Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Haoyuan Zhang | + 1751 word(s) | 1751 | 2022-01-14 08:03:20 | | | |
| 2 | Rita Xu | Meta information modification | 1751 | 2022-01-25 09:17:04 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zhang, H. ACSL4 Directs Intramuscular Adipogenesis. Encyclopedia. Available online: https://encyclopedia.pub/entry/18751 (accessed on 07 February 2026).
Zhang H. ACSL4 Directs Intramuscular Adipogenesis. Encyclopedia. Available at: https://encyclopedia.pub/entry/18751. Accessed February 07, 2026.
Zhang, Haoyuan. "ACSL4 Directs Intramuscular Adipogenesis" Encyclopedia, https://encyclopedia.pub/entry/18751 (accessed February 07, 2026).
Zhang, H. (2022, January 25). ACSL4 Directs Intramuscular Adipogenesis. In Encyclopedia. https://encyclopedia.pub/entry/18751
Zhang, Haoyuan. "ACSL4 Directs Intramuscular Adipogenesis." Encyclopedia. Web. 25 January, 2022.
Copy Citation
In the livestock industry, intramuscular fat content is an important indicator of the meat quality of domestic animals. The variations of the Acyl-CoA Synthetase Long-Chain Family Member 4 (ACSL4) gene locus are associated with intramuscular fat content in different pig populations, but the detailed molecular function of ACSL4 in pig intramuscular adipogenesis remains obscure.
ACSL4
intramuscular
adipocyte
pig
1. Introduction
Intramuscular fat (IMF) content is an integral part of meat quality and directly influences meat tenderness, juiciness, and flavor. IMF refers to the chemically extractable fat inside the muscle, predominantly from intramuscular adipocytes, which are derived from preadipocytes that reside in the muscle [1]. However, the underlying mechanisms controlling the adipogenic differentiation and fat deposition of porcine intramuscular preadipocytes remain poorly understood, and obviously involve genetic, nutritional, and environmental factors [2].
The IMF content varies in different pig breeds and even in different individuals within the same breed populations. Dozens of functional or candidate genes have been identified and genetic polymorphisms associated with IMF have also been revealed [3]. Among them, the ACSL4 is one of the most frequently identified candidate genes related to IMF content in different population-based association studies in pigs [4][5][6][7][8]. The genomic variations around the ACSL4 locus control the ACSL4 transcription, but whether the fluctuations of ACSL4 expression levels could impact the intramuscular fat deposition remains unclear. A previous study reported that ACSL4 was involved in preadipocyte differentiation in pigs [9]. However, detailed functional validation of pig ACSL4 in the intramuscular adipogenesis was not thoroughly investigated.
The ACSL4 gene encodes fatty acid-CoA ligase 4, an isozyme of the long-chain fatty-acid-coenzyme ligase family. It converts free long-chain fatty acids into fatty acyl-CoA esters, and thereby plays a key role in lipid biosynthesis and fatty acid degradation [10]. Five isoforms of ACSL have been identified in humans and rodents, and they perform individual functions in fatty acid metabolism. ACSL4 showed a marked preference for arachidonic and eicosapentaenoic acid as substrates and play important roles in metabolic regulation of cell proliferation, differentiation and migration [11]. Dysregulation of ACSL4 has been demonstrated to promote ferroptosis in different cell types [12][13], and cause diverse diseases and disorders, such as hepatocellular carcinoma [14], breast cancer [15], intellectual disability [16], and mental dysfunction [17]. However, the expression dynamics and regulatory function of ACSL4 in adipogenesis or lipogenesis have not been thoroughly investigated.
