
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mohd Imran | + 1943 word(s) | 1943 | 2022-01-25 07:13:23 | | | |
| 2 | Lindsay Dong | Meta information modification | 1943 | 2022-01-25 10:35:16 | | | | |
| 3 | Lindsay Dong | Meta information modification | 1943 | 2022-01-25 10:35:36 | | |
Video Upload Options
Human African trypanosomiasis (HAT or ‘sleeping sickness’) is a neglected tropical disease. If untreated, it is always fatal and leads to death. A few treatments are available for HAT, but most of them require a skilled professional, which increases the financial burden on the patient. Recently, fexinidazole (FEX) has been approved by the European Medicine Agency (EMA) and the United States Food and Drug Administration (USFDA) as the first all-oral therapy for the treatment of stage-1 (hemolymphatic) as well as stage-2 (meningoencephalitic) of HAT. Before the FEX approval, there were separate treatments for stage-1 and stage-2 of HAT.
1. Introduction
| Drug (Dosage Form and Administration) |
Comments |
|---|---|
| Treatment of Stage 1 of Human African Trypanosomiasis | |
| Pentamidine (Solution for inhalation/Injection) |
A skilled and trained professional is needed for drug administration. It is administered as a single daily intramuscular/intravenous injection for seven days. It can cause severe hypotension after intramuscular/intravenous administration, hypoglycemia, acute pancreatitis, and cardiac arrhythmias, and is effective against stage-1 of g-human African trypanosomiasis only because it does not cross the blood–brain barrier efficiently [8]. |
| Suramin (Intravenous, injection) |
A skilled and trained professional is needed for drug administration. It is mainly used for stage-1 of r-human African trypanosomiasis, and rarely used for stage-1 g-human African trypanosomiasis. It can cause renal toxicity and anaphylactic reactions [9]. |
| Treatment of Stage 2 of Human African Trypanosomiasis | |
| Nifurtimox (Tablet, Oral) |
The combination of nifurtimox with eflornithine is the first-line treatment for stage-2 of human African trypanosomiasis. It has potential for genotoxicity, carcinogenicity, fetal toxicity, worsening of neurological and psychiatric conditions, hypersensitivity, decreased appetite and weight loss, and porphyria [10]. |
| Eflornithine (Intravenous, injection) |
A skilled and trained professional is needed for drug administration and requires long therapy. It can cause fever, pruritus, hypertension, cough, anorexia, nausea, vomiting, diarrhea, abdominal pain, headaches, and is the second-line treatment for stage-2 of g-human African trypanosomiasis [11]. |
| Melarosoprol (Intravenous, injection) |
A skilled and trained professional is needed for drug administration and is effective for stage-2 g-human African trypanosomiasis. Its administration is painful and toxic. The adverse events may be life-threatening including encephalopathic syndrome [12]. |
| Nifurtimox-eflornithine combination therapy (Oral Nifurtimox + Intravenous Eflornithine) |
A skilled and trained professional is needed for drug administration. It needs systematic hospitalization and is mainly used for stage-2 of g-human African trypanosomiasis [13]. |
It is evident from Table 1 that few treatments are available for HAT, wherein nifurtimox and pentamidine are USFDA approved drugs. Most of these treatments are injectable and are effective either for stage-1 or stage-2 of HAT [8][9][10][11][12][13]. None of the available treatments are effective in treating stage-1 as well as stage-2 of HAT. Most of the treatments require a skilled person for IV administration, which increases the financial burden on the patient.
