Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marija Lesjak | + 3814 word(s) | 3814 | 2022-01-17 09:08:22 | | | |
| 2 | Camila Xu | Meta information modification | 3814 | 2022-01-27 02:30:34 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Lesjak, M. Polyphenols (PCs). Encyclopedia. Available online: https://encyclopedia.pub/entry/18808 (accessed on 07 February 2026).
Lesjak M. Polyphenols (PCs). Encyclopedia. Available at: https://encyclopedia.pub/entry/18808. Accessed February 07, 2026.
Lesjak, Marija. "Polyphenols (PCs)" Encyclopedia, https://encyclopedia.pub/entry/18808 (accessed February 07, 2026).
Lesjak, M. (2022, January 26). Polyphenols (PCs). In Encyclopedia. https://encyclopedia.pub/entry/18808
Lesjak, Marija. "Polyphenols (PCs)." Encyclopedia. Web. 26 January, 2022.
Copy Citation
Polyphenols, a diverse group of naturally occurring molecules commonly found in higher plants, have been heavily investigated over the last two decades due to their potent biological activities—among which the most important are their antioxidant, antimicrobial, anticancer, anti-inflammatory and neuroprotective activities.
polyphenols
metabolites
ferroptosis inhibition
1. Overview on Polyphenols
Polyphenols (PCs) are a large heterogeneous group of naturally occurring molecules commonly found in higher plants. PCs have been studied extensively during the past 40 years due to their large spectrum of potential beneficial effects on human health, improving quality of life and prolonging lifespans. PCs are products of plant secondary metabolism that are synthesized to protect plants from different environmental factors and to enable their communication with the environment. More than 10,000 PCs have been identified in various plant species [1]. There is a great chemical structural diversity among PCs, but their common characteristic is the presence of more than one phenolic hydroxy group attached to one or more aromatic rings. According to the number of phenolic rings and the structural elements that link these rings, PCs are subdivided into several classes. The basic classification of PCs would include five main polyphenolic classes: phenolic acids (benzoic (C6-C1) and cinnamic acids (C6-C3)); flavonoids (C6-C3-C6); stilbenes (C6-C2-C6), lignans ((C6-C3)2); and others (coumarins (C6-C3), diarylheptanoids (C6-C7-C6), anthraquinones (C6-C2-C6), naphthoquinones (C6-C4), xanthones (C6-C1-C6), and condensed tannins (C6-C3-C6)n; Figure 1; [2][3][4][5].
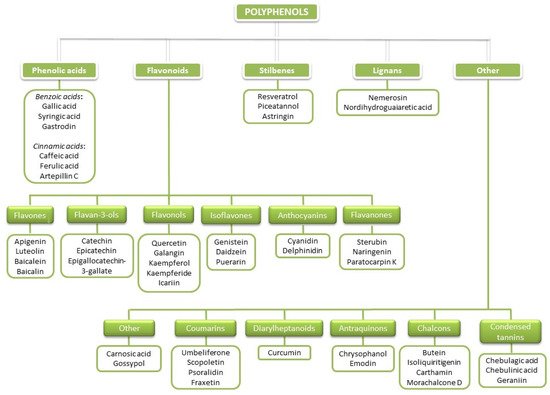
Figure 1. Basic classification of PCs with representatives of particular classes.
Phenolic acids and flavonoids are widely distributed in the plant kingdom, while other classes such as lignans, stilbenes, anthraquinones, coumarins, or diarylheptanoids are more specific to particular plant genera. Phenolic acids include hydroxycinnamic acids and derivatives of benzoic acid. Flavonoids represent a subclass of PCs with a C6-C3-C6 backbone structure, and they can be subdivided into two major groups according to whether the central heterocyclic ring is unsaturated or not. Flavonoids with an unsaturated central ring include anthocyanins, flavones, and flavanols, while flavanones and flavans belong to a group with saturated rings. Flavonoids in which the 2-phenyl side chain is isomerized at the 3-position are known as isoflavonoids. Plant PCs are synthetized from carbohydrates via the shikimate pathway. The biosynthetic relationship of flavonoids is shown in Figure 2. [6].
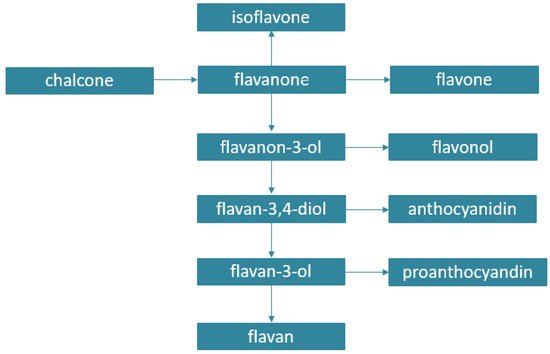
Figure 2. Biosynthetic origin of flavonoids.
The majority of flavonoids are monomeric, while flavan-3-ols (catechins) can oligomerize—giving proanthocyanidins—or polymerize, giving non-hydrolysable tannins.
PCs mostly occur in a conjugated form as glycosides, with sugar residues linked predominantly to the hydroxy groups or directly to an aromatic carbon, or as esters with organic acids. They can also be present in plants in a free form, but much less frequently.