2. The Isolation, Proliferation, and Differentiation of Porcine Intramuscular Preadipocytes
The differential velocity adherent technique was employed to isolate the intramuscular preadipocytes from the pig muscles. After their attachment to the surface of the culture dishes, the cells stretched out like fibroblasts in morphology and staggered protrusions between adjacent cells were easily observed (Figure 1A). In the growth media, the intramuscular fat precursor cells showed an S-shaped growth curve (Figure 1B). The cells proliferated robustly during the first 2 to 3 days of inoculation, and reached the plateau phase after 4 days. In order to examine the fat deposition and lipid droplet morphology in the cultured intramuscular adipocytes, the cell differentiation was induced and stained with Oil Red O. After 3 days of induction, a small amount of lipid droplets was detected (Figure 1C). The abundance of lipid droplets gradually increased from day 3 to day 9, and a large number of lipid droplets appeared on day 9 (Figure 1C). Therefore, we successfully established the intramuscular adipogenesis system with porcine intramuscular pre-adipocytes for subsequent molecular and cellular functional studies.
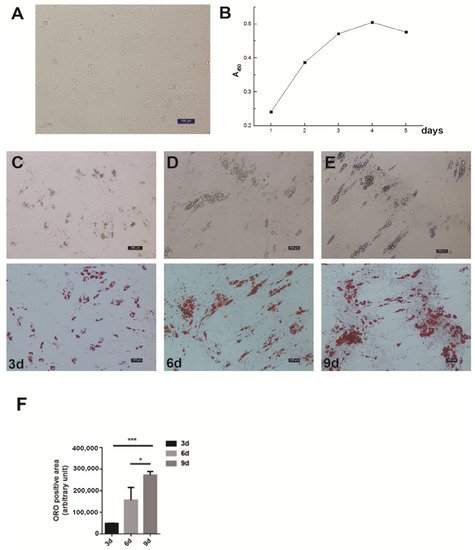
Figure 1. Isolation, proliferation and differentiation of pig intramuscular preadipocytes. (A) Purified intramuscular preadipocytes from pig skeletal muscle. (B) The proliferation dynamics of porcine intramuscular preadipocytes in culture. (C–E) The adipogenic differentiation of porcine intramuscular preadipocytes at 3, 6, and 9 days. The upper panels are bright view and the lower panels are Oil Red O staining. (Scale bars, 50 µm). (F) The quantitation of Oil Red O (ORO)-positive region for porcine intramuscular preadipocytes at 3, 6, and 9 days. Data are expressed as means + SEM. * indicated p < 0.05, *** indicated p < 0.001.
3. The Spatial-Temporal Expression Pattern of Pig ACSL4 Gene
We first examined the expression of ACSL4 gene in different tissues from the developing animals which are depositing intramuscular fat aggressively. We found relatively high expression of ACSL4 in the liver, lung and spleen (Figure 2A), suggesting that the liver is the major organ for fatty acid synthesis. However, the ACSL4 expression in the muscle tissues was much lower compared to other organs, indicating that ACSL4 is only expressed in specific type of cells within the bulk muscle tissue, or it is not the predominant fatty acid-CoA ligase isoform in the developing muscle. Therefore, we further validated the ACSL4 expression in the purified intramuscular preadipocytes during the adipogenic differentiation program. We detected both the mRNA and protein expression of ACSL4 in the preadipocytes purified from the pig skeletal muscle. The mRNA and protein levels of ACSL4 gradually increased and peaked on day 3 during the adipogenic differentiation process (Figure 2B,C), when most of preadipocytes differentiated into adipocytes. Subsequently, the expression of ACSL4 showed a steady declining trend, suggesting that ACSL4 was induced in the early stage of adipogenic differentiation and remained low during adipocyte maturation.
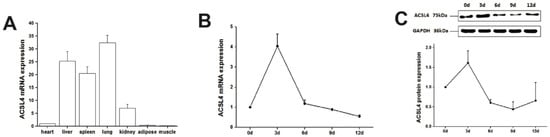
Figure 2. The pig ACSL4 expression pattern in different tissues and during adipogenic differentiation. (A) The ACSL4 gene expression in different pig tissues including heart, liver, spleen, lung, kidney, fat, and skeletal muscle. (B) The mRNA expression levels of pig ACSL4 during adipogenic differentiation of intramuscular preadipocytes. (C) The protein levels of pig ACSL4 during adipogenic differentiation of intramuscular preadipocytes as shown by Western blot. Data are expressed as means + SEM.
