.31 | C. albicans C. glabrata C. krusei C. parapsilosis |
Synergism | [78] |
| Fluconazole Amphotericin B | Citrus aurantium | 0.36 and 0.24 | Candida albicans | Synergism | [79] |
| Fluconazole, Ciprofloxacin Vancomycin |
Laurus nobilis Prunus armeniaca |
0.258–0.75 | M. luteus,S. aureus, B. subtilis, E. coli, P. aeruginosa, K. pneumoniae andC. parapsilosis,Candida albicans, Candida glabrata, Candida krusei | Synergism | [80] |
| Fluconazole, Econazole, Ketoconazole Itraconazole | Melaleuca leucadendra | 0.35–0.46 | C. albicans | Synergism | [81] |
| Octenidine dihydrochloride | Lavender | 0.11–0.26 | MRSA | Synergism | [82] |
| Oxacillin, Amoxicillin, Gentamicin, Ciprofloxacin, Tetracycline, Erythromycin, Clindamycin | coriander oil | 0.25–1 | MRSA S. epidermidis P. aeruginosa E. coli |
Synergism—coriander oil with amoxicillin, gentamicin, oxacillin and tetracycline against MRSA; coriander oil with gentamicin against P. aeruginosa; coriander oil with erythromycin and tetracycline against E. coli Additive—coriander oil with amoxicillin and clindamycin against MRSA; coriander oil with gentamicin and ciprofloxacin against E. coli |
[83] |
| Polymyxin B | Cinnamomum cassia | 0.006 | carbapenemase-producing Klebsiella pneumoniae and Serratia marcescens | Synergism | [84] |
| Sarafloxacin, Levofloxacin, Polymycin, Lincomycin, Amoxicillin, Ceftiofur, Ceftriaxone, Maquindox, Florfenicol, Doxycycline, Kanamycin |
Oregano | 0.375–1.5 | E. coli | Synergism—oregano oil with Sarafloxacin, Levofloxacin, Maquindox, Florfenicol, Doxycycline Additive—oregano oil with Polymycin, Lincomycin, Amoxicillin, Ceftiofur, Ceftriaxone Independent—oregano oil with Kanamycin |
[85] |
| Streptomycin Ampicillin Chloramphenicol |
Cinnamomum cassia | 0.38–0.125 | E. coli, S. aureus, and P. aeruginosa | Synergism—EO with chloramphenicol against E. coli and S. aureus Additive—EO with Streptomycin and Ampicillin against E. coli, S. aureus and P. aeruginosa |
[86] |
| β-lactam antibiotics (methicillin, penicillin G) | 1,8-cineole, eugenol, carvacrol, linalool, linalyl acetate, trans-anethole, thymol, menthone, menthol, β-caryophyllene | 0.2–5.0 | MSRA | Synergism—linalyl acetate with methicillin and 1,8-cineole with penicillin G Additive—linalyl acetate with penicillin G Antagonism—methicillin with thymol and methicillin with menthone |
[87] |
* Fractional Inhibitory Concentration Index.
References
- de Kraker, M.E.A.; Stewardson, A.J.; Harbarth, S. Will 10 million people die a year due to antimicrobial resistance by 2050? PLoS Med. 2016, 13, e1002184.
- Miguel, M.G.; Lourenço, J.P.; Faleiro, M.L. Superparamagnetic iron oxide nanoparticles and essential oils: A new tool for biological applications. Int. J. Mol. Sci. 2020, 21, 6633.
- Rai, M.; Paralikar, P.; Jogee, P.; Agarkar, G.; Ingle, A.P.; Derita, M.; Zacchino, S. Synergistic antimicrobial potential of essential oils in combination with nanoparticles: Emerging trends and future perspectives. Int. J. Pharm. 2017, 519, 67–78.
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial activity of some essential oils—Present status and future perspectives. Medicines 2017, 4, 58.