Food Sources of PCs
PCs are widely present in foods of plant origin. They are found in all fruits and vegetables, but with a qualitative and quantitative distribution that varies between different plants. Since PCs are present in plants as complex mixtures, only a small number of species have been examined systematically for their phenolic composition; thus, the data are usually incomplete and there is no comprehensive database on PC amounts in food [3]. Thus, it is hard to precisely estimate the dietary intake of PCs in a particular diet or to assess the dietary intake in a population. The most abundant PCs in the diet are phenolic acids and flavonoids. According to some rough estimations, the mean daily total flavonoid intake in the U.S. is 189.7 mg/d, from which there are listed classes present in different percentages: flavan-3-ols (83.5%), followed by flavanones (7.6%), flavanols (6.8%), anthocyanidins (1.6%), flavones (0.8%), and isoflavones (0.6%) [7].
Quercetin and kaempferol are the most abundant flavanols in plant diet. Flavanols are mainly present in plants in their highly hydrophilic glycosylated forms, mainly as β-glycosides of various sugars (monosaccharides—galactose, glucose, arabinose, xylose and rhamnose, and the disaccharide rutinose) [8]. The dominant quercetin glycosides are quercetin-3-O-galactoside (hyperoside), quercetin-3-O-rhamnoside (quercitrin), quercetin-3-O-rutinoside (rutin), quercetin-3-O-glucoside (isoquercitrin), and quercetin-4′-O-glucoside [9].
High concentrations of quercetin are found in onions, tea, apples, asparagus, berries and red wine, while the richest plant sources of kaempferol are green leafy vegetables, including spinach and kale, and herbs such as dill, chives, and tarragon [10][11]. Quercetin can also be taken as a dietary supplement with a daily recommended doses of 200–1200 mg, as well as through functional foods with a concentration range of 10–125 mg per serving. Dietary supplementation with quercetin and its addition into food is highly supported by data on its safety [12].
Apigenin and luteolin are the main representatives of flavones, the less common class of flavonoids. Rich natural sources of these compounds are green leafy herbs, such as celery, parsley, and spinach, as well as chamomile, green paper, eggplant, oranges, and red wine [13]. In these sources, apigenin is commonly found as a 7-O-glucoside, 6-C-glucoside, or 8-C-glucoside. The daily intake of apigenin in the human diet is between 0.45 and 1.17 mg [14].
Naringenin and hesperidin are flavanones specific to citrus fruits. For citrus flavonoids, anti-inflammatory, anticarcinogenic, and antitumor activities have been reported. Naringin mainly occurs as glycosides such as naringenin-7-rhamnoglucoside (naringin) or naringenin-7-glucoside. Naringin is present in grapefruit and grapefruit juices in high amounts (from 100 to 500 mg/L of juice) and is responsible for the bitterness of grapefruit juices [15].
Catechin and epicatechin are most commonly found monomeric flavan-3-ols. Their main food sources are fruits (berries, cherries, apple, grapes, plums, apricots), teas, red wine, and cocoa. The average daily monomeric catechin and epicatechin intake is 9–13 mg and 11–17 mg, respectively [16][17]. Catechin and epicatechin are often found in fruits and vegetables in a polymerized form, such as oligomeric and polymeric proanthocyandins (condensed tannins).
Resveratrol is the most investigated polyphenol from the stilbene family. It is found in more than 70 plant species—often as a glucoside—particularly in grape skin and seeds, berries, apples, plums, and peanuts, as well as in food products such as red wine. The total dietary intake of resveratrol is up to 4 mg/day [18]. Resveratrol can also be taken as a dietary supplement, with daily recommended doses of 250–1000 mg for up to 3 months. Numerous beneficial health effects have been confirmed for resveratrol, including anticancer, antimicrobial, neuroprotective, antiaging, anti-inflammatory, cardioprotective, and blood-sugar lowering properties, as well as life-prolonging effects [19].
Diarylheptanoid curcumin, the major bioactive component of the rhizome of turmeric (Curcuma longa L.) has been extensively studied over the past few decades. The average intake of turmeric in the Indian diet is approximately 2–2.5 g, which corresponds to a daily intake of approximately 60–100 mg of curcumin [20]. It can also be taken as a dietary supplement, with daily doses of up to 12 g. Numerous biological activities, such as anti-inflammatory, antioxidant, hypoglycaemic, wound healing, antimicrobial, and antitumor activities have been confirmed for this compound [21].
For most PCs, dietary recommendations for their intake have not been established yet.