4. Knockdown of ACSL4 in Intramuscular Preadipocytes Impairs Fat Deposition and Cell Development
Next, we wondered whether the knockdown of ACSL4 gene in intramuscular preadipocytes cells affects the gene expression associated with fat deposition. We performed RNA-seq to measure the expression profiles of intramuscular preadipocyte cells transfected with siRNA-NC and siRNA-ACSL4. The RT-qPCR and Western blot showed that the knockdown efficiency could reach more than 70% and 80% for mRNA and protein levels, respectively (Figure 3A,B). Next, we performed transcriptome analysis in the ACSL4 knockdown cells and the control cells to discover the differentially expressed genes (DEG) and related pathways caused by the ACSL4 loss-of-function. A total of six RNA-seq libraries were constructed. Compared to the control, 134 genes were differentially expressed in the ACSL4 knockdown cells (Fold Change ≥ 2, p < 0.05). Among the DEGs, we identified that many adipogenesis-related genes were downregulated, including MOGAT1 [18], IL10 [19], TFAP2B [20], and ACSL6 [21], while several negative regulators of adipogenesis, such as FABP4 [22] and SST [23], were upregulated (Figure 3C). The KEGG pathway analysis of the DEGs shows that the majority of differentially expressed genes were enriched in signaling pathways that are important during adipogenesis, such as “response to ATP” and “cellular response to growth factor stimulus” [24]. Interestingly, several cellular development and differentiation pathways, such as “cell exploration behavior” and “multicellular organismal response to stress” were also enriched in the KEGG analysis of the DEGs (Figure 3D), indicating that ACSL4 could be involved in basic cell development and differentiation processes. These results indicate that ACSL4 is necessary for the normal adipocyte differentiation and could be responsible for lipid composition of cell membrane by fatty acid oxidation or lipid production.
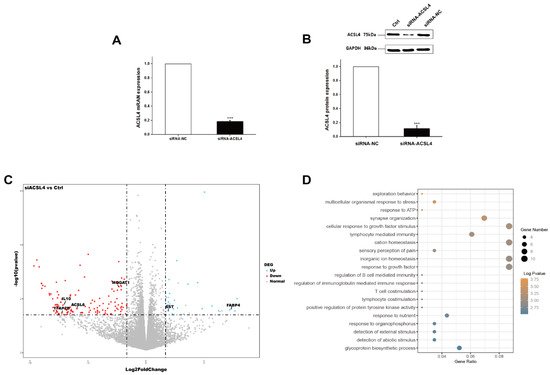
Figure 3. Transcriptome analysis of siRNA mediated ACSL4 knockdown in the pig intramuscular preadipocytes. (A) Realtime PCR showed ACSL4 gene was dramatically downregulated after siRNA knockdown. (B) Western blot indicated ACSL4 protein was dramatically reduced after siRNA knockdown compared to control. (C) Differentially expressed genes after ACSL4 knockdown as shown by the volcano plot. (D) KEGG pathway analysis of the differentially expressed genes after ACSL4 knockdown. Data are presented as the mean ± SEM. Comparisons were performed by unpaired two-tailed Student’s t-tests. *** indicated p < 0.001.
5. Overexpression of ACSL4 Results in Enhanced Lipid Deposition and Elevated Polyunsaturated Fatty Acids Synthesis in Pig Intramuscular Adipocyte
To further illustrate the function of ACSL4 in intramuscular adipogenesis and lipogenesis, we performed an adenovirus-mediated overexpression of ACSL4 and evaluated the lipid deposition and composition in pig intramuscular preadipocytes. Compared to the control, the transduction of the cells with Adeno-ACSL4 dramatically increased the ACSL4 protein expression and lipid deposition, as shown by the Western blot (Figure 4A) and Oil Red O staining (Figure 4B). We also observed significantly elevated adipogenic marker gene expression including FASN, ACACB, and C/EBPα (Figure 4C). Thus, it is concluded that ACSL4 is a positive regulator of intramuscular adipogenesis in pigs.