- de Groot, A.C.; Schmidt, E. Essential oils, part I: Introduction. Dermatitis 2016, 27, 39–42.
- De Groot, A.C.; Schmidt, E. Essential oils, part III: Chemical composition. Dermatitis 2016, 27, 161–169.
- Eslahi, H.; Fahimi, N.; Sardarian, A.R. Chemical composition of essential oils. Essent. Oils Food Process. Chem. Saf. Appl. 2017, 119–171.
- Ramsey, J.T.; Shropshire, B.C.; Nagy, T.R.; Chambers, K.D.; Li, Y.; Korach, K.S. Focus: Plant-based medicine and pharmacology: Essential oils and health. Yale J. Biol. Med. 2020, 93, 291.
- Wongkattiya, N.; Sanguansermsri, P.; Fraser, I.H.; Sanguansermsri, D. Antibacterial activity of cuminaldehyde on food-borne pathogens, the bioactive component of essential oil from Cuminum cyminum L. collected in Thailand. J. Complement. Integr. Med. 2019, 16.
- Singh, A.; Deepika; Chaudhari, A.K.; Das, S.; Singh, V.K.; Dwivedy, A.K.; Shivalingam, R.K.; Dubey, N.K. Assessment of preservative potential of Bunium persicum (Boiss) essential oil against fungal and aflatoxin contamination of stored masticatories and improvement in efficacy through encapsulation into chitosan nanomatrix. Environ. Sci. Pollut. Res. 2020, 27, 27635–27650.
- Chahota, R.K.; Sharma, V.; Ghani, M.; Sharma, T.R.; Rana, J.C.; Sharma, S.K. Genetic and phytochemical diversity analysis in Bunium persicum populations of north-western Himalaya. Physiol. Mol. Biol. Plants 2017, 23, 429–441.
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules 2019, 24, 2471.
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.K.; Ezzat, M.O.; Majid, A.S.A.; Majid, A.M.S.A. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-caryophyllene from the essential oil of aquilaria crassna. Molecules 2015, 20, 11808–11829.
- Tan, L.T.H.; Lee, L.H.; Yin, W.F.; Chan, C.K.; Kadir, H.A.; Chan, K.G.; Goh, B.H. Traditional uses, phytochemistry, and bioactivities of Cananga odorata (Ylang-Ylang). Evid.-Based Complement. Altern. Med. 2015, 2015, 1–30.
- Mbekou, M.I.K.; Dize, D.; Yimgang, V.L.; Djague, F.; Toghueo, R.M.K.; Sewald, N.; Lenta, B.N.; Boyom, F.F. Antibacterial and mode of action of extracts from endophytic fungi derived from Terminalia mantaly, Terminalia catappa, and Cananga odorata. BioMed Res. Int. 2021, 2021, 6697973.
- Beatović, D.; Krstić-Milošević, D.; Trifunović, S.; Šiljegović, J.; Glamočlija, J.; Ristić, M.; Jelačić, S. Chemical composition, antioxidant and antimicrobial activities of the essential oils of twelve Ocimum basilicum L. cultivars grown in Serbia. Rec. Nat. Prod. 2015, 9, 62–75.
- Xu, J.; Zhou, F.; Ji, B.-P.; Pei, R.-S.; Xu, N. The antibacterial mechanism of carvacrol and thymol against Escherichia coli. Lett. Appl. Microbiol. 2008, 47, 174–179.
- Ghadimian, S.; Esmaeili, F. Chemical composition of the essential oils of Carum copticum. J. Essent. Oil Bear. Plants 2016, 19, 1834–1836.
- Marchese, A.; Arciola, C.R.; Barbieri, R.; Silva, A.S.; Nabavi, S.M.; Sokeng, A.J.T.; Izadi, M.; Jafari, N.J.; Suntar, I.; Daglia, M.; et al. Update on monoterpenes as antimicrobial agents: A particular focus on p-cymene. Materials 2017, 10, 947.
