Colorectal cancer (CRC) is a serious disease that affects millions of people throughout the world, despite considerable advances in therapy. The formation of colorectal adenomas and invasive adenocarcinomas is the consequence of a succession of genetic and epigenetic changes in the normal colonic epithelium. Genetic and epigenetic processes associated with the onset, development, and metastasis of sporadic CRC have been studied in depth, resulting in identifying biomarkers that might be used to predict behaviour and prognosis beyond staging and influence therapeutic options. A novel biomarker, or a group of biomarkers, must be discovered in order to build an accurate and clinically useful test that may be used as an alternative to conventional methods for the early detection of CRC and to identify prospective new therapeutic intervention targets. To minimise the mortality burden of colorectal cancer, new screening methods with higher accuracy and nano-based diagnostic precision are needed.
1. Introduction
Colorectal cancer (CRC) is a serious health issue in developed and developing countries alike, with the third highest incidence rate of all tumour-causing diseases
[1]. This kind of cancer is a leading cause of morbidity and mortality in Western nations. Only a small percentage of CRC instances are caused by basic genetic abnormalities, with most cases occurring due to specific regular factors and growing age
[2]. If left untreated, it can spread to the intestinal wall and the muscles beneath the surface of the skin. Environmental and genetic variables can also combine in many ways to accelerate cancer development and make it more difficult to treat.
In most cases, patients appear beyond the age of 60, with precursor starting adenomas that develop into cancer over for 1 to 2 decades
[3]. Generalised screening of this population at age 50 years and older has been shown to reduce death from the disease, detect cancer at an earlier stage, and reduce the incidence of the disease. There have been four major hypotheses put out on the aetiology of colorectal cancer. To begin, the same genetic and epigenetic changes that cause colon cancer also increase the risk of developing colorectal cancer. Second, cancer develops in a staged manner, beginning with a precancerous condition and progressing to malignancy. Third, cancer development necessitates a lack of genetic stability. Fourth, in hereditary cancer syndromes, the germline forms of significant genetic abnormalities are generally associated with the development of rare colon cancers, which are caused by somatic occurrences of hereditary diseases
[4].
Epigenetics, defined as heritable changes in gene expression without permanent changes in DNA sequence, plays an important role in the aetiology of different malignancies, including colorectal cancer. Recent epigenetic research has found a missing connection between certain gene expression patterns associated with CRC and the lack of genetic abnormalities. This missing link has now been discovered during the last two decades
[5]. As an example, microsatellite in-stability (MSI), which is a hallmark of a molecular subgroup of CRC, results from an inability to repair DNA mismatches (MMRs)
[6] properly. A genetic mutation can cause this defect in one of the MMR genes, the hypermethylation of the MLH1 gene’s promoter, or both. Chromosome instability (CIN) in CRC has also been linked to global hypomethylation
[7]. There is further evidence that cancer-related pathways at the post-transcriptional level are influenced by microRNAs (miRNAs), which play a role in nearly all phases of CRC, from initiation through progression and metastasis. Cell development is inhibited by miR-143, which downregulates in CRC because it tar-gets the KRAS mRNA transcript
[8]. Aside from improving our knowledge of CRC pathogenesis, these discoveries have also provided new opportunities to find disease biomarkers and treatment targets. The various CRC biomarkers such as BRAF mutations, NRAS, and KRAS, microsatellite instability (MSI), DNA mismatch repair (MMR) status, and CpG island methylation have been studied. A KRAS or NRAS mutation is associated with a worse prognosis, and anti-epidermal growth factor receptor (anti-EGFR) antibody treatment will be ineffective
[9]. Mutation in BRAF V600E is seen in 8–10% of individuals with CRC. These patients have a more aggressive illness and a worse prognosis in the adjuvant and metastatic context. People with BRAF mutation and microsatellite instability-high (MSI-H) tumours had a better overall prognosis than people with BRAF mutation and microsatellite stable (MSS) illness, and it turns out, using MSI to screen for Lynch syndrome and identify patients who could benefit from immunotherapy is becoming increasingly important in CRC
[10].
