You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Rong, S. Zinc-Alpha2-Glycoprotein (AZGP1) and Fibrotic Kidney Disease. Encyclopedia. Available online: https://encyclopedia.pub/entry/18767 (accessed on 22 December 2025).
Rong S. Zinc-Alpha2-Glycoprotein (AZGP1) and Fibrotic Kidney Disease. Encyclopedia. Available at: https://encyclopedia.pub/entry/18767. Accessed December 22, 2025.
Rong, Song. "Zinc-Alpha2-Glycoprotein (AZGP1) and Fibrotic Kidney Disease" Encyclopedia, https://encyclopedia.pub/entry/18767 (accessed December 22, 2025).
Rong, S. (2022, January 25). Zinc-Alpha2-Glycoprotein (AZGP1) and Fibrotic Kidney Disease. In Encyclopedia. https://encyclopedia.pub/entry/18767
Rong, Song. "Zinc-Alpha2-Glycoprotein (AZGP1) and Fibrotic Kidney Disease." Encyclopedia. Web. 25 January, 2022.
Copy Citation
Chronic Kidney Disease (CKD) is a condition characterized by a gradual loss of kidney function over time. It is typically accompanied by progressive fibrosis of the tubulointerstitial compartment.
AZGP1
chronic kidney disease
kidney fibrosis
renal lipid metabolism
1. Administration of Recombinant AZGP1 Attenuates Renal Fibrosis Development
On the basis of previous data [1][2], it was hypothesized that exogenous administration of AZGP1 may inhibit fibrotic processes in the kidney. To test this, it was induced experimental renal fibrosis by UUO in male C57Bl/6J mice treated with recombinant AZPG1 (rAZGP1) or with vehicle. After surgery, mice were injected intravenously with rAZGP1 (150 µg per injection) or vehicle every other day until day 11 (Figure 1A). On day 14, kidneys were harvested, and blood was taken to determine AZGP1 serum levels. Three days after the last injection, serum AZGP1 levels were still significantly higher in rAZGP1 treated mice (Figure 1B). This was accompanied by better preservation of proximal tubular integrity as shown by Lotus tetragonolobus lectin (LTL) binding (Figure 1C,D) and by reduced collagen deposition as shown by Picrosirius Red staining (Figure 1C,E). In parallel, it was observed significantly lower expression of Havcr1 (coding for kidney injury marker KIM-1), Acta2 (coding for the fibroblast activation marker α-smooth muscle actin; αSMA), and Col1a1 (coding for collagen type I) in kidneys of AZGP1 treated mice (Figure 1F–H). Together, these data indicated anti-fibrotic effects of rAZGP1 administration in kidneys challenged by UUO stress.
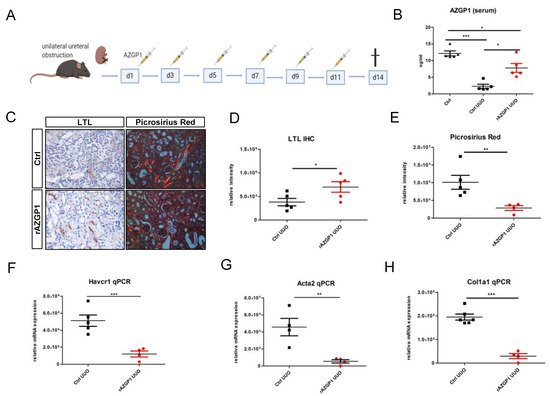
Figure 1. (A): Experimental design for the treatment with recombinant AZGP1 (rAZGP1) after UUO. (B): AZGP1 levels in serum of untreated mice without surgery and untreated and treated mice 14 days after UUO. (C): Lotus tetragonolobus lectin (LTL) staining and Picrosirius Red staining after 14 days UUO. (D). Quantification of LTL staining. (E): Quantification of Picrosirius Red staining. (F–H): Damage and fibrosis marker mRNA expression after 14 days UUO. Significance was tested by student’s t-test. * p < 0.05; ** p < 0.01; *** p < 0.001.