2. Health Benefits of PCs
Epidemiological studies have indicated that intake of polyphenol-rich food, such as fruits, vegetables, and cereals, is directly related to declines in the incidence of chronic diseases in the population, such as cardiovascular disease, obesity, diabetes mellitus, asthma, liver disorders, and cancer [22]. For instance, the relatively low incidence of coronary heart disease among the French, despite a diet rich in saturated fats, is attributed to the consumption of red wine, which is rich in PCs. These studies attracted considerable public attention, increased consumers awareness of the importance of a “healthy diet” in everyday life, and food industries started to continuously develop new products, defined as “functional food” due to the presence of specific PCs. This inspired scientists to intensively study PCs from plants in terms of their chemical characterization and the evaluation of their biological activities. Thousands of scientific papers related to PCs have been published up to now (according to [22], more than 120,000 articles from 2000 to 2016), and there are several databases providing detailed information on the PC content in different foods (e.g., “Phenol-Explorer” version 1.5.7, INRA and Wishart Research Group, 2009). The most popular journals for covering original research on PCs are The Journal of Agriculture and Food Chemistry, Food Chemistry, PLOS ONE, and Planta Medica. There is an enormous amount of data regarding the biological activities of naturally occurring PCs, among which the most important are antioxidant [23], antimicrobial [24], anticancer [25], anti-inflammatory [26], and neuroprotective activity [27]. Additionally, PCs could be involved in the regulation of different processes in an organism, such as: expression of cell cycle regulatory proteins, signal transduction, or enzyme activity [28]. In addition to being highly bioactive, they express low toxicity, which makes their medicinal use very attractive.
PCs can affect enzymatic and signalling systems involved in the inflammatory processes, and thus express anti-inflammatory activity. For example, genistein was proven to inhibit tyrosine protein kinase, thus indirectly preventing T-cell proliferation and activation of B cells [22]. Additionally, luteolin, kaempferol, apigenin, and quercetin are powerful inhibitors of β-glucuronidase and lysosomal enzymes released from neutrophils. At the same time, these PCs inhibit phospholipase A2 and prevent the release of arachidonic acid from cell membranes, which is a precursor of proinflammatory mediators [22]. PCs also inhibit enzymes involved in the metabolism of arachidonic acid, such as cyclooxygenases (COX) and lipoxygenases (LOX), and consequently inhibit the production of prostaglandins and leukotrienes, which are essential for the development of inflammatory diseases [23][29][30].
One of the cardioprotective mechanisms of PCs is their suppression of platelet activity. Numerous studies have confirmed that PCs can inhibit platelet adhesion, activation, aggregation, and degranulation by acting on different thrombogenic pathways, such as glycoprotein VI (GPVI)–collagen, COX-1–thromboxane, P2Y1/P2Y12–ADP, and protease-activated receptor 1 (PAR1)–thrombin pathways [31].
It has been reported in numerous studies that certain PCs, such as flavan-3-ols, flavanols, and tannins, possess significant antimicrobial (antiviral, antifungal, and antibacterial) activity against many human pathogens, or express synergistic effects with various antibiotics [8]. Additionally, it is known that phenolic acids such as p-hydroxybenzoic acid, syringic acid, and gallic, ferulic, and caffeic acid possess high antibacterial activity against several different bacteria [31]. However, the structure–activity relationships and mechanisms underlying the antibacterial activity of PCs, as well as their antibiofilm and antiquorum-sensing activity, have been poorly investigated [32][33].
PCs induce apoptosis and cell cycle arrest and express antiproliferative effects against many human cancer cell lines [9][34]. Thus, certain PCs could be considered as safe and effective agents in cancer prevention and therapy. Additionally, PCs improve the efficacy of chemotherapeutic therapy, reduce the side-effects of chemotherapy, and help to by-pass cancer drug resistance, as well as express antiangiogenic and antimetastatic effects [35][36]. Several studies have confirmed that their anti-cancer efficacy can be enhanced by combining several different PCs instead of using a single one, since they have multiple targets and can exhibit synergistic effects [37].
However, most of the data confirming the biological activity of PCs have been obtained from in vitro studies which were performed using pure phenolic compounds (“parent” PCs) or plant extracts rich in PCs. In these studies, the bioavailability and biotransformation of PCs after ingestion were not taken into consideration. It is known that the bioavailability of many PCs after oral intake is very low and that they are extensively metabolized. Thus, it is difficult to obtain reliable conclusions for any “real” health-promoting properties of PCs based on in vitro studies that were carried out with “parent” PCs compounds. Both aspects—biological activity and bioavailability—are rarely investigated at the same time, meaning that the biological activities of PC metabolites, which are actually present in human blood and tissues, are almost fully unknown. Claiming a high biological activity of PCs based on in vitro studies of parent PCs can mislead companies from the food and pharmaceutical industries to develop products rich in PCs that will not exhibit the desired health benefits in humans after oral consumption. Thus, further studies confirming the biological activities of PC metabolites need to be carried out.
PCs as Antioxidants in Human Health and Disease
Due to their high antioxidant activity, PCs have for a long time been considered powerful agents for protection from oxidative stress and associated pathologies such as cancers, inflammation, cardiovascular, and neurodegenerative diseases. Free radical species occur in the course of numerous physiological processes or under different exogenous factors and can initiate the damage of nucleic acids, lipids, and proteins, resulting in the disturbance of vital cellular functions and causing a wide range of disorders. PCs can exert antioxidant activity by directly scavenging a wide range of highly toxic reactive oxygen species (ROS), suppressing ROS formation by inhibiting enzymes or by complexing metal ions involved in ROS production, and upregulating or protecting the cellular antioxidant defence system [10]. The influence of PCs on the cellular antioxidant defence system is based on the inhibition of xanthine oxidase, the induction of glutathione peroxidase (GPX), catalase, and superoxide dismutase (SOD), and the elevation of endogenous antioxidants [38][39]. By inhibiting lipid peroxidation, PCs protect cell membranes from degradation and enhance the stability of cells against lysis [40]. Additionally, PCs may inhibit the oxidation of low-density lipoprotein (LDL), thus preventing the occurrence of arthrosclerosis [41].