- Condò, C.; Anacarso, I.; Sabia, C.; Iseppi, R.; Anfelli, I.; Forti, L.; de Niederhäusern, S.; Bondi, M.; Messi, P. Antimicrobial activity of spices essential oils and its effectiveness on mature biofilms of human pathogens. Nat. Prod. Res. 2020, 34, 567–574.
- Feng, J.; Zhang, S.; Shi, W.; Zubcevik, N.; Miklossy, J.; Zhang, Y. Selective essential oils from spice or culinary herbs have high activity against stationary phase and biofilm borrelia burgdorferi. Front. Med. 2017, 4, 169.
- Navarra, M.; Mannucci, C.; Delbò, M.; Calapai, G. Citrus bergamia essential oil: From basic research to clinical application. Front. Pharmacol. 2015, 6, 36.
- Liu, X.; Cai, J.; Chen, H.; Zhong, Q.; Hou, Y.; Chen, W.; Chen, W. Antibacterial activity and mechanism of linalool against Pseudomonas aeruginosa. Microb. Pathog. 2020, 141, 103980.
- Song, X.; Liu, T.; Wang, L.; Liu, L.; Li, X.; Wu, X. Antibacterial effects and mechanism of mandarin (Citrus reticulata L.) essential oil against Staphylococcus aureus. Molecules 2020, 25, 4956.
- Sharma, S.; Habib, S.; Sahu, D.; Gupta, J. Chemical properties and therapeutic potential of citral, a monoterpene isolated from lemongrass. Med. Chem. 2020, 17, 2–12.
- Shi, C.; Song, K.; Zhang, X.; Sun, Y.; Sui, Y.; Chen, Y.; Jia, Z.; Sun, H.; Sun, Z.; Xia, X. Antimicrobial activity and possible mechanism of action of citral against cronobacter sakazakii. PLoS ONE 2016, 11, e0159006.
- Aldoghaim, F.S.; Flematti, G.R.; Hammer, K.A. Antimicrobial activity of several cineole-rich western australian eucalyptus essential oils. Microorganisms 2018, 6, 122.
- Sebei, K.; Sakouhi, F.; Herchi, W.; Khouja, M.L.; Boukhchina, S. Chemical composition and antibacterial activities of seven Eucalyptus species essential oils leaves. Biol. Res. 2015, 48, 7.
- Gonçalves, S.M.; Motta, J.F.G.; dos Santos, R.R.; Chávez, D.W.H.; de Melo, N.R. Functional and antimicrobial properties of cellulose acetate films incorporated with sweet fennel essential oil and plasticizers. Curr. Res. Food Sci. 2020, 3, 1–8.
- Kubo, I.; Fujita, K.-I.; Nihei, K.-I. Antimicrobial activity of anethole and related compounds from aniseed. J. Sci. Food Agric. 2008, 88, 242–247.
- Rozman, N.A.S.; Yenn, T.W.; Tan, W.-N.; Ring, L.C.; Yusof, F.A.B.M.; Sulaiman, B. Homalomena pineodora, a novel essential oil bearing plant and its antimicrobial activity against diabetic wound pathogens. J. Essent. Oil Bear. Plants 2018, 21, 963–971.
- Yang, L.; Zhan, C.; Huang, X.; Hong, L.; Fang, L.; Wang, W.; Su, J. Durable antibacterial cotton fabrics based on natural borneol-derived Anti-MRSA agents. Adv. Health Mater. 2020, 9, e2000186.
- Verma, R.; Rahman, L.; Chanotiya, C.; Verma, R.; Chauhan, A.; Yadav, A.; Singh, A.; Yadav, A. Essential oil composition of Lavandula angustifolia Mill. cultivated in the mid hills of Uttarakhand, India. J. Serb. Chem. Soc. 2010, 75, 343–348.
- de Oliveira, E.F.; DE Paula, H.C.; de Paula, R.C. Alginate/cashew gum nanoparticles for essential oil encapsulation. Colloids Surf. B Biointerfaces 2014, 113, 146–151.