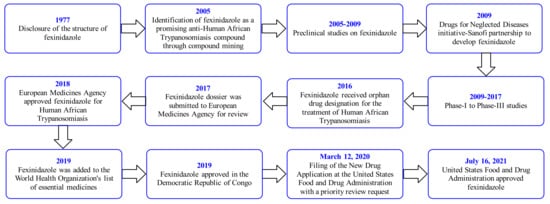
2. Fexinidazole (FEX)
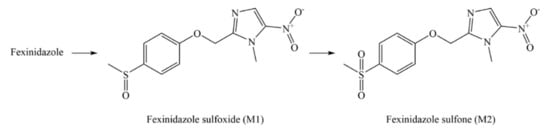
3. Pharmacology of FEX
| Condition | Phase (Number Enrolled) |
Status (Study Start Date (SSD); Study Completion Date (SCD); Last Update Date (LUD)) |
National Clinical Trial (NCT) Number/Other IDs (Sponsor/Collaborators; Funder Type; Location) |
|---|---|---|---|
| Chagas Disease and South American Trypanosomiasis | Phase 2 (140) |
Unknown (SSD: July 2014; SCD: February 2016; LUD: 15 July 2015) |
NCT02498782/DNDi-CH-FEXI-001 (Drugs for Neglected Diseases initiative; Other; Bolivia) |
| Trypanosomiasis (African) | Phase 1 (30) |
Terminated (SSD: September 2011; SCD: February 2012; LUD: 31 March 2017) |
NCT01483170/DNDiFEX003 (Drugs for Neglected Diseases initiative; Other; France) |
| Visceral Leishmaniasis | Phase 2 (14) |
Terminated (SSD: November 2013; SCD: September 2015; LUD: 30 October 2015) |
NCT01980199/FEXI VL001 (Drugs for Neglected Diseases initiative; Other; Sudan) |
| r-Human African Trypanosomiasis | Phase 2/3 (50) |
Recruiting (SSD: 29 September 2019; SCD: March 2023; LUD: 30 August 2021) |
NCT03974178/DNDi-FEX-07-HAT (Drugs for Neglected Diseases initiative; Other; Malawi and Uganda) |
| Trypanosomiasis (African) | Phase 1 (30) |
Completed (SSD: March 2015; SCD: June 2015; LUD: 8 October 2015) |
NCT02571062/DNDiHATFEX008 (Drugs for Neglected Diseases initiative; Other; France) |
| Human African Trypanosomiasis | Phase 2/3 (394) |
Completed (SSD: October 2012; SCD: 26 April 2017; LUD: 20 February 2018) |
NCT01685827/DNDiFEX004 (Drugs for Neglected Diseases initiative; Other; Batangafo, Bagata, Congo, etc.) |
| Human African Trypanosomiasis | Phase 2/3 (125) |
Completed (SSD: May 3, 2014; SCD: 27 June 2017; LUD: 24 June 2020) |
NCT02184689/DNDiHATFEX006 (Drugs for Neglected Diseases initiative; Other; Congo) |
| Human African Trypanosomiasis | Phase 2/3 (230) |
Completed (SSD: 30 April 2014; SCD: 25 April 2017; LUD: 24 June 2020) |
NCT02169557/DNDiHATFEX005 (Drugs for Neglected Diseases initiative; Other; Congo) |
| Human African Trypanosomiasis | Phase 1 (108) |
Completed (SSD: September 2009; SCD: October 2010; LUD: 6 April 2017) |
NCT00982904/DNDiFEX001 (Drugs for Neglected Diseases initiative and Sanofi; Other/Industry; France) |
| Human African Trypanosomiasis and Trypanosomiasis (Gambian) | Phase 3 (174) |
Completed (SSD: 17 November 2016; SCD: 1 February 2021; LUD: 11 October 2021) |
NCT03025789/DNDi-FEX-09-HAT (Drugs for Neglected Diseases initiative and Sanofi; Other/Industry; Congo, Mbuji-Mayi, Bagata, etc.) |
| Pharmacokinetic in Healthy Volunteers | Phase 1 (12) |
Completed (SSD: February 2011; SCD: April 2011; LUD: 31 March 2017) |
NCT01340157/DNDiFEX002 (Drugs for Neglected Diseases initiative and Sanofi; Other/Industry; France) |
| Chagas’ Disease (Chronic) | Phase 2 (45) |
Completed (SSD: 13 November 2017; SCD: 28 August 2019; LUD: 23 September 2020) |
NCT03587766/DNDi-FEX-12-CH (Drugs for Neglected Diseases initiative; Other; Spain) |
References
- Bagcchi, S. WHO manual on neglected tropical diseases. Lancet Infect. Dis. 2021, 21, E1498.
- Kennedy, P.G.E. Update on human African trypanosomiasis (sleeping sickness). J. Neurol. 2019, 266, 2334–2337.
- Kennedy, P.G. Clinical features, diagnosis, and treatment of human African trypanosomiasis (sleeping sickness). Lancet Neurol. 2013, 12, 186–194.
- Büscher, P.; Cecchi, G.; Jamonneau, V.; Priotto, G. Human African trypanosomiasis. Lancet 2017, 390, 2397–2409.
- Kennedy, P.G. Human African trypanosomiasis of the CNS: Current issues and challenges. J. Clin. Investig. 2004, 113, 496–504.