Due to their strong antioxidant activity, PCs have many healthful actions in the human body, such as lowering blood pressure and the risk of cardiovascular diseases, and decreasing the occurrence of neurodegeneration and carcinogenesis [28].
The glucuronidation of PCs is considered to be the dominant type of metabolism in the small intestine; thus, glucuronides are the major circulating forms of PCs. However, β-glucuronidases, which cleave glucuronides back to aglycones, have been confirmed in different tissues and body fluids, such as the lungs, liver, and serum, as well as in macrophages and other blood cells [42][43]. Therefore, it is likely that active parent aglycones are present in vivo in a variety of different tissues and cell populations via the action of β-glucuronidases, which reconvert metabolites [44][45].
Additionally, recirculation via enterohepatic pathways (hepatic excretion, enteric deconjugation, and intestinal reabsorption) can be responsible for the slower excretion and longer presence of active PCs in the body [46].
3. Bioavailability of PCs
The health properties of PCs are highly dependent on their metabolic transformation in the human body. Since the first data indicating that the bioavailability of PCs can be very low, the interest in evaluating the bioavailability of PCs has increased. For many PCs, their metabolites are identified and quantified in in vitro and in vivo studies [47]. It has been concluded that the chemical structure of PCs dictates their solubility, absorption in the gut, metabolic transformation, and bioavailability. It is, therefore, essential to know the exact chemical structure of the PCs and the amounts in which they are consumed in order to predict their bioavailability. Bioavailability refers to the rate and extent to which a polyphenol is absorbed and becomes available at the site of action. In general, PCs have low bioavailability, meaning that low amounts of unmetabolized polyphenol—the parent molecule—enters the systemic blood circulation and reaches the target tissue. The bioavailability of PCs can vary drastically among different PCs classes, as well as between individual compounds in a particular class.
It is considered that less than 5% of the total PC intake is absorbed and reaches the plasma unchanged [48]. In most cases, these unchanged PCs are undetectable even using highly-sensitive analytical techniques, since their plasma levels are too low to supply target cells with an efficient concentration of PCs [49]. Instead of this, PCs undergo extensive metabolic changes in the intestine or colon, forming a wide variety of new chemical structures (metabolites) [50][51]. After this, the metabolites are absorbed and further metabolized within enterocytes and in the liver by phase I and phase II enzymes (Figure 3). Thus, after ingestion, the PCs metabolites, whose structures can be very different from their parent molecules, reach the target tissue and may or may not be bioactive. PCs are mainly excreted through bile, while renal excretion is usually a minor route of elimination [52].
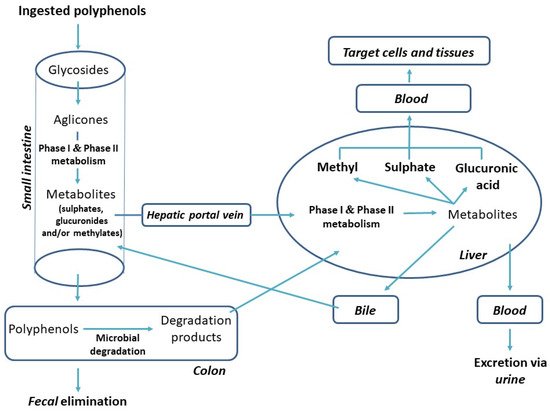
Figure 3. Biotransformation of PCs after oral intake [13].
Following their ingestion, most polyphenol aglycones are absorbed in the small intestine unmetabolized, via passive diffusion from the intestinal lumen into the enterocytes, as they are non-polar molecules (Figure 3). However, PCs are predominantly present in plants in the form of glycosides. Glycosylation patterns determine the digestion, absorption, and metabolism of PC glycosides. Most of them are partially hydrolysed via the enzymatic activity of lactase phloridizin hydrolase (LPH), which is part of the apical brush-border epithelial cells (enterocytes) present in the small intestine. This is then followed by the entrance of the released aglycones into the epithelial cells via the process of passive diffusion. Alternatively, glucosides use sodium-dependent glucose transporter SGLT1 to get imported into enterocytes, where cytosolic β-glucosidase (CBG) hydrolyses them to aglycones. In epithelial cells, the aglycones are partially transformed into sulphate, glucuronide, or methyl conjugates. These aglycone metabolites are then transported to the hepatic portal vein by ATP-binding cassette (ABC) transporters and carried by the blood into the liver, where the conjugation processes are continued. The conjugation of PCs is a metabolic detoxification process that facilitates their biliary and urinary elimination by increasing their hydrophilic character. The resulting conjugates are then transported, together with other intestinal metabolites, into the systemic circulation, from where they will be distributed to the different organs and tissues. The PCs that are not absorbed in the small intestine reach the colon, where the colonic microflora hydrolyses glycosides into aglycones and degrades them into more simple degradation products—for example, 3-hydroxyphenylacetic, 3,4-dihydroxybenzoic, 3,4-dihydroxyphenylacetic, 3-(3-hydroxyphenyl)-propionic, 3-phenylpropionic, and 3-(4-hydroxyphenyl)-propionic acids [10][47][53]. These listed metabolites are further excreted out of the body via the faeces or taken into the blood circulation, where after conjugation in the liver, they would ready again to reach target tissues and express their biological activity.