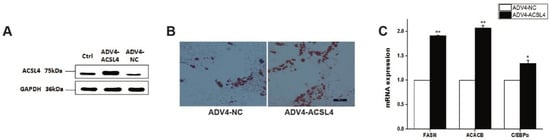
Figure 4. Overexpression of pig ACSL4 gene increased adipogenesis and lipid deposition in pig intramuscular preadipocytes. (A) Western blot showed that ACSL4 protein increased after adenovirus mediated overexpression. (B) Oil Red O staining showed that ACSL4 overexpression stimulated lipid deposition. (C) Realtime PCR showed that adipogenic genes FASN, ACACB, C/EBPa increased significantly after ACSL4 overexpression. Bars are presented as the mean ± SEM. Comparisons were performed by unpaired two-tailed Student’s t-tests. * indicated p < 0.05 and ** indicated p < 0.01.
Next, to understand how ACSL4 was involved in the de novo lipogenesis in pig muscle, we applied mass spectrometry-based lipidomics to determine how ACSL4 overexpression affects the composition and distribution of fatty acids in intramuscular preadipocytes. The quantification of the lipid classes revealed dramatic ACSL4-induced changes in the abundance of lipid species in several of the analyzed lipid classes. The overall abundance of saturated fatty acids (SFAs), which are the most abundant lipid classes in intramuscular adipocytes, was not affected by ACSL4 overexpression (Table 1). By contrast, we detected an approximately 22% and 29% increase in the concentration of mono- and polyunsaturated fatty acids (MUFA and PUFA, respectively) in response to ACSL4 overexpression (Table 1). Further analysis showed that this increase was caused by increased levels of MUFA containing C16:1, C18:1, and C24:1 and PUFA containing C18:2, C18:3N6, and C20:4N6 (Table 1). Both the n-3 fatty acid and n-6 fatty acid increased after ACSL4 overexpression but the n-6/n-3 ratio remained stable.
Table 1. The fatty acid composition of samples.
| Fatty Acid | ADV4-ACSL4 (μg/1 × 107 Cells) |
ADV4-NC (μg/1 × 107 Cells) |
|---|---|---|
| Saturated fatty acid | 50.63 | 49.07 |
| Monounsaturated fatty acid | 24.38 | 19.98 |
| Polyunsaturated fatty acid | 37.87 | 29.27 |
| n-3 fatty acid | 19.35 | 14.55 |
| n-6 fatty acid | 15.00 | 12.023 |
| n-6/n-3 fatty acid | 0.78 | 0.83 |
| C14:0 (Myristic acid) | 0.83 | 0.63 |
| C14:1 (Myristoleic acid) | 0.22 | 0.23 |
| C15:0 (Pentadecanoic acid) | 0.80 | 0.62 |
| C15:1 (Pentadecenoic acid) | 0.14 | 0.14 |
| C16:0 (Palmitic acid) | 21.84 | 21.99 |
| C16:1 (Palmitoleic acid) | 1.69 | 1.30 |
| C17:0 (Heptadecanoic acid) | 1.30 | 1.04 |
| C17:1 (Heptadecenoic acid) | 0.58 | 0.41 |
| C18:0 (Stearic acid) | 24.54 | 23.61 |
| C18:1 (Oleic acid) | 16.63 | 13.73 |
| C18:2 (Linoleic acid) | 2.58 | 1.89 |
| C18:3N6 (γ- linolenic acid) | 0.24 | 0.16 |
| C18:3N3 (Linolenic acid) | 0.13 | 0.11 |
| C20:0 (Arachidic acid) | 0.69 | 0.64 |
| C20:1 (Eicosenoic acid) | 1.08 | 0.99 |