2. Transgenic AZGP1 Expression in Proximal Tubule Provides Protection in UUO
To consolidate the observed effects by an independent method, it was generated a mouse model with transgenic AZGP1 expression driven by a proximal tubule-specific promoter. Transgenic mice carrying murine Azgp1 cDNA with an upstream loxP-Stop-loxP sequence were crossed with Slc34a1-CreERT2 mice to achieve tamoxifen-inducible AZGP1 overexpression in proximal tubular cells (Figure 2A). Successful Cre recombination in proximal tubules was confirmed by additional tdTomato reporter gene co-expression (Figure 2B). Tamoxifen-induced overexpression of AZGP1 resulted in a mean 4,6-fold increase of Azgp1 transcript levels in control kidney homogenates. (Figure 2C). This increase in healthy control mice was smaller than anticipated and was only associated with a small but non-significant increase in serum AZGP1 (Figure 2D). Tamoxifen-induced transgenic mice and control mice underwent UUO surgery, and kidneys were examined after two weeks. AZGP1 transcript, as well as serum levels, showed a significant decrease after UUO (Figure 2C,D). While it was found no significant differences in tubular LTL binding or Picrosirius Red staining in the AZGP1 overexpressing mice (Figure 2E–G), it observed significantly reduced up-regulation of Havcr1, Acta2, and Col1a1 transcripts in the tamoxifen-treated group (Figure 2H–J). These data are consistent with a protective effect of transgenic tubular AZGP1 expression, although the observed differences were smaller as compared to the effects obtained with systemic rAZGP1 treatment.
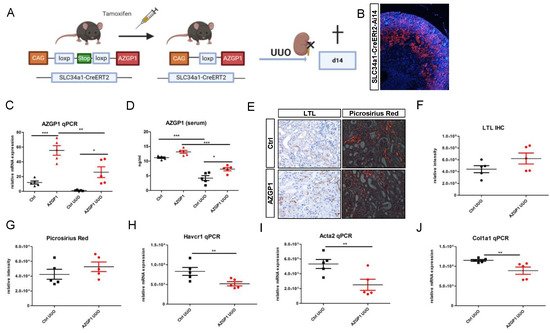
Figure 2. (A): Schematic of transgenic mouse model for conditional overexpression of AZGP1 in the proximal tubule. (B): tdTomato positive tubules showing Ai14 reporter expression of successful Cre recombination after tamoxifen administration. (C): AZGP1 mRNA expression levels in control and UUO kidneys at 2 weeks after tamoxifen treatment. (D): AZGP1 serum levels of AZGP1 overexpressing mice as measured by ELISA. (E): LTL staining and Picrosirius Red staining after 14 days of UUO. (F): Quantification of LTL staining (G): Quantification of Picrosirius Red. (H–J): Damage and fibrosis marker mRNA expression after 14 days UUO. Significance was tested by student’s t-test. * p < 0.05; ** p < 0.01; *** p < 0.001.
3. AZGP1 Reduces Intrarenal Lipid Accumulation and Acts as an Inducer of Key Players in Lipid Metabolism
It previously linked anti-fibrotic activities of AZGP1 to TGF-beta antagonizing mechanisms and reduced fibroblast activation [1][3][4]. Additional mechanisms, in particular a potential involvement of AZGP1 in altered lipid metabolism of the kidney, have not been explored. Given its properties as a lipid degradation factor, it evaluated changes in lipid accumulation in UUO kidneys. Quantification of Oil Red O staining revealed a significant reduction of tubular lipid droplet deposition in rAZGP1 treated kidneys (Figure 3A,B). Concomitantly, the expression of key players of tubular fatty acid oxidation (FAO) was significantly different between the groups. Quantitative PCR revealed elevated transcripts for carnitine palmitoyl-transferase 1A (Cpt1), peroxisome-proliferator-activated receptor α (Ppara), and PPARgamma coactivator-1a (Ppargc1a) in contralateral (con) as well as ligated (UUO) rAZGP1 treated kidneys (Figure 3C–E). These data suggested that AZGP1 plays a role in stabilizing renal tubular lipid balance and FAO during stress conditions. Accordingly, it was observed an inverse pattern in kidneys from AZGP1 knockout mice, which showed a significantly decreased expression of Cpt1, Ppara, and Ppargc1a (Figure 3F–H).