In in vivo studies, 23 metabolites of quercetin have been identified. The main metabolites of quercetin in the plasma are quercetin-3′-sulfate, quercetin-3-sulfate, and quercetin-3-glucuronide. However, the urinary metabolites of quercetin have been found to be quercetin-3′-glucuronide, quercetin diglucuronide, isorhamnetin-glucuronide sulphate, isorhamnetin-glucuronide, and isorhamnetin-methyl quercetin diglucuronide [54]. Quercetin-3-O-glucoside is absorbed mainly in its native form—the same as rutin (also known as quercetin-3-O-rutinoside), which cannot be hydrolysed or glucuronidated in the small intestine at all [55]. The half-lives of elimination of quercetin and its glycosides from the body are considered to be very slow, ranging from 15 to 28 h—indicating that these compounds could be accumulated in tissues long enough to express biological activity [56].
After ingestion of food rich in kaempferol, four different metabolites can be identified, where kaempferol mono- and di-sulphates have been detected only in urine, while kaempferol aglycone and kaempferol-3-glucuronide were found in plasma [10].
Apigenin-glycosides are hydrolysed by β-glucosidases in the stomach and small intestines to generate apigenin. Apigenin can be directly absorbed into the systemic blood circulation, or may firstly undergo downstream phase I and II metabolism in the small intestines and liver to generate hydroxylated metabolites such as luteolin, or glucuronidated and sulfonated metabolites [14]. Luteolin 7-O-β-glucoside is first hydrolysed to luteolin in the small intestine and then converted to glucuronides by passing through intestinal mucosa, and are absorbed in the form glucuronides, while a certain amount of luteolin is absorbed in the form of free aglycone [57].
After oral intake of naringenin-7-O-glucoside, it is hydrolysed by an intestinal enzyme to aglycone naringenin and then glucuronidated within the epithelium, and afterwards, is largely esterified to sulphate in the liver [15][58]. Naringenin-7-rhamnoglucoside (naringin) cannot be hydrolysed by β-glucosidase in the small intestine, but rather only by colonic microflora. Liberated aglycone is then absorbed in the colon [15].
Concerning the bioavailability of flavan-3-ols, it has been observed that after consumption of red wine containing catechins and epicatechins, the concentration of 3′-O-methyl-catechin, sulphate, and glucuronide metabolites in plasma increases [59]. It has been shown that epicatechin and catechin are extensively O-methylated during their transfer across the jejunum, due to the activity of catechol-O-methyltransferases [45]. After absorption by epithelial cells, they may be further glucuronidated, so that they enter the blood circulation in O-methylated and glucuronidated forms. Procyanidins (oligomers and polymers of catechin and epicatechin) are unstable under the acidic environment conditions of the gastric milieu and essentially decompose to epicatechin or catechin monomers and dimmers, which are further metabolized by the previously mentioned pathway [60].
Stilbene resveratrol is absorbed after oral consumption to a very high extent (75%) [61]. After absorption, resveratrol is conjugated with glucuronic acid, forming mainly resveratrol-4′-O-glucuronide or resveratrol-3-O-glucuronide, followed by the formation of resveratrol-4′-O-sulfate, resveratrol-3-O-sulfate, and resveratrol disulfates in the liver [21][44]. Intestinal bacteria also contribute to resveratrol metabolism, converting it to dihydroresveratrol (which is partially absorbed and conjugated) and two other microbial-derived metabolites—3,4′-dihydroxy-trans-stilbene and 3,4′-dihydroxybibenzyl. Resveratrol is mainly excreted in the urine and faeces [21].
After oral administration, curcumin undergoes limited absorption in the small intestine, followed by metabolism in enterocytes and hepatocytes to dihydrocurcumin, tetrahydrocurcumin (THC—the major metabolite), hexahydrocurcumin (HHC), and octahydrocurcumin (OHC) by alcohol dehydrogenases. Curcumin and its reductive metabolites can be easily conjugated by phase II metabolism to monoglucuronides, monosulphates, and mixed glucuronide/sulphates. Curcumin is excreted through faeces, with minimal elimination in the urine [21].
PCs are commonly mixed with different macromolecules such as carbohydrates, lipids, and proteins, forming a food matrix. There is evidence indicating that food microstructures affect the bio-accessibility and bioavailability of PCs. Thus, urgent research is needed to determine PCs’ bioavailability from different foods [62].
Despite their poor bioavailability, PCs are undoubtedly responsible for many biological effects, which have been proven in vivo—mainly in animals. This low bioavailability/high bioactivity paradox can be explained by the high activity of PC metabolites. The biological activities of PC metabolites have rarely been investigated. As recent studies have shown, metabolites derived from certain dietary PCs elicit significant intrinsic bioactivities that could explain the effects observed for their parent compounds [63][64]. Additionally, the antioxidant and anti-inflammatory activities of some quercetin metabolites (quercetin-3-O-glucuronide, 4′-O-methylquercetin, and 3′-O-methylquercetin (isorhamnetin)) have been investigated. It was shown that all the investigated metabolites demonstrated a notable antioxidant activity, but one lower than that of quercetin aglycone, while 4′-O-methylquercetin expressed a significantly superior anti-inflammatory potential compared with quercetin and other metabolites, via its inhibition of the COX and 12-LOX enzymes [65].