| C20:2 (Eicosadienoic acid) | 0.45 | 0.36 |
| C20:3N6 (Eicosatrienoic acid triglyceride N6) | 3.93 | 3.22 |
| C20:3N3 (Eicosatrienoic acid triglyceride N3) | 0.15 | 0.14 |
| C20:4N6 (Arachidonic acid) | 10.83 | 8.64 |
| C20:5N3 (Eicosapentaenoic acid) | 1.75 | 1.14 |
| C21:0 (Heneicosanoic acid) | 0.28 | 0.22 |
| C22:0 (Behenic acid) | 0.25 | 0.23 |
| C22:2 (Docosadienoic acid) | 0.49 | 0.45 |
| C22:1N9 (Erucic Acid) | 0.44 | 0.41 |
| C22:6N3 (Docosahexaenoic Acid) | 17.32 | 13.17 |
| C24:0 (Lignoceric acid) | 0.11 | 0.10 |
| C24:1 (Nervonic acid) | 3.59 | 2.79 |
Note: Cell sample is 1 × 107 cells and was resuspended in 1 mL of chloroform-methanol solution. Units in the table means μg/1 × 107 cells.
References
- Hausman, G.J.; Basu, U.; Du, M.; Fernyhough-Culver, M.; Dodson, M.V. Intermuscular and intramuscular adipose tissues: Bad vs. good adipose tissues. Adipocyte 2014, 3, 242–255.
- Gao, S.-Z.; Zhao, S.-M. Physiology, affecting factors and strategies for control of pig meat intramuscular fat. Recent Patents Food Nutr. Agric. 2009, 1, 59–74.
- Quintanilla, R.; Pena, R.N.; Gallardo, D.; Cánovas, A.; Ramírez, O.; Díaz, I.; Noguera, J.L.; Amills, M. Porcine intramuscular fat content and composition are regulated by quantitative trait loci with muscle-specific effects1. J. Anim. Sci. 2011, 89, 2963–2971.
- Chen, J.; Jiang, Y.; Cen, W.; Xing, S.; Zhu, L.; Tang, G.; Li, M.; Jiang, A.; Lou, P.; Wen, A.; et al. Distribution of H-FABP and ACSL4 gene polymorphisms and their associations with intramuscular fat content and backfat thickness in different pig populations. Genet. Mol. Res. 2014, 13, 6759–6772.
- Liu, X.N. Polymorphism Analysis of the 3′UTR Region in ACSL4 Gene of Wild Pigs, Domestic Pigs and Their Hybrids. J. Anhui Agric. Sci. 2008, 36, 5327–5328.
- Mercade, A.; Estelle, J.; Perez-Enciso, M. Characterization of the porcine acyl-CoA synthetase long-chain 4 gene and its association with growth and meat quality traits. Anim. Genet. 2006, 37, 219–224.
- Rusc, A.; Sieczkowska, H.; Krzecio, E. The association between acyl-CoA synthetase (ACSL4) polymorphism and intramuscular fat content in (Landrace x Yorkshire) x Duroc pigs. Meat Sci. 2011, 89, 440–443.
- Corominas, J.; Ramayo-Caldas, Y.; Castelló, A.; Muñoz, M.; Ibanez-Escriche, N.; Folch, J.M.; Ballester, M. Evaluation of the porcine ACSL4 gene as a candidate gene for meat quality traits in pigs. Anim. Genet. 2012, 43, 714–720.
- Wang, W.; Li, X.; Ding, N.; Teng, J.; Zhang, S.; Zhang, Q.; Tang, H. miR-34a regulates adipogenesis in porcine intramuscular adipocytes by targeting ACSL4. BMC Genet. 2020, 21, 33.
- Mashek, D.G.; Li, L.O.; Coleman, R.A. Long-chain acyl-CoA synthetases and fatty acid channeling. Future Lipidol. 2007, 2, 465–476.