A variety of drug-loaded nanoparticles in the 20–400-nm size range have made significant contributions to chemotherapeutic drug delivery in recent years (e.g., liposomes, dendrimers, polymeric nanoparticles, and micelles)
[11]. Many potential nanomedicine advancements are based on these systems, which have evolved from basic drug-loaded nanoparticles to multifunctional nanoparticles that target particular cancer cells by attaching them to specific cell-surface proteins. Antigens such as integrin and folic acid receptors, which are differentially expressed on the surface of cancer cells, can be targeted using targeted nanoparticles
[12]. Several of these nanoparticles are now in clinical trials. A great deal has been learned about using nanoparticles as therapeutic platforms for treating malignancies ranging from the prostate to the ovary. The clinical development of nanoparticles for cancer therapy is restricted, despite the high death and morbidity rates associated with CRC.
2. Pathogenesis of Colorectal Cancer
Genetic alterations in oncogenes and tumour suppressor genes occur sequentially from normal to the dysplastic epithelium in the adenoma–carcinoma process. This results in the development of CRC. Genomic changes, both genetic and epigenetic, convert normal glandular epithelium into adenocarcinoma when they accumulate
[13]. While molecular mechanisms behind the cancer’s unabated development and spread remain a mystery, several genetic pathways have been proposed to explain CRC aetiology (
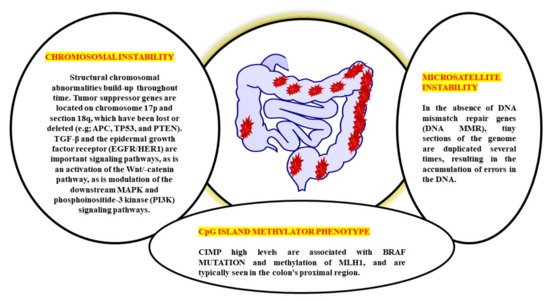
Figure 1). CRC involves numerous molecular pathways, including the three major pathways: CIN, MSI, and CIMP, all of which play a crucial role in the development of CRC due to genetic and epigenetic changes (
Table 1). Three molecular pathways that indicate progression from cancer have been identified: microsatellite instability (associated with hypermutated group), chromosomal instability (completely within the non-hypermutated category), and the CpG Island Methylator Phenotype (CIMP; associated with both the hypermutated and non-hypermutated groups)
[14]. These molecular pathways may dictate tumour development and metastatic time, as the epidemiology, mutational trials, and immune responsiveness information vary by route. The methods used to treat an illness might also differ. A biomarker known as “EMAST” refers to elevated microsatellite alterations at selected tetranucleotide repeats recently demonstrated to predict patient survival as a modulator of the three biological pathways. Individuals with sporadic CRC cannot predict whether they develop a metachronous adenoma or cancer based on the previously identified pathways
[15][16]. Clinical monitoring is critical in detecting and eliminating metachronous lesions in these patients.
Figure 1. Multiple genetic pathways of CRC pathogenesis.
Table 1. An assessment of the MSI-H, EMAST, CIMP, and CIN CRC indices.
| Pathophysiological Routes |
Genomic
Instability |
Inflammation |
Prognosis |
Pathogenesis |
| CIN |
Causes to the mutation and copy number variation; MSS; aneuploid |
Tumour margin, lamina propria, and intraepithelial sites all have varying degrees of differentiation |
Referent |
Genetic changes that lead to heterozygosity loss |
| CIMP |
Leading hypermethylation at DNA loci |
Without hMLH1 hypermethylation: varied |
Poor survival without hMLH1 hypermethylation |
Without hMLH1 hypermethylation: unknown |
| MSI-H |
Microsatellite instability (MSI) and diploid is appeared |
Crohn’s-like around tumour (tumour margin) |
Better survival; early stage |
Target gene frameshift mutation; BRAFV600E |
| EMAST |
Instability found in mostly at MSS and MSI-L, includes MSI-H |
Tumour nests surrounding epithelial components have been linked to this condition. |
Poor survival; later stage |
Chromosome instability in combination with a frameshift mutation on a target gene |
The biological behaviour, prognosis, and therapeutic response of CRC are all very variable. CRC has historically been divided into three subgroups based on the three pathophysiological routes of carcinogenesis: CIN, MSI, and CIMP from a molecular perspective
[17]. CIN is the distinguishing feature for most CRCs, accounting for ~80–85% percent of the cases. CIN is characterised by the activation of growth-promoting pathways while simultaneously decreasing the activity of apoptotic pathways, with the latter being more common
[18]. These tumours begin as adenomatous polyps due to the APC gene’s deactivation mutation [encodes the WNT pathway effector adenomatous polyposis coli (APC)].