The protective effects of epicatechin and one of its major metabolites—3′-O-methyl epicatechin—against cell death induced by H2O2 has been evaluated [60]. The results indicated that both compounds similarly protected cells from death via suppression of caspase-3 activity. Resveratrol metabolites were found to possess in vitro cytotoxic, anti-inflammatory, antioxidant, and delipidating properties [21]. The reductive metabolites of curcumin THC, HHC, and OHC express stronger antioxidant activity than curcumin. Curcumin metabolites also possess anti-inflammatory activity, but weaker than that of curcumin. Furthermore, THC exhibits antiproliferative, antiangiogenic, and chemo-preventive effects, as well as HHC anticarcinogenic effects, while curcumin glucuronide and curcumin sulphate show lower antitumor activity than curcumin. Additionally, THC possesses antidiabetic activity, while HHC contributes to curcumin’s cardioprotective effects, as it possesses anti-platelet aggregation properties and can enhance its anti-atherosclerotic effects [21].
To establish a conclusion on the efficiency of PCs in the prevention of human disease and improvement of human well-being, data regarding its bioavailability and the biological activity of its metabolites are needed. However, in the literature, these data can be found only for very small number of polyphenolic compounds.
References
- Li, A.N.; Li, S.; Zhang, Y.J.; Xu, X.R.; Chen, Y.M.; Li, H.B. Resources and biological activities of natural polyphenols. Nutrients 2014, 6, 6020–6047.
- Grosso, G.; Stepaniak, U.; Topor-Mądry, R.; Szafraniec, K.; Pająk, A. Estimated dietary intake and major food sources of polyphenols in the Polish arm of the HAPIEE study. Nutrition 2014, 30, 1398–1403.
- Robards, K. Strategies for the determination of bioactive phenols in plants, fruit and vegetables. J. Chromatogr. A 2003, 1000, 657–691.
- Lattanzio, V.; Lattanzio, V.M.T.; Cardinali, A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. In Phytochemistry Advances in Research; Imperato, F., Ed.; Research Signpost: Trivandrum, India, 2006; pp. 23–67.
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20.
- Dictionary of Natural Products. Available online: https://dnp.chemnetbase.com/HelpFiles/DNP_Introduction.pdf (accessed on 14 December 2021).
- Chun, O.K.; Chung, S.J.; Song, W.O. Estimated dietary flavonoid intake and major food sources of U.S. adults. J. Nutr. 2007, 137, 1244–1252.
- Xiao, J.; Muzashvili, T.S.; Georgiev, M.I. Advances in the biotechnological glycosylation of valuable flavonoids. Biotechnol. Adv. 2014, 32, 1145–1156.
- Lee, J.; Mitchell, A.E. Pharmacokinetics of quercetin absorption from apples and onions in healthy humans. J. Agric. Food Chem. 2012, 60, 3874–3881.
- Dabeek, W.M.; Marra, M.V. Dietary quercetin and kaempferol: Bioavailability in humans. Nutrients 2019, 11, 2288.
- Bai, W.; Wang, C.; Ren, C. Intakes of total and individual flavonoids by US adults. Int. J. Food Sci. Nutr. 2014, 65, 9–20.
- Okamoto, T. Safety of quercetin for clinical application. Int. J. Mol. Med. 2005, 16, 275–278.
- Thilakarathna, S.H.; Rupasinghe, H.P.V. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 2013, 5, 3367–3387.
- De-Rango-Adem, E.F.; Blay, J. Does oral apigenin have real potential for a therapeutic effect in the context of human gastrointestinal and other cancers? Front. Pharmacol. 2021, 12, 1196.
- Felgines, C.; Texier, O.; Morand, C.; Manach, C.; Scalbert, A.; Regerat, F.; Remesy, C. Bioavailability of the flavanone naringenin and its glycosides in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 279, G1148–G1154.
- Somerset, S.M.; Johannot, L. Dietary flavonoid sources in Australian adults. Nutr. Cancer 2008, 60, 442–449.
- Zamora-Ros, R.; Andres-Lacueva, C.; Lamuela-Raventós, R.M.; Berenguer, T.; Jakszyn, P.; Barricarte, A.; Ardanaz, E.; Amiano, P.; Dorronsoro, M.; Larrañaga, N.; et al. Estimation of dietary sources and flavonoid intake in a Spanish adult population (EPIC-Spain). J. Am. Diet. Assoc. 2010, 110, 390–398.
- Vang, O.; Ahmad, N.; Baile, C.A.; Baur, J.A.; Brown, K.; Csiszar, A.; Das, D.K.; Delmas, D.; Gottfried, C.; Lin, H.Y.; et al. What is new for an old molecule? Systematic review and recommendations on the use of resveratrol. PLoS ONE 2011, 6, 19881.
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A double-edged sword in health benefits. Biomedicines 2018, 6, 91.