- Bisser, S.; Lumbala, C.; Nguertoum, E.; Kande, V.; Flevaud, L.; Vatunga, G.; Boelaert, M.; Büscher, P.; Josenando, T.; Bessell, P.R.; et al. Sensitivity and specificity of a prototype rapid diagnostic test for the detection of Trypanosoma brucei gambiense infection: A multi-centric prospective study. PLoS Negl. Trop. Dis. 2016, 10, e0004608.
- Lumbala, C.; Biéler, S.; Kayembe, S.; Makabuza, J.; Ongarello, S.; Ndung’u, J.M. Prospective evaluation of a rapid diagnostic test for Trypanosoma brucei gambiense infection developed using recombinant antigens. PLoS Negl. Trop. Dis. 2018, 12, e0006386.
- Hafiz, S.; Kyriakopoulos, C. Pentamidine. 19 June 2021. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557586/ (accessed on 20 December 2021).
- Wiedemar, N.; Hauser, D.A.; Mäser, P. 100 years of Suramin. Antimicrob. Agents Chemother. 2020, 64, e01168-19.
- Thakare, R.; Dasgupta, A.; Chopra, S. Update on nifurtimox for treatment of Chagas disease. Drugs Today 2021, 57, 251–263.
- Jobanputra, K.S.; Rajpal, A.V.; Nagpur, N.G. Eflornithine. Indian J. Dermatol. Venereol. Leprol. 2007, 73, 365–366.
- Fairlamb, A.H.; Horn, D. Melarsoprol resistance in African trypanosomiasis. Trends Parasitol. 2018, 34, 481–492.
- Hidalgo, J.; Ortiz, J.F.; Fabara, S.P.; Eissa-Garcés, A.; Reddy, D.; Collins, K.D.; Tirupathi, R. Efficacy and toxicity of fexinidazole and nifurtimox plus eflornithine in the treatment of African trypanosomiasis: A systematic review. Cureus 2021, 13, 16881.
- Fairlamb, A.H. Fexinidazole for the treatment of human African trypanosomiasis. Drugs Today 2019, 55, 705–712.
- Neau, P.; Hänel, H.; Lameyre, V.; Strub-Wourgaft, N.; Kuykens, L. Innovative partnerships for the elimination of human African Trypanosomiasis and the development of fexinidazole. Trop. Med. Infect. Dis. 2020, 5, 17.
- Deeks, E.D. Fexinidazole: First global approval. Drugs 2019, 79, 215–220.
- Quality Review of Fexinidazole. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/214429Orig1s000ChemR.pdf (accessed on 2 November 2021).
- Wyllie, S.; Patterson, S.; Stojanovski, L.; Simeons, F.R.; Norval, S.; Kime, R.; Read, K.D.; Fairlamb, A.H. The anti-trypanosome drug fexinidazole shows potential for treating visceral leishmaniasis. Sci. Transl. Med. 2012, 4, 119re1.
- Prescribing Information of Fexinidazole. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214429s000lbl.pdf (accessed on 2 November 2021).
- Patterson, S.; Wyllie, S. Nitro drugs for the treatment of trypanosomatid diseases: Past, present, and future prospects. Trends Parasitol. 2014, 30, 289–298.
- Tarral, A.; Blesson, S.; Mordt, O.V.; Torreele, E.; Sassella, D.; Bray, M.A.; Hovsepian, L.; Evene, E.; Gualano, V.; Felices, M.; et al. Determination of an optimal dosing regimen for fexinidazole, a novel oral drug for the treatment of human African trypanosomiasis: First-in-human studies. Clin. Pharmacokinet. 2014, 53, 565–580.
- Torreele, E.; Bourdin Trunz, B.; Tweats, D.; Kaiser, M.; Brun, R.; Mazue, G.; Bray, M.A.; Pecoul, B. Fexinidazole—A new oral nitroimidazole drug candidate entering clinical development for the treatment of sleeping sickness. PLoS Negl. Trop. Dis. 2010, 4, e923.
- Kaiser, M.; Bray, M.A.; Cal, M.; Bourdin Trunz, B.; Torreele, E.; Brun, R. Antitrypanosomal activity of fexinidazole, a new oral nitroimidazole drug candidate for treatment of sleeping sickness. Antimicrob. Agents Chemother. 2011, 55, 5602–5608.




