
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Theodora Noely Tambaria | + 2027 word(s) | 2027 | 2022-01-17 08:48:57 | | | |
| 2 | Bruce Ren | Meta information modification | 2027 | 2022-01-26 09:42:08 | | |
Video Upload Options
Enhanced coal bed methane recovery using gas injection can provide increased methane extraction depending on the characteristics of the coal and the gas that is used. Accurate prediction of the extent of gas adsorption by coal are therefore important. Both experimental methods and modeling have been used to assess gas adsorption and its effects, including volumetric and gravimetric techniques, as well as the Ono–Kondo model and other numerical simulations. Thermodynamic parameters may be used to model adsorption on coal surfaces while adsorption isotherms can be used to predict adsorption on coal pores. In addition, density functional theory and grand canonical Monte Carlo methods may be employed.
1. Introduction
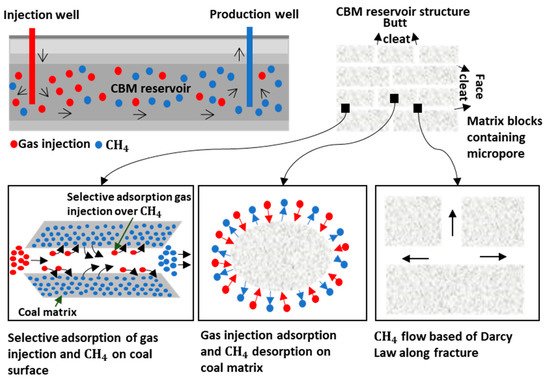
2. Methane in Coal
3. Gas Adsorption Characteristic of Coal
3.1. Effects of Sample Condition
3.2. Moisture Effects
3.3. Ash Yield Effects
3.4. Maceral Effects
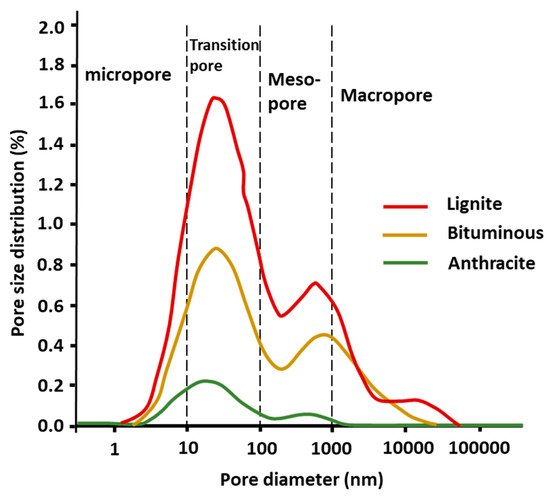
3.5. Coal Pore Effects
4. Gas Injection for ECBM Recovery
4.1. CO2 Injection
4.2. N2 Injection
4.3. Mixed Gas (CO2-N2) Injection
 (1)
(1)where SCO2/N2 is the adsorption selectivity and, x and y represent the mole fractions of each gas in the adsorbed and bulk phase, respectively. An adsorption selectivity of 1 indicates that N2 is adsorbed more strongly than CO2 while a value greater than 1 indicates the opposite.
References
- Alexis, D.A.; Karpyn, Z.T.; Ertekin, T.; Crandall, D. Fracture permeability and relative permeability of coal and their dependence on stress conditions. J. Unconv. Oil Gas Resour. 2015, 10, 1–10.
- Crosdale, P.J.; Beamish, B.; Valix, M. Coalbed methane sorption related to coal composition. Int. J. Coal Geol. 1998, 35, 147–158.
- Wang, R.; Zhang, N.; Liu, X.; Wu, X.; Chen, J.; Ma, L. Characteristics of Pore Volume Distribution and Methane Adsorption on Shales. Adsorpt. Sci. Technol. 2015, 33, 915–938.
- Bae, J.-S.; Bhatia, S.K.; Rudolph, V.; Massarotto, P. Pore Accessibility of Methane and Carbon Dioxide in Coals. Energy Fuels 2009, 23, 3319–3327.
- Lu, T.; Yu, H.; Zhou, T.; Mao, J.; Guo, B. Improvement of methane drainage in high gassy coal seam using waterjet technique. Int. J. Coal Geol. 2009, 79, 40–48.