- McKay, D.L.; Blumberg, J.B. A Review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L.). Phytother. Res. 2006, 20, 519–530.
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (Tea Tree) oil: A review of antimicrobial and other medicinal properties. Clin. Microbiol. Rev. 2006, 19, 50–62.
- Zhang, Y.; Feng, R.; Li, L.; Zhou, X.; Li, Z.; Jia, R.; Song, X.; Zou, Y.; Yin, L.; He, C.; et al. The Antibacterial mechanism of terpinen-4-ol against streptococcus agalactiae. Curr. Microbiol. 2018, 75, 1214–1220.
- Saharkhiz, M.J.; Motamedi, M.; Zomorodian, K.; Pakshir, K.; Miri, R.; Hemyari, K. Chemical Composition, antifungal and antibiofilm activities of the essential oil of Mentha piperita L. ISRN Pharm. 2012, 2012, 718645.
- Dawaba, A.M.; Dawaba, H.M. Application of optimization technique to develop nano-based carrier of nigella sativa essential oil: Characterization and assessment. Recent Pat. Drug Deliv. Formul. 2020, 13, 228–240.
- Goel, S.; Mishra, P. Thymoquinone inhibits biofilm formation and has selective antibacterial activity due to ROS generation. Appl. Microbiol. Biotechnol. 2018, 102, 1955–1967.
- Rodriguez-Garcia, I.; Silva-Espinoza, B.; Ortega-Ramirez, L.; Leyva, J.; Siddiqui, M.W.; Valenzuela, M.R.C.; Gonzalez-Aguilar, G.; Zavala, J.F.A. Oregano essential oil as an antimicrobial and antioxidant additive in food products. Crit. Rev. Food Sci. Nutr. 2016, 56, 1717–1727.
- Ellahi, H.; Sadrabad, E.K.; Hekmatimoghaddam, S.H.; Jebali, A.; Sadeghizadeh-Yazdi, J.; Rastiani, F.; Mohajeri, F.A. Antimicrobial activity and chemical composition of Pistachia Atlantica Gum Sub Sp. Kurdica. essential oil. J. Nutr. Food Secur. 2019, 4, 186–190.
- Ghavam, M.; Manca, M.L.; Manconi, M. Chemical Composition and Antimicrobial Activity of Essential Oils Obtained from Leaves and Fowers of Salvia Hydrangea DC. ex Benth. Available online: https://www.nature.com/articles/s41598-020-73193-y.pdf?origin=ppub (accessed on 4 January 2022).
- Paraschos, S.; Mitakou, S.; Skaltsounis, A.-L. Chios gum mastic: A review of its biological activities. Curr. Med. Chem. 2012, 19, 2292–2302.
- Gonçalves, F.A.; Neto, M.A.; Bezerra, J.N.S.; Macrae, A.; de Sousa, O.V.; Fonteles-Filho, A.A.; Vieira, R.H. Antibacterial activity of GUAVA, Psidium guajava Linnaeus, leaf extracts on diarrhea-causing enteric bacteria isolated from Seabob shrimp, Xiphopenaeus kroyeri (Heller). Rev. Inst. Med. Trop. São Paulo 2008, 50, 11–15.
- Fathi, N.; Lotfipour, F.; Dizaj, S.M.; Hamishehkar, H.; Mohammadi, M. Antimicrobial activity of nanostructured lipid carriers loaded punica granatum seed oil against Staphylococcus epidermidis. Pharm. Nanotechnol. 2020, 8, 485–494.
- Yemis, G.P.; Bach, S.; Delaquis, P. Antibacterial activity of polyphenol-rich pomegranate peel extract against Cronobacter sakazakii. Int. J. Food Prop. 2019, 22, 985–993.