- Mahmood, K.; Zia, K.M.; Zuber, M.; Salman, M.; Anjum, M.N. Recent developments in curcumin and curcumin based polymeric materials for biomedical applications: A review. Int. J. Biol. Macromol. 2015, 81, 877–890.
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659.
- Rasouli, H.; Farzaei, M.H.; Khodarahmi, R. Polyphenols and their benefits: A review. Int. J. Food Prop. 2017, 20, 1700–1741.
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159.
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotech. 2012, 174–181.
- Han, X.; Shen, T.; Lou, H. Dietary polyphenols and their biological significance. Int. J. Mol. Sci. 2007, 8, 950–988.
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797.
- Butterfield, D.; Castegna, A.; Pocernich, C.; Drake, J.; Scapagnini, G.; Calabrese, V. Nutritional approaches to combat oxidative stress in Alzheimer’s disease. J. Nutr. Biochem. 2002, 13, 444–461.
- Sharma, R. Polyphenols in health and disease. Practice and Mechanisms of Benefits. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Acedemic Press: Cambridge, MA, USA, 2014; Volume 1, pp. 757–778.
- Beara, I.N.; Orcić, D.Z.; Lesjak, M.M.; Mimica-Dukić, N.M.; Peković, B.A.; Popović, M.R. Liquid chromatography/tandem mass spectrometry study of anti-inflammatory activity of plantain (Plantago L.) species. J. Pharm. Biomed. Anal. 2010, 52, 701–706.
- Simin, N.; Orcic, D.; Cetojevic-Simin, D.; Mimica-Dukic, N.; Anackov, G.; Beara, I.; Mitic-Culafic, D.; Bozin, B. Phenolic profile, antioxidant, anti-inflammatory and cytotoxic activities of small yellow onion (Allium flavum L. subsp. flavum, Alliaceae). LWT Food Sci. Technol. 2013, 54, 139–146.
- Nignpense, B.E.; Chinkwo, K.A.; Blanchard, C.L.; Santhakumar, A.B. Polyphenols: Modulators of platelet function and platelet microparticle generation? Int. J. Mol. Sci. 2020, 21, 146.
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356.
- Slobodníková, L.; Fialová, S.; Rendeková, K.; Kováč, J.; Mučaji, P. Antibiofilm activity of plant polyphenols. Molecules 2016, 21, 1717.
- Horáková, Ľ. Flavonoids in prevention of diseases with respect to modulation of Ca-pump function. Interdiscip. Toxicol. 2011, 4, 114–124.
- Pavic, A.; Mitic-Culafic, D.; Jasnic, N.; Nikolic, B.; Simin, N.; Vasiljevic, B.; Knezevic-Vukcevic, J. Wild edible onions—Allium flavum and Allium carinatum—successfully prevent adverse effects of chemotherapeutic drug doxorubicin. Biomed. Pharmacother. 2019, 109, 2482–2491.
- Rengarajan, Y.; Yaacob, N.S. The flavonoid fisetin as an anticancer agent targeting the growth signaling pathways. Eur. J. Pharmacol. 2016, 789, 8–16.
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer efficacy of polyphenols and their combinations. Nutrients 2016, 8, 552.
- Disilvestro, R.A. Flavonoids as antioxidants. In Handbook of Nutraceuticals and Functional Foods; Wildman, R.E.C., Ed.; CRC Press: Boca Raton, FL, USA, 2001; pp. 127–142.
- Mitic-Culafic, D.; Nikolic, B.; Simin, N.; Jasnić, N.; Četojević-Simin, D.; Krstić, M.; Knežević-Vukčević, J. Effect of Allium flavum L. and Allium melanantherum Panč. extracts on oxidative DNA damage and antioxidative enzymes superoxide dismutase and catalase. Plant Foods Hum. Nutr. 2016, 71, 28–34.
- Chaudhuri, S.; Banerjee, A.; Basu, K.; Sengupta, B.; Sengupta, P.K. Interaction of flavonoids with red blood cell membrane lipids and proteins: Antioxidant and antihemolyic effects. Int. J. Biol. Macromol. 2007, 41, 42–48.
- Lian, T.W.; Wang, L.; Lo, Y.H.; Huang, I.J.; Wu, M.J. Fisetin, morin and myricetin attenuate CD36 expression and oxLDL uptake in U937-derived macrophages. Biochim. Biophys. (BBA)-Mol. Cell. Biol. Lipids 2008, 1781, 601–609.
- Sperker, B.; Backman, T.; Kroemer, H.K. The role of β-glucuronidase in drug disposition and drug targeting in humans. Clin. Pharmacokinet. 1997, 33, 18–31.
- Paigen, K. Mammalian β-glucuronidase: Genetics, molecular biology and cell biology. Prog. Nucleic Acid Res. Mol. Biol. 1989, 37, 155–205.
- Kuhnle, G.; Spencer, J.P.; Chowrimootoo, G.; Schroeter, H.; Debnam, E.S.; Srai, S.K.; Rice-Evans, C.; Hahn, U. Resveratrol is absorbed in the small intestine as resveratrol glucuronide. Biochem. Biophys. Res. Commun. 2000, 272, 212–217.