- Keshavarz, A.; Badalyan, A.; Carageorgos, T.; Bedrikovetsky, P.; Johnson, R. Stimulation of coal seam permeability by micro-sized graded proppant placement using selective fluid properties. Fuel 2015, 144, 228–236.
- Umezaki, T.; Kawamura, T.; Okamoto, K.; Hattori, A.; Kobayashi, Y. Swelling properties and coefficient of permeability of friction-reducing polymer for pull-out of temporary sheet piles. Soils Found. 2018, 58, 797–807.
- Ahamed, M.; Perera, M.; Dong-Yin, L.; Ranjith, P.; Matthai, S. Proppant damage mechanisms in coal seam reservoirs during the hydraulic fracturing process: A review. Fuel 2019, 253, 615–629.
- Lyu, S.; Wang, S.; Chen, X.; Shah, S.; Li, R.; Xiao, Y.; Dong, Q.; Gu, Y. Experimental study of a degradable polymer drilling fluid system for coalbed methane well. J. Pet. Sci. Eng. 2019, 178, 678–690.
- Li, Z.; Wei, G.; Liang, R.; Shi, P.; Wen, H.; Zhou, W. LCO2-ECBM technology for preventing coal and gas outburst: Integrated effect of permeability improvement and gas displacement. Fuel 2021, 285, 119219.
- Zarrouk, S.J.; Moore, T. Preliminary reservoir model of enhanced coalbed methane (ECBM) in a subbituminous coal seam, Huntly Coalfield, New Zealand. Int. J. Coal Geol. 2009, 77, 153–161.
- Shimada, S.; Li, H.; Oshima, Y.; Adachi, K. Displacement behavior of CH4 adsorbed on coals by injecting pure CO2, N2, and CO2–N2 mixture. Environ. Earth Sci. 2005, 49, 44–52.
- Seomoon, H.; Lee, M.; Sung, W. Analysis of methane recovery through CO 2 –N 2 mixed gas injection considering gas diffusion phenomenon in coal seam. Energy Explor. Exploit. 2016, 34, 661–675.
- Oudinot, A.Y.; Riestenberg, D.E.; Koperna, G.J. Enhanced Gas Recovery and CO2 Storage in Coal Bed Methane Reservoirs with N2 Co-Injection. Energy Procedia 2017, 114, 5356–5376.
- Cho, S.; Kim, S.; Kim, J. Life-cycle energy, cost, and CO2 emission of CO2-enhanced coalbed methane (ECBM) recovery framework. J. Nat. Gas Sci. Eng. 2019, 70, 102953.
- Qi, L.; Tang, X.; Wang, Z.; Peng, X. Pore characterization of different types of coal from coal and gas outburst disaster sites using low temperature nitrogen adsorption approach. Int. J. Min. Sci. Technol. 2017, 27, 371–377.
- Godec, M.; Koperna, G.; Gale, J. CO2-ECBM: A Review of its Status and Global Potential. Energy Procedia 2014, 63, 5858–5869.
- Mukherjee, M.; Misra, S. A review of experimental research on Enhanced Coal Bed Methane (ECBM) recovery via CO2 sequestration. Earth-Sci. Rev. 2018, 179, 392–410.
- Harpalani, S.; Ouyang, S. A New Laboratory Technique to Estimate Gas Diffusion Characteristics of Coals. In Proceedings of the International Coalbed Methane Symposium, Tuscaloosa, AL, USA, 11–17 June 1999; pp. 141–149.
- Vishal, V.; Mahanta, B.; Pradhan, S.; Singh, T.; Ranjith, P. Simulation of CO2 enhanced coalbed methane recovery in Jharia coalfields, India. Energy 2018, 159, 1185–1194.
- Pan, Z.; Connell, L.D. A theoretical model for gas adsorption-induced coal swelling. Int. J. Coal Geol. 2007, 69, 243–252.
- Kim, H.J.; Shi, Y.; He, J.; Lee, H.-H.; Lee, C.-H. Adsorption characteristics of CO2 and CH4 on dry and wet coal from subcritical to supercritical conditions. Chem. Eng. J. 2011, 171, 45–53.
- Hol, S.; Peach, C.J.; Spiers, C.J. Applied stress reduces the CO2 sorption capacity of coal. Int. J. Coal Geol. 2011, 85, 128–142.
- Pan, Z.; Connell, L.D. Modelling permeability for coal reservoirs: A review of analytical models and testing data. Int. J. Coal Geol. 2012, 92, 1–44.