Further, the progress to adenocarcinomas by activating mutations in the KRAS (responsible for the receptor tyrosine kinase signalling) and deactivating mutations in SMAD4 (accounting for the TGFβ, it played an important role in the cell cycle control). Due to the initial event, this pathway is sometimes referred to as the APC pathway
[19]. There are no mutations of MSI, MLH1, methylation, or BRAF in the CIN pathway. Moreover, Lynch syndrome (hereditary CRC’s most frequent type) is caused by the germline mutations in the MMR genes, including MLH1, MSH2, MSH6, and PMS2. It is believed that MSI is initiated by a lack in the MMR DNA, causing mutations in the MLH1, MSH2, and MSH6 MMR genes. It is possible to have MMR deficiency due to MLH1 deactivation induced by biallelic hypermethylation of the MLH1 promoter or by dual somatic mutations in the MMR genes
[20]. It is noticed that the individual with MMR-deficient and MSI-high CRC (dMMR–MSI-H) demonstrated a good prognosis but did not acquire therapeutics benefits from 5-fluorouracil (5-FU) and has already been characterised based on MSI status
[21]. A subset of colorectal cancers known as CIMPs, which are defined by a substantial percentage of CpG island hypermethylation around the promoter of multiple tumour-suppressor genes, is identified by epigenetics rather than by CIN or MSI. Due to its close association with colorectal carcinogenesis’s serrated route, hypermethylation of the MLH1 promoter, as well as its association with female gender, advanced age, and poor histology, CIMP has a significant risk of being found in patients with the disease
[22]. However, the lack of a defined definition of CIMP-high makes it difficult to translate this pathophysiological feature into treatment. Although CIMP testing is most commonly done on MLH1, MINT1, CACNA1G, and CDKN2A (could potentially up to 16 distinct genes identified), there is currently does not agree about which cut-off values will be applied to distinguish between CIMP high and CIMP+ on certain scientific procedures must be employed to conduct assessments for CIMP tests
[23].
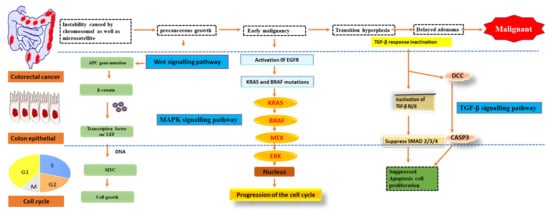
As early as lesions in the polyp cancer development sequence, gene alterations have a role in cancer creation and progression. The abnormal crypt focus is the initial histological lesion linked to CRC development
[24]. Tumour suppressor gene APC is often altered in colorectal cancers, and dysplastic aberrant crypt foci can spread mutations. The Wingless/Wnt pathway is activated by inhibiting APC, a systematic approach to start the polyp cancer development sequence. The Wnt signalling pathway harbours the clonal development of polyp cells to cancer due to subsequent mutations in genes like KRAS or TP53 (
Figure 2). The transforming growth factor b (TGFB1)-mediated cell signalling pathway may also be modulated by KRAS and TP53 mutations, further accelerating CRC development
[25].
Figure 2. Pathways of signalling that have been genetically changed by CRC.
Oncogene activation and tumour suppressor gene inactivation are attributed to genetic and epigenetic alterations that occur during colon cancer development. B-Raf sends EGFR signals to KRAS, a proto-oncogene that activates the MAPK pathway
[26]. Over half of all CRCs have mutations in KRAS or B-Raf, which trigger the MAPK signalling pathway and promote proliferation while also stopping cell death. The PI3 K, WNT-APC-CTNNB1, and TGFB1-SMAD signalling pathways are also part of CRC. It also includes the RAS–RAF–MAPK pathway
[27].