- Xu, Y.; Shi, C.; Wu, Q.; Zheng, Z.; Liu, P.; Li, G.; Peng, X.; Xia, X. Antimicrobial activity of punicalagin against Staphylococcus aureus and its effect on biofilm formation. Foodborne Pathog. Dis. 2017, 14, 282–287.
- Ojeda-Sana, A.M.; van Baren, C.M.; Elechosa, M.A.; Juárez, M.A.; Moreno, S. New insights into antibacterial and antioxidant activities of rosemary essential oils and their main components. Food Control 2013, 31, 189–195.
- Wu, K.; Lin, Y.; Chai, X.; Duan, X.; Zhao, X.; Chun, C. Mechanisms of vapor-phase antibacterial action of essential oil from Cinnamomum camphora var. linaloofera Fujita against Escherichia coli. Food Sci. Nutr. 2019, 7, 2546–2555.
- Feyzioglu, G.C.; Tornuk, F. Development of chitosan nanoparticles loaded with summer savory (Satureja hortensis L.) essential oil for antimicrobial and antioxidant delivery applications. LWT 2016, 70, 104–110.
- Kaur, K.; Kaushal, S.; Rani, R. Chemical composition, antioxidant and antifungal potential of clove (Syzygium aromaticum) essential oil, its major compound and its derivatives. J. Essent. Oil Bear. Plants 2019, 22, 1195–1217.
- Aldosary, S.K.; El-Rahman, S.N.A.; Al-Jameel, S.S.; Alromihi, N.M. Antioxidant and antimicrobial activities of Thymus vulgaris essential oil contained and synthesis thymus (Vulgaris) silver nanoparticles. Braz. J. Biol. 2021, 83, e244675.
- Vinciguerra, V.; Rojas, F.; Tedesco, V.; Giusiano, G.; Angiolella, L. Chemical characterization and antifungal activity of Origanum vulgare, Thymus vulgaris essential oils and carvacrol against Malassezia furfur. Nat. Prod. Res. 2019, 33, 3273–3277.
- Sajed, H.; Sahebkar, A.; Iranshahi, M. Zataria multiflora Boiss. (Shirazi thyme)—An ancient condiment with modern pharmaceutical uses. J. Ethnopharmacol. 2013, 145, 686–698.
- Tenover, F.C. Mechanisms of antimicrobial resistance in bacteria. Am. J. Med. 2006, 119, S3–S10.
- Gavanji, S.; Mohammadi, E.; Larki, B.; Bakhtari, A. Antimicrobial and cytotoxic evaluation of some herbal essential oils in comparison with common antibiotics in bioassay condition. Integr. Med. Res. 2014, 3, 142–152.
- Duarte, M.C.T.; Leme, E.E.; Delarmelina, C.; Soares, A.A.; Figueira, G.M.; Sartoratto, A. Activity of essential oils from Brazilian medicinal plants on Escherichia coli. J. Ethnopharmacol. 2007, 111, 197–201.
- Reichling, J.; Schnitzler, P.; Suschke, U.; Saller, R. Essential oils of aromatic plants with antibacterial, antifungal, antiviral, and cytotoxic properties—An overview. Complement. Med. Res. 2009, 16, 79–90.
- Radunz, M.; da Trindade, M.L.M.; Camargo, T.M.; Radünz, A.L.; Borges, C.D.; Gandra, E.A.; Helbig, E. Antimicrobial and antioxidant activity of unencapsulated and encapsulated clove (Syzygium aromaticum, L.) essential oil. Food Chem. 2019, 276, 180–186.
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Antimicrobial mechanism of clove oil on Listeria monocytogenes. Food Control 2018, 94, 140–146.
- Astani, A.; Reichling, J.; Schnitzler, P. Comparative study on the antiviral activity of selected monoterpenes derived from essential oils. Phytother. Res. 2009, 24, 673–679.
- Fani, M.; Kohanteb, J. In vitro antimicrobial activity of thymus vulgaris essential oil against major oral pathogens. J. Evid.-Based Integr. Med. 2017, 22, 660–666.