- White, C.M.; Smith, D.H.; Jones, K.L.; Goodman, A.L.; Jikich, S.A.; LaCount, R.B.; DuBose, S.B.; Ozdemir, E.; Morsi, A.B.I.; Schroeder, K.T. Sequestration of Carbon Dioxide in Coal with Enhanced Coalbed Methane Recovery—A Review. Energy Fuels 2005, 19, 659–724.
- Busch, A.; Gensterblum, Y. CBM and CO2-ECBM related sorption processes in coal: A review. Int. J. Coal Geol. 2011, 87, 49–71.
- Susilawati, R.; Esterle, J.S.; Golding, S.D.; Mares, T.E. Microbial Methane Potential for the South Sumatra Basin Coal: Formation Water Screening and Coal Substrate Bioavailability. Energy Procedia 2015, 65, 282–291.
- Saghafi, A. Potential for ECBM and CO2 storage in mixed gas Australian coals. Int. J. Coal Geol. 2010, 82, 240–251.
- Ahmed, M.; Smith, J. Biogenic methane generation in the degradation of eastern Australian Permian coals. Org. Geochem. 2001, 32, 809–816.
- Moore, T.A. Coalbed methane: A review. Int. J. Coal Geol. 2012, 101, 36–81.
- Scott, A.R.; Kaiser, W.R.; Ayers, W.B. Thermogenic and secondary biogenic gases, San Juan Basin, Colorado and New Mexico—Implications for coalbed gas producibility. Am. Assoc. Pet. Geol. Bull. 1994, 78, 1186–1209.
- Rice, D.D. Composition and Origins of Coalbed Gas. AAPG Bull. 1993, 77.
- Al-Mahmoud, M.J.; Inan, S.; Al-Duaiji, A.A. Coal occurrence in the Jurassic Dhruma Formation in Saudi Arabia: Inferences on its gas and surface mining potential. Int. J. Coal Geol. 2014, 124, 5–10.
- Zheng, Y.; Li, Q.; Yuan, C.; Tao, Q.; Zhao, Y.; Zhang, G.; Liu, J. Influence of temperature on adsorption selectivity: Coal-based activated carbon for CH4 enrichment from coal mine methane. Powder Technol. 2019, 347, 42–49.
- Merkel, A.; Gensterblum, Y.; Krooss, B.; Amann-Hildenbrand, A. Competitive sorption of CH4, CO2 and H2O on natural coals of different rank. Int. J. Coal Geol. 2015, 150–151, 181–192.
- Levine, J.R. Coalification: The Evolution of Coal as a Source Rock and Reservoir Rock for Oil and Gas. In Hydrocarbon in Coal; Law, B.E., Rice, D.D., Eds.; American Association of Petroleum Geologists Studies in Geology: Tuscaloosa, AL, USA, 1993; Volume 38, pp. 39–77.
- Li, Y.; Zhang, C.; Tang, D.; Gan, Q.; Niu, X.; Wang, K.; Shen, R. Coal pore size distributions controlled by the coalification process: An experimental study of coals from the Junggar, Ordos and Qinshui basins in China. Fuel 2017, 206, 352–363.
- Olajossy, A. Some parameters of coal methane system that cause very slow release of methane from virgin coal beds (CBM). Int. J. Min. Sci. Technol. 2017, 27, 321–326.
- Pone, J.D.N.; Halleck, P.M.; Mathews, J.P. Sorption Capacity and Sorption Kinetic Measurements of CO2 and CH4 in Confined and Unconfined Bituminous Coal. Energy Fuels 2009, 23, 4688–4695.
- Kim, D.; Seo, Y.; Kim, J.; Han, J.; Lee, Y. Experimental and Simulation Studies on Adsorption and Diffusion Characteristics of Coalbed Methane. Energies 2019, 12, 3445.
- Busch, A.; Krooss, B.; Gensterblum, Y.; van Bergen, F.; Pagnier, H. High-pressure adsorption of methane, carbon dioxideand their mixtures on coals with a special focus on the preferential sorption behaviour. J. Geochem. Explor. 2003, 78–79, 671–674.
- Mastalerz, M.; Gluskoter, H.; Rupp, J. Carbon dioxide and methane sorption in high volatile bituminous coals from Indiana, USA. Int. J. Coal Geol. 2004, 60, 43–55.