3. Biomarkers Based on Epigenetic Changes for CRC
When CRC progresses from an adenomas or serrated lesions stage, genetic and epigenetic changes accumulate in the lesions, giving progressively dysplastic characteristics until they become an adenocarcinoma
[28]. To avoid this illness, it is critical to find precancerous lesions and early-onset colorectal cancer in people at average risk who are otherwise healthy during routine screening. As far as CRC screening is concerned, colonoscopy is the gold standard, since it can detect and eradicate precursor lesions
[29]. For these reasons, many people choose not to get colonoscopies. They are painful, costly with low compliance rates, and can cause complications, including haemorrhage and perforation. For precursor lesions like adenomas, non-invasive screening procedures like the faecal occult blood test (FOBT) and faecal immunochemical test (FIT), widely used in Europe and other Western nations, have lower sensitivity and specificity than colonoscopy. As a result of these drawbacks, new non-invasive detection methods for precursor lesions and early-stage CRCs are urgently required
[30].
At intermediate stages of CRC staging, the currently used tumour–node–metastasis (TNM) classification approach is inadequate for prognostication and clinical decision-making
[31]. Individuals with a high risk of illness recurrence or mortality (prognostic biomarkers) and those who would benefit from chemotherapy, immunotherapy, or targeted therapy are urgently in need of biomarkers that aid identify these patients (which may be unnecessary). Many epigenetic biomarkers, including DNA methylation, histone alterations, miRNAs, and long noncoding RNA, have shown promise as clinically significant biomarkers for CRC diagnosis, prognosis, and therapy response prediction (lncRNA)
[32].
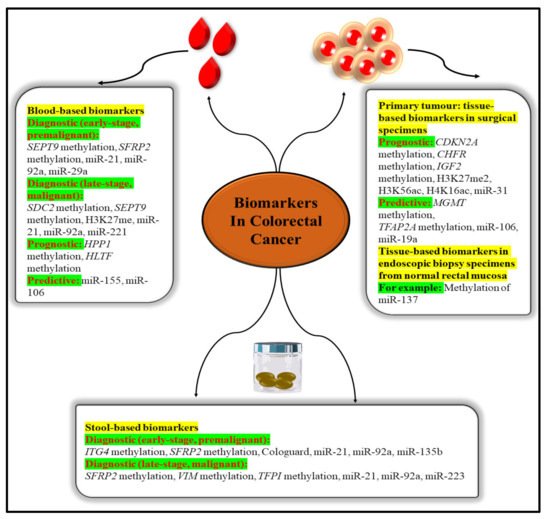
Blood and other body fluids and tissues can include quantifiable indicators of pathological or physiological processes or conditions or diseases, such as molecular markers (biomarkers). Molecular markers can be used to identify conditions or diseases. Biomarkers are important diagnostic, prognostic, and therapeutic tools for cancer detection, diagnosis, and therapy selection (
Figure 3). It can also be used to pinpoint the exact location of a tumour and identify any areas that are at an advanced stage of growth or are receptive to a particular therapy
[33]. These molecular markers represent the mechanisms of neoplastic cell exfoliation and mucus secretion of aberrant glycoproteins in CRC by reflecting the mechanisms of cell exfoliation and mucus secretion. Shortly, researchers hope to find novel, non-invasive DNA, RNA, or protein-based molecular markers that may be used to detect colorectal cancer in the blood, faeces, and other body fluid. The prognostic, predictive, and diagnostic markers have been developed for the determination of the CRC. Diagnostic biomarkers indicate the likelihood of the illness progressing. Patient treatment measures can be predicted with the use of these molecular markers. Predictive biomarkers are utilised for forecasting the efficacy of treatment in cancer patients
[34]. Colorectal polyps can be detected early with the use of the following biomarkers.
Figure 3. Epigenetic biomarkers in CRC.
3.1. Molecular Markers for Diagnosis
3.1.1. HNPCC (Hereditary Nonpolyposis CRC)
HNPCC is the most well-known autosomal dominant hereditary colon cancer condition. It is a screening test for the early identification of colorectal cancer (CRC). HNPCC is present when a germline mutation is identified in one of the four MMR genes, such as MSH6, MLH1, MSH2, and PMS2, all found in the human body. Many young patients with a new genetic condition come from families where colon cancer runs in the blood.