- Alexa, E.; Sumalan, R.M.; Danciu, C.; Obistioiu, D.; Negrea, M.; Poiana, M.-A.; Rus, C.; Radulov, I.; Pop, G.; Dehelean, C. Synergistic antifungal, allelopatic and anti-proliferative potential of Salvia officinalis L., and Thymus vulgaris L. essential oils. Molecules 2018, 23, 185.
- (PDF) Anti-Influenza Virus Activity of Essential Oils and Vapors. Available online: https://www.researchgate.net/publication/267035381_Anti-influenza_virus_activity_of_essential_oils_and_vapors (accessed on 5 December 2021).
- Jiang, Y.; Wang, D.; Li, F.; Li, D.; Huang, Q. Cinnamon essential oil Pickering emulsion stabilized by zein-pectin composite nanoparticles: Characterization, antimicrobial effect and advantages in storage application. Int. J. Biol. Macromol. 2020, 148, 1280–1289.
- Carson, C.F.; Ashton, L.; Dry, L.; Smith, D.W.; Riley, T.V. Melaleuca alternifolia (tea tree) oil gel (6%) for the treatment of recurrent herpes labialis. J. Antimicrob. Chemother. 2001, 48, 450–451.
- Powers, C.N.; Osier, J.L.; McFeeters, R.L.; Brazell, C.B.; Olsen, E.L.; Moriarity, D.M.; Satyal, P.; Setzer, W.N. Antifungal and cytotoxic activities of sixty commercially-available essential oils. Molecules 2018, 23, 1549.
- Brun, P.; Bernabè, G.; Filippini, R.; Piovan, A. In vitro antimicrobial activities of commercially available tea tree (Melaleuca alternifolia) essential oils. Curr. Microbiol. 2019, 76, 108–116.
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53.
- Stevanovic, Z.D.; Sieniawska, E.; Glowniak, K.; Obradovic, N.; Pajic-Lijakovic, I. Natural Macromolecules as carriers for essential oils: From extraction to biomedical application. Front. Bioeng. Biotechnol. 2020, 8, 563.
- Langeveld, W.T.; Veldhuizen, E.J.A.; Burt, S.A. Synergy between essential oil components and antibiotics: A review. Crit. Rev. Microbiol. 2013, 40, 76–94.
- Grădinaru, A.C.; Trifan, A.; Şpac, A.; Brebu, M.; Miron, A.; Aprotosoaie, A.C. Antibacterial activity of traditional spices against lower respiratory tract pathogens: Combinatorial effects of Trachyspermum ammi essential oil with conventional antibiotics. Lett. Appl. Microbiol. 2018, 67, 449–457.
- Ben-Khalifa, R.; Gaspar, F.B.; Pereira, C.; Chekir-Ghedira, L.; Rodríguez-Rojo, S. Essential oil and hydrophilic antibiotic co-encapsulation in multiple lipid nanoparticles: Proof of concept and in vitro activity against Pseudomonas aeruginosa. Antibiotics 2021, 10, 1300.
- Nafis, A.; Iriti, M.; Ouchari, L.; El Otmani, F.; Marraiki, N.; Elgorban, A.M.; Syed, A.; Mezrioui, N.; Hassani, L.; Custódio, L. New insight into the chemical composition, antimicrobial and synergistic effects of the moroccan endemic Thymus atlanticus (ball) roussine essential oil in combination with conventional antibiotics. Molecules 2021, 26, 5850.
- Nafis, A.; Saad, F.E.; El Khalloufi, F.; Kasrati, A.; Abbad, A.; Mezrioui, N.; Oudra, B.; Vasconcelos, V.; Hassani, L. New insight into antimicrobial activities of Linaria ventricosa essential oil and its synergetic effect with conventional antibiotics. Arch. Microbiol. 2021, 203, 4361–4366.