- Kuhnle, G.; Spencer, J.P.E.; Schroeter, H.; Shenoy, B.; Debnam, E.S.; Srai, S.K.; Rice-Evans, C.; Hahn, U. Epicatechin and catechin are o-methylated and glucuronidated in the small intestine. Biochem. Biophys. Res. Commun. 2000, 277, 507–512.
- Gambini, J.; Ingles, M.; Olaso, G.; Lopez-Grueso, V.R.; Bonet-Costa, L.; Gimeno-Mallench, C.; Mas-Bargues, K.M.; Abdelaziz, M.C.; Gomez- Cabrera, J.; Vina, B.C. Properties of resveratrol: In vitro and in vivo studies about metabolism, bioavailability, and biological effects in animal models and humans. Oxid. Med. Cell. Longev. 2015, 2015, 837042.
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342.
- Cao, H.; Jia, X.; Shi, J.; Xiao, J.; Chen, X. Non-covalent interaction between dietary stilbenoids and human serum albumin: Structure-affinity relationship, and its influence on the stability, free radical scavenging activity and cell uptake of stilbenoids. Food Chem. 2016, 202, 383–388.
- Chiou, Y.S.; Wu, J.C.; Huang, Q.; Shahidi, F.; Wang, Y.J.; Ho, C.T.; Pan, M.H. Metabolic and colonic microbiota transformation may enhance the bioactivities of dietary polyphenols. J. Funct. Foods 2014, 7, 3–25.
- Zeka, K.; Ruparelia, K.; Arroo, R.R.J.; Budriesi, R.; Micucci, M. Flavonoids and their metabolites: Prevention in cardiovascular diseases and diabetes. Diseases 2017, 5, 19.
- Williamson, G.; Clifford, M.N. Role of the small intestine, colon and microbiota in determining the metabolic fate of polyphenols. Biochem. Pharmacol. 2017, 139, 24–39.
- Choudhury, R.; Srai, S.K.; Debnam, E.; Rice-Evans, C.A. Urinary excretion of hydroxycinnamates and flavonoids after oral and intravenous administration. Free Radic. Biol. Med. 1999, 278–286.
- Rechner, A.R.; Smith, M.A.; Kuhnle, G.; Gibson, G.R.; Debnam, E.S.; Srai, S.K.; Moore, K.P.; Rice-Evans, C.A. Colonic metabolism of dietary polyphenols: Influence of structure on microbial fermentation products. Free Radic. Biol. Med. 2004, 36, 212–225.
- Mullen, W.; Edwards, C.A.; Crozier, A. Absorption, excretion and metabolite profiling of methyl-, glucuronyl-, glucosyl- and sulpho-conjugates of quercetin in human plasma and urine after ingestion of onions. Br. J. Nutr. 2006, 96, 107–116.
- Spencer, J.P.; Chowrimootoo, G.; Choudhury, R.; Debnam, E.S.; Srai, S.K. The small intestine can both absorb and glucuronidate luminal flavonoids. FEBS Lett. 1999, 458, 224–230.
- Erlund, I.; Kosonen, T.; Alfthan, G.; Mäenpää, J.; Perttunen, K.; Kenraali, J.; Parantainen, J.; Aro, A. Pharmacokinetics of quercetin from quercetin aglycone and rutin in healthy volunteers. Eur. J. Clin. Pharmacol. 2000, 56, 545–553.
- Shimoi, K.; Okada, H.; Furugori, M.; Goda, T.; Takase, S.; Suzuki, M.; Hara, Y.; Yamamoto, H.; Kinae, N. Intestinal absorption of luteolin and luteolin 7-O-β-glucoside in rats and humans. FEBS Lett. 1998, 438, 220–224.
- Choudhury, R.; Chowrimootoo, G.; Srai, K.; Debnam, E.; Rice-Evans, C.A. Interactions of the flavonoid naringenin in the gastrointestinal tract and the influence of glycosylation. Biochem. Biophys. Res. Commun. 1999, 265, 410–415.
- Donovan, J.L.; Bell, J.R.; Kasim-Karakas, S.; German, J.B.; Walzem, R.L.; Hansen, R.J.; Waterhouse, A.L. Catechin is present as metabolites in human plasma after consumption of red wine. J. Nutr. 1999, 129, 1662–1668.
- Spencer, J.P.; Schroeter, H.; Kuhnle, G.; Srai, S.K.; Tyrrell, R.M.; Hahn, U.; Rice-Evans, C. Epicatechin and its in vivo metabolite, 3h-O-methyl epicatechin, protect human fibroblasts from oxidative-stress-induced cell death involving caspase-3 activation. Biochem. J. 2001, 354, 493–500.
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E., Jr.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377–1382.
- Palafox-Carlos, H.; Ayala-Zavala, F.; Gonzalez-Aguilar, G.A. The Role of Dietary Fiber in the bioaccessibility and bioavailability of fruit and vegetable antioxidants. J. Food Sci. 2011, 76, 6–15.
- Heleno, S.A.; Martins, A.; Queiroz, M.J.; Ferreira, I.C. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513.
- Teng, H.; Chen, L. Polyphenols and bioavailability: An update. Crit. Rev. Food Sci. Nutr. 2019, 59, 2040–2051.
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75.
More
Information
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Revisions:
2 times
(View History)
Update Date:
27 Jan 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