- Battistutta, E.; van Hemert, P.; Lutynski, M.; Bruining, H.; Wolf, K.-H. Swelling and sorption experiments on methane, nitrogen and carbon dioxide on dry Selar Cornish coal. Int. J. Coal Geol. 2010, 84, 39–48.
- Ozdemir, E.; I Morsi, B.; Schroeder, K. CO2 adsorption capacity of argonne premium coals. Fuel 2004, 83, 1085–1094.
- Skoczylas, N.; Pajdak, A.; Młynarczuk, M. CO2 Adsorption–Desorption Kinetics from the Plane Sheet of Hard Coal and Associated Shrinkage of the Material. Energies 2019, 12, 4013.
- Švábová, M.; Weishauptová, Z.; Přibyl, O. The effect of moisture on the sorption process of CO2 on coal. Fuel 2012, 92, 187–196.
- Busch, A.; Gensterblum, Y.; Krooss, B.; Littke, R. Methane and carbon dioxide adsorption–diffusion experiments on coal: Upscaling and modeling. Int. J. Coal Geol. 2004, 60, 151–168.
- Crosdale, P.J.; Moore, T.; Mares, T. Influence of moisture content and temperature on methane adsorption isotherm analysis for coals from a low-rank, biogenically-sourced gas reservoir. Int. J. Coal Geol. 2008, 76, 166–174.
- Chen, M.-Y.; Cheng, Y.-P.; Li, H.-R.; Wang, L.; Jin, K.; Dong, J. Impact of inherent moisture on the methane adsorption characteristics of coals with various degrees of metamorphism. J. Nat. Gas Sci. Eng. 2018, 55, 312–320.
- Guo, H.; Cheng, Y.; Wang, L.; Lu, S.; Jin, K. Experimental study on the effect of moisture on low-rank coal adsorption characteristics. J. Nat. Gas Sci. Eng. 2015, 24, 245–251.
- Hao, D.; Zhang, L.; Li, M.; Tu, S.; Zhang, C.; Bai, Q.; Wang, C. Experimental study of the moisture content influence on CH4 adsorption and deformation characteristics of cylindrical bituminous coal core. Adsorpt. Sci. Technol. 2018, 36, 1512–1537.
- Cai, Y.; Liu, D.; Pan, Z.; Yao, Y.; Li, J.; Qiu, Y. Pore structure and its impact on CH4 adsorption capacity and flow capability of bituminous and subbituminous coals from Northeast China. Fuel 2013, 103, 258–268.
- Laxminarayana, C.; Crosdale, P.J. Role of coal type and rank on methane sorption characteristics of Bowen Basin, Australia coals. Int. J. Coal Geol. 1999, 40, 309–325.
- Li, J.; Li, B. Evolution features of coal matrix porosity with the variation in temperature and stress. IOP Conf. Ser. Mater. Sci. Eng. 2017, 191, 12050.
- Siemons, N.; Busch, A. Measurement and interpretation of supercritical CO2 sorption on various coals. Int. J. Coal Geol. 2007, 69, 229–242.
- Kumar, H.; Mishra, M.K.; Mishra, S. Sorption capacity of Indian coal and its variation with rank parameters. J. Pet. Explor. Prod. Technol. 2019, 9, 2175–2184.
- Yalçin, E.; Durucan, Ş. Methane capacities of Zonguldak coals and the factors affecting methane adsorption. Min. Sci. Technol. 1991, 13, 215–222.
- Faiz, M.M.; Aziz, N.I.; Hutton, A.C.; Jones, B.G. Porosity and gas sorption capacity of some eastern Australian coals in relation to coal rank and composition. Coalbed Methane Symp. 1992, 19, 9–13.
- Karayiğit, A.I.; Mastalerz, M.; Oskay, R.G.; Buzkan, I. Bituminous coal seams from underground mines in the Zonguldak Basin (NW Turkey): Insights from mineralogy, coal petrography, Rock-Eval pyrolysis, and meso-and microporosity. Int. J. Coal Geol. 2018, 199, 91–112.
- Fitzgerald, J.; Pan, Z.; Sudibandriyo, M.; Robinson, J.R.; Gasem, K.; Reeves, S. Adsorption of methane, nitrogen, carbon dioxide and their mixtures on wet Tiffany coal. Fuel 2005, 84, 2351–2363.
- Bustin, R.; Clarkson, C. Geological controls on coalbed methane reservoir capacity and gas content. Int. J. Coal Geol. 1998, 38, 3–26.