- Soupene, E.; Kuypers, F.A. Mammalian Long-Chain Acyl-CoA Synthetases. Exp. Biol. Med. 2008, 233, 507–521.
- Yuan, H.; Li, X.; Zhang, X.; Kang, R.; Tang, D. Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem. Biophys. Res. Commun. 2016, 478, 1338–1343.
- Doll, S.; Proneth, B.; Tyurina, Y.; Panzilius, E.; Kobayashi, S.; Ingold, I.; Irmler, M.; Beckers, M.I.J.; Aichler, M.; Walch, M.A.A.; et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 2017, 13, 91–98.
- Xia, H.; Lee, K.W.; Chen, J.; Kong, S.N.; Sekar, K.; Deivasigamani, A.; Seshachalam, V.P.; Goh, B.K.P.; Ooi, L.L.; Hui, K.M. Simultaneous silencing of ACSL4 and induction of GADD45B in hepatocellular carcinoma cells amplifies the synergistic therapeutic effect of aspirin and sorafenib. Cell Death Discov. 2017, 3, 1–10.
- Belkaid, A.; Ouellette, R.J.; Surette, M.E. 17beta-estradiol-induced ACSL4 protein expression promotes an invasive phenotype in estrogen receptor positive mammary carcinoma cells. Carcinogenesis 2017, 38, 402–410.
- Gazou, A.; Riess, A.; Grasshoff, U.; Schäferhoff, K.; Bonin, M.; Jauch, A.; Riess, O.; Tzschach, A. Xq22.3-q23 deletion includingACSL4in a patient with intellectual disability. Am. J. Med. Genet. A 2013, 161, 860–864.
- Zhang, Y.; Chen, D.; Wang, Z. Analyses of mental dysfunction-related ACSl4 in Drosophila reveal its requirement for Dpp/BMP production cvisual wiring in the brain. Hum. Mol. Genet. 2009, 18, 3894–3905.
- Liss, K.H.; Lutkewitte, A.J.; Pietka, T.; Finck, B.N.; Franczyk, M.; Yoshino, J.; Klein, S.; Hall, A.M. Metabolic importance of adipose tissue monoacylglycerol acyltransferase 1 in mice and humans. J. Lipid Res. 2018, 59, 1630–1639.
- Rajbhandari, P.; Thomas, B.J.; Feng, A.-C.; Hong, C.; Wang, J.; Vergnes, L.; Sallam, T.; Wang, B.; Sandhu, J.; Seldin, M.M.; et al. IL-10 Signaling Remodels Adipose Chromatin Architecture to Limit Thermogenesis and Energy Expenditure. Cell 2018, 172, 218–233.e17.
- Ikeda, K.; Maegawa, H.; Ugi, S. Transcription factor activating enhancer-binding protein: A negative regulator of adiponectin gene expression. J. Biol. Chem. 2006, 281, 31245–31253.
- Soupene, E.; Dinh, N.P.; Siliakus, M. Activity of the acyl-CoA synthetase ACSL6 isoforms: Role of the fatty acid Gate-domains. BMC Biochem. 2010, 11, 18.
- Garin-Shkolnik, T.; Rudich, A.; Hotamisligil, G.S. FABP4 attenuates PPAR gamma and adipogenesis and is inversely correlated with PPARgamma in adipose tissues. Diabetes 2014, 63, 900–911.
- Kumar, U.; Singh, S. Role of Somatostatin in the Regulation of Central and Peripheral Factors of Satiety and Obesity. Int. J. Mol. Sci. 2020, 21, 2568.
- Moseti, D.; Regassa, A.; Kim, W.-K. Molecular Regulation of Adipogenesis and Potential Anti-Adipogenic Bioactive Molecules. Int. J. Mol. Sci. 2016, 17, 124.
More
Information
Subjects:
Agriculture, Dairy & Animal Science
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
956
Revisions:
2 times
(View History)
Update Date:
25 Jan 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