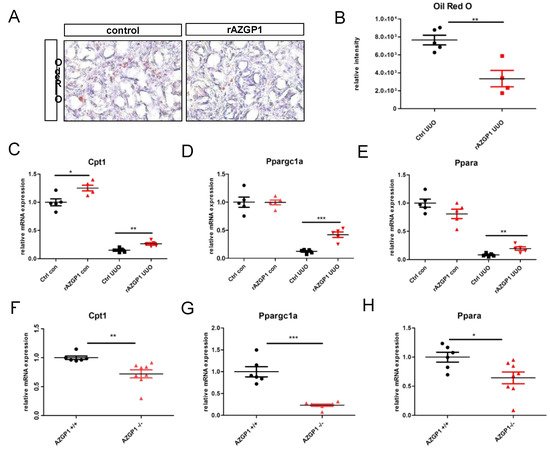
Figure 3. (A): Representative pictures of Oli Red O staining on rAZGP1 treated and control kidney cryosections after 14 days of UUO. (B): Quantification of Oil Red staining. (C–E). mRNA expression of carnitine palmitoyl-transferase 1A (Cpt1), PPARgamma coactivator-1a (Ppargc1a), and peroxisome-proliferator-activated receptor α (Ppara) in kidneys of AZGP1 treated mice. (F–H): mRNA expression of Cpt1, Ppargc1a, and Ppara in AZGP1 deficient mice. Significance was tested by student’s t-test. * p < 0.05; ** p < 0.01; *** p < 0.001.
4. Altered Lipid Metabolism in AZGP1 Deficient Tubular Cells
To further differentiate primary effects of AZGP1 from secondary changes due to UUO, it analyzed isolated primary tubular epithelial cells (PTEC) from kidneys of AZGP1 knockout and wildtype mice. In AZGP1 deficient PTEC, it was observed increased lipid droplets by Oil Red O staining (Figure 4A,B). The increased lipid accumulation was accentuated when PTEC were challenged with 0.4 mM palmitic acid (PA) for 24 h (Figure 4A,B). On the transcriptional level, knockout PTEC showed significantly lower levels of Cpt1 and Ppargc1a, as well as a trend for lower Ppara expression (Figure 4C–E). Together, these results provide evidence for the involvement of AZGP1 in tubular epithelial lipid metabolism.
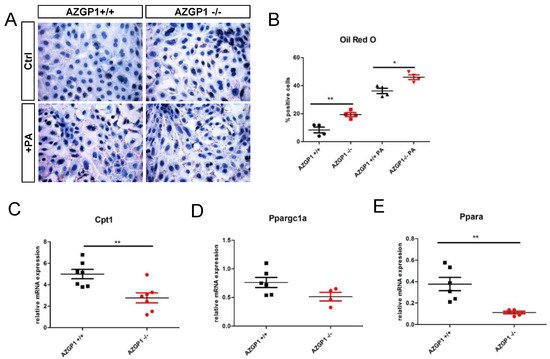
Figure 4. (A): Oil Red O staining in primary tubular epithelial cells (PTEC) isolated from wildtype or AZGP1 deficient mice untreated or treated with palmitic acid. (B): Quantification of Oil Red O staining in PTEC. (C–E): mRNA expression of Cpt1, Ppargc1a, and Ppara in wildtype and AZGP1 deficient PTEC. Significance was tested by student’s t-test. * p < 0.05; ** p < 0.01.
References
- Sörensen-Zender, I.; Bhayana, S.; Susnik, N.; Rolli, V.; Batkai, S.; Baisantry, A.; Bahram, S.; Sen, P.; Teng, B.; Lindner, R.; et al. Zinc-α2-glycoprotein exerts antifibrotic effects in kidney and heart. J. Am. Soc. Nephrol. 2015, 26, 2659–2668.
- Schmitt, R.; Marlier, A.; Cantley, L.G. Zag expression during aging suppresses proliferation after kidney injury. J. Am. Soc. Nephrol. 2008, 19, 2375–2383.
- Xu, M.Y.; Chen, R.; Yu, J.X.; Liu, T.; Qu, Y.; Lu, L.G. AZGP1 suppresses epithelial-to-mesenchymal transition and hepatic carcinogenesis by blocking TGFβ1-ERK2 pathways. Cancer Lett. 2016, 374, 241–249.
- Lin, B.; He, H.; Zhang, Q.; Zhang, J.; Xu, L.; Zhou, L.; Zheng, S.; Wu, L. Long non-coding RNA00844 inhibits MAPK signaling to suppress the progression of hepatocellular carcinoma by targeting AZGP1. Ann. Transl. Med. 2020, 8, 1365.
More
Information
Subjects:
Medicine, Research & Experimental
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
831
Revisions:
2 times
(View History)
Update Date:
26 Jan 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