- Shen, J.; Qin, Y.; Zhao, J. Maceral Contribution to Pore Size Distribution in Anthracite in the South Qinshui Basin. Energy Fuels 2019, 33, 7234–7243.
- Rodrigues, C.F.A.; de Sousa, M.J.L. The measurement of coal porosity with different gases. Int. J. Coal Geol. 2002, 48, 245–251.
- Beamish, B.; Crosdale, P.J. Instantaneous outbursts in underground coal mines: An overview and association with coal type. Int. J. Coal Geol. 1998, 35, 27–55.
- Karacan, C.; Mitchell, G.D. Behavior and effect of different coal microlithotypes during gas transport for carbon dioxide sequestration into coal seams. Int. J. Coal Geol. 2003, 53, 201–217.
- Larsen, J.W. The effects of dissolved CO2 on coal structure and properties. Int. J. Coal Geol. 2004, 57, 63–70.
- Unsworth, J.F.; Fowler, C.S.; Jones, L.F. Moisture in coal: 2. Maceral effects on pore structure. Fuel 1989, 68, 18–26.
- Keshavarz, A.; Sakurovs, R.; Grigore, M.; Sayyafzadeh, M. Effect of maceral composition and coal rank on gas diffusion in Australian coals. Int. J. Coal Geol. 2017, 173, 65–75.
- Brandani, S.; Mangano, E.; Sarkisov, L. Net, excess and absolute adsorption and adsorption of helium. Adsorption 2016, 22, 261–276.
- Zhou, Y.; Zhang, R.; Huang, J.; Li, Z.; Zhao, Z.; Zeng, Z. Effects of pore structure and methane adsorption in coal with alkaline treatment. Fuel 2019, 254, 115600.
- Bakshi, T.; Prusty, B.; Pathak, K.; Nayak, B.; Mani, D.; Pal, S. Source rock characteristics and pore characterization of Indian shale. J. Nat. Gas Sci. Eng. 2017, 45, 761–770.
- Qin, L.; Li, S.; Zhai, C.; Lin, H.; Zhao, P.; Yan, M.; Ding, Y.; Shi, Y. Joint analysis of pores in low, intermediate, and high rank coals using mercury intrusion, nitrogen adsorption, and nuclear magnetic resonance. Powder Technol. 2020, 362, 615–627.
- Li, S.; Tang, D.; Xu, H.; Yang, Z. The pore-fracture system properties of coalbed methane reservoirs in the Panguan Syncline, Guizhou, China. Geosci. Front. 2012, 3, 853–862.
- Li, Z.; Liu, D.; Cai, Y.; Wang, Y.; Teng, J. Adsorption pore structure and its fractal characteristics of coals by N2 adsorption/desorption and FESEM image analyses. Fuel 2019, 257, 116031.
- Clarkson, C.; Bustin, R. The effect of pore structure and gas pressure upon the transport properties of coal: A laboratory and modeling study. 1. Isotherms and pore volume distributions. Fuel 1999, 78, 1333–1344.
- Parkash, S.; Chakrabartty, S. Microporosity in Alberta Plains coals. Int. J. Coal Geol. 1986, 6, 55–70.
- Sun, W.; Feng, Y.; Jiang, C.; Chu, W. Fractal characterization and methane adsorption features of coal particles taken from shallow and deep coalmine layers. Fuel 2015, 155, 7–13.
- Yao, Y.; Liu, D. Effects of igneous intrusions on coal petrology, pore-fracture and coalbed methane characteristics in Hongyang, Handan and Huaibei coalfields, North China. Int. J. Coal Geol. 2012, 96–97, 72–81.
- Staib, G.; Sakurovs, R.; Gray, E.M.A. A pressure and concentration dependence of CO2 diffusion in two Australian bituminous coals. Int. J. Coal Geol. 2013, 116–117, 106–116.
- Zhou, Y.; Li, Z.; Zhang, R.; Wang, G.; Yu, H.; Sun, G.; Chen, L. CO2 injection in coal: Advantages and influences of temperature and pressure. Fuel 2018, 236, 493–500.
- Yamazaki, T.; Aso, K.; Chinju, J. Japanese potential of CO2 sequestration in coal seams. Appl. Energy 2006, 83, 911–920.