- Valcourt, C.; Saulnier, P.; Umerska, A.; Zanelli, M.; Montagu, A.; Rossines, E.; Joly-Guillou, M. Synergistic interactions between doxycycline and terpenic components of essential oils encapsulated within lipid nanocapsules against gram negative bacteria. Int. J. Pharm. 2016, 498, 23–31.
- Soulaimani, B.; Varoni, E.; Iriti, M.; Mezrioui, N.-E.; Hassani, L.; Abbad, A. Synergistic anticandidal effects of six essential oils in Combination with Fluconazole or Amphotericin B against four clinically isolated Candida Strains. Antibiotics 2021, 10, 1049.
- Nidhi, P.; Rolta, R.; Kumar, V.; Dev, K.; Sourirajan, A. Synergistic potential of Citrus aurantium L. essential oil with antibiotics against Candida albicans. J. Ethnopharmacol. 2020, 262, 113135.
- Nafis, A.; Kasrati, A.; Jamali, C.A.; Custódio, L.; Vitalini, S.; Iriti, M.; Hassani, L. A comparative study of the in vitro antimicrobial and synergistic effect of essential oils from Laurus nobilis L. and Prunus armeniaca L. from Morocco with antimicrobial drugs: New approach for health promoting products. Antibiotics 2020, 9, 140.
- Zhang, J.; Wu, H.; Jiang, D.; Yang, Y.; Tang, W.; Xu, K. The antifungal activity of essential oil from Melaleuca leucadendra (L.) L. grown in China and its synergistic effects with conventional antibiotics against Candida. Nat. Prod. Res. 2019, 33, 2545–2548.
- Kwiatkowski, P.; Łopusiewicz, Ł.; Kostek, M.; Drozłowska, E.; Pruss, A.; Wojciuk, B.; Sienkiewicz, M.; Zielińska-Bliźniewska, H.; Dołęgowska, B. The antibacterial activity of lavender essential oil alone and in combination with octenidine dihydrochloride against MRSA strains. Molecules 2019, 25, 95.
- Aelenei, P.; Rimbu, C.M.; Guguianu, E.; Dimitriu, G.; Aprotosoaie, A.C.; Brebu, M.; Horhogea, C.; Miron, A. Coriander essential oil and linalool-interactions with antibiotics against Gram-positive and Gram-negative bacteria. Lett. Appl. Microbiol. 2018, 68, 156–164.
- Vasconcelos, N.G.; Queiroz, J.H.F.D.S.; Da Silva, K.E.; Vasconcelos, P.C.D.P.; Croda, J.; Simionatto, S. Synergistic effects of Cinnamomum cassia L. essential oil in combination with polymyxin B against carbapenemase-producing Klebsiella pneumoniae and Serratia marcescens. PLoS ONE 2020, 15, e0236505.
- Si, H.; Hu, J.; Liu, Z.; Zeng, Z.-L. Antibacterial effect of oregano essential oil alone and in combination with antibiotics against extended-spectrum β-lactamase-producing Escherichia coli: Table 1. FEMS Immunol. Med. Microbiol. 2008, 53, 190–194.
- El Atki, Y.; Aouam, I.; El Kamari, F.; Taroq, A.; Nayme, K.; Timinouni, M.; Lyoussi, B.; Abdellaoui, A. Antibacterial activity of cinnamon essential oils and their synergistic potential with antibiotics. J. Adv. Pharm. Technol. Res. 2019, 10, 63–67.
- Kwiatkowski, P.; Łopusiewicz, Ł.; Pruss, A.; Kostek, M.; Sienkiewicz, M.; Bonikowski, R.; Wojciechowska-Koszko, I.; Dołęgowska, B. Antibacterial activity of selected essential oil compounds alone and in combination with β-lactam antibiotics against MRSA strains. Int. J. Mol. Sci. 2020, 21, 7106.
More
Information
Subjects:
Allergy
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Revisions:
2 times
(View History)
Update Date:
27 Jan 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