3.1.2. Telomerase
The telomerase is key enzyme that preserves the telomere (repeating DNA (TTAGGG/AATCCC in the human telomeres) that protects from chromosomal breakdown, elimination of operational genes, and cell death. Telomerase is necessary for the maintenance of the telomere. The activation of telomerase is an independent diagnostic and prognostic marker for malignant tumours that can be used independently.
3.1.3. Insulin Like Growth Factor Binding Protein 2 (IGFBP2)
According to research, compared to healthy individuals, CRC patients had higher serum and plasma levels of IGFBP2. It has emerged as a diagnostic device for the primary identification and development of CRC, since it is implicated in cancer cell proliferation, migration, and invasion.
3.1.4. Pyruvate Kinase M2 (PKM2)
PKM2 refers to a cytosolic enzyme expressed in both normal and malignant cells and plays an important function in energy metabolism. PKM2 expression is upregulated in CRC and other gastrointestinal malignancies. It may also be relevant for CRC inquiry and/or diagnosis based on stool or blood samples as a dismal prognostic sign.
3.2. Molecular Markers for Prognostic
3.2.1. p53
The existence of the tumour inhibitor p53 is also considered an independent prognostic factor for colon cancer. The alteration of the tumour-suppressor gene p53 is an initial and critical step in ulcerative colitis-associated carcinogenesis (sometimes referred to as TP53). The development of carcinoma in an adenoma coincides with a p53 mutation and a loss of heterozygosity (LOH) in the wild-type allele, further demonstrating its function in the regulation of malignancy. Patients with CRC who are missing this gene have a bad prognosis.
3.2.2. 18 q Loss of Heterozygosity
Patients with colorectal cancer (CRC) who have chromosomal 18q LOH have a reduced chance of survival. Patients with stage II or stage III colon cancer who lose their somatic heterozygosity have a worse prognosis than those whose tumours retain both of their parents’ alleles on chromosome 18 q. Patients with stage II or stage III colon cancer with this 18qLOH have a poorer prognosis than those with normal alleles on chromosome 18q.
3.2.3. MLH1 Methylation
DNA microsatellite unstable recognition or the absence of the MLH1 protein production on immunohistochemical investigation confirm somatic MLH1 inactivation in primary colorectal malignancies, which is more common in early-stage colorectal cancers than in late illness. A more indolent illness or a better prognosis might result from this inactivation in the absence of adjuvant treatment.
3.2.4. VEGF
One of the angiogenic agents in CRC is vascular endothelial growth factor (VEGF), which is expressed in roughly 50% of CRCs but only in extremely low amounts in normal colonic mucosa and adenomas. As a result, VEGF-1 expression appears to deliver useful predictive information in CRC patients.
3.3. Predictive Molecular Markers
3.3.1. KRAS
The discovery of KRAS mutations is employed as the foremost potential prognostic marker in anti-EGFR (epidermal growth factor receptor) antibody-based therapy in CRC, such as panitumumab and cetuximab.
3.3.2. B-Raf V600E
The activation of this kinase by somatic mutation leads to an unconstrained MAPK signalling pathway in humans. The BRAF V600E activating mutation does not respond to EGFR inhibitor treatment in individuals with stage IV CRC. Although the significance of BRAF mutant level as a predicting marker gene is uncertain, BRAF mutant as a biomarker of anti-EGFR antibody resistance is perhaps the extensively researched predicting potential of BRAF alteration.
3.3.3. PIK3CA Status
The PIK3CA mutation causes an abnormal stimulation of the AKT pathway, leading to cancer cell growth. If the PI3K/AKT pathway is persistently activated, it is likely to affect CRC progression significantly. Colon cancer cells often with an induced PIK3CA mutant were more sensitive to cetuximab in preclinical studies even than PIK3CA wild-type cells.
3.3.4. ERCC—1
Repair protein for DNA excision ERCC-1 is a nucleotide excision repair gene that is involved in DNA repair. A new analysis of metastasis and stage II or III CRC and evaluating ERCC-1 as a possible predictive biomarker in direct response to oxaliplatin by studying dysregulation of ERCC-1 expression in between oxaliplatin treatment has been presented
[35][36][37][38].