- Busch, A.; Gensterblum, Y.; Krooss, B.M. High-Pressure Sorption of Nitrogen, Carbon Dioxide, and their Mixtures on Argonne Premium Coals. Energy Fuels 2007, 21, 1640–1645.
- Cui, X.; Bustin, R.; Dipple, G. Selective transport of CO2, CH4, and N2 in coals: Insights from modeling of experimental gas adsorption data. Fuel 2004, 83, 293–303.
- Zheng, G.; Pan, Z.; Tang, S.; Ling, B.; Lv, D.; Connell, L.D. Laboratory and Modeling Study on Gas Diffusion with Pore Structures in Different-Rank Chinese Coals. Energy Explor. Exploit. 2013, 31, 859–877.
- Bhowmik, S.; Dutta, P. Adsorption rate characteristics of methane and CO2 in coal samples from Raniganj and Jharia coalfields of India. Int. J. Coal Geol. 2013, 113, 50–59.
- Charrière, D.; Pokryszka, Z.; Behra, P. Effect of pressure and temperature on diffusion of CO2 and CH4 into coal from the Lorraine basin (France). Int. J. Coal Geol. 2010, 81, 373–380.
- Zhao, J.; Tang, D.; Qin, Y.; Xu, H.; Liu, Y.; Wu, H. Characteristics of Methane (CH4) Diffusion in Coal and Its Influencing Factors in the Qinshui and Ordos Basins. Energy Fuels 2018, 32, 1196–1205.
- Li, X.; Yan, X.; Kang, Y. Effect of temperature on the permeability of gas adsorbed coal under triaxial stress conditions. J. Geophys. Eng. 2017, 15, 386–396.
- Fang, H.; Sang, S.; Liu, S. The coupling mechanism of the thermal-hydraulic-mechanical fields in CH4-bearing coal and its application in the CO2-enhanced coalbed methane recovery. J. Pet. Sci. Eng. 2019, 181, 106177.
- Wang, X.; Zhang, D.; Su, E.; Jiang, Z.; Wang, C.; Chu, Y.; Ye, C. Pore structure and diffusion characteristics of intact and tectonic coals: Implications for selection of CO2 geological sequestration site. J. Nat. Gas Sci. Eng. 2020, 81, 103388.
- Dutta, A. Multicomponent Gas Diffusion and Adsorption in Coals for Enhanced Methane Recovery. Master’s Thesis, Stanford University, Stanford, USA, June 2009.
- Abunowara, M.; Sufian, S.; Bustam, M.A.; Eldemerdash, U.; Suleman, H.; Bencini, R.; Assiri, M.A.; Ullah, S.; Al-Sehemi, A.G. Experimental measurements of carbon dioxide, methane and nitrogen high-pressure adsorption properties onto Malaysian coals under various conditions. Energy 2020, 210, 118575.
- George, J.S.; Barakat, M. The change in effective stress associated with shrinkage from gas desorption in coal. Int. J. Coal Geol. 2001, 45, 105–113.
- Joewondo, N. Pore Sturucture of Micro and Mesoporous Mudrocks Based on Nitrogen and Carbon Sorption. Master’s Thesis, Colorado School of Mines, Golden, Colorado, 2018.
- Aleghafouri, A.; Mohsen-Nia, M.; Mohajeri, A.; Mahdyarfar, M.; Asghari, M. Micropore Size Analysis of Activated Carbons Using Nitrogen, Carbon Dioxide and Methane Adsorption Isotherms: Experimental and Theoretical Studies. Adsorpt. Sci. Technol. 2012, 30, 307–316.
- Wang, H.; Fu, X.; Jian, K.; Li, T.; Luo, P. Changes in coal pore structure and permeability during N 2 injection. J. Nat. Gas Sci. Eng. 2015, 27, 1234–1241.
- Fang, Z.; Li, X.; Hu, H. Gas mixture enhance coalbed methane recovery technology: Pilot tests. Energy Procedia 2011, 4, 2144–2149.
- Reeves, S. The Coal-Seq Project: Key Results from Field, Laboratory, and Modeling Studies. Greenh. Gas Control Technol. 2005, II, 1399–1403.
- Zhu, H.; He, X.; Xie, Y.; Guo, S.; Huo, Y.; Wang, W. A Study on the Effect of Coal Metamorphism on the Adsorption Characteristics of a Binary Component System: CO2 and N2. ACS Omega 2020, 6, 523–532.




