Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Seunho Jung | + 2124 word(s) | 2124 | 2022-01-17 07:00:53 | | | |
| 2 | Nora Tang | Meta information modification | 2124 | 2022-01-24 06:41:37 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Jung, S. Physical Properties of Succinoglycan. Encyclopedia. Available online: https://encyclopedia.pub/entry/18676 (accessed on 08 February 2026).
Jung S. Physical Properties of Succinoglycan. Encyclopedia. Available at: https://encyclopedia.pub/entry/18676. Accessed February 08, 2026.
Jung, Seunho. "Physical Properties of Succinoglycan" Encyclopedia, https://encyclopedia.pub/entry/18676 (accessed February 08, 2026).
Jung, S. (2022, January 24). Physical Properties of Succinoglycan. In Encyclopedia. https://encyclopedia.pub/entry/18676
Jung, Seunho. "Physical Properties of Succinoglycan." Encyclopedia. Web. 24 January, 2022.
Copy Citation
Succinoglycan is a type of bacterial anionic exopolysaccharide produced from Rhizobium, Agrobacterium, and other soil bacteria. The exact structure of succinoglycan depends in part on the type of bacterial strain, and the final production yield also depends on the medium composition, culture conditions, and genotype of each strain. Various bacterial polysaccharides, such as cellulose, xanthan, gellan, and pullulan, that can be mass-produced for biotechnology are being actively studied.
succinoglycan
bacterial polysaccharides
application
hydrogels
biomaterials
1. Conformational Analysis
The structure of succinoglycans was examined using the X-ray diffraction (XRD) technique. The results of XRD patterns of the succinoglycans produced by wild type strain, Rhizobium radiobacter ATCC 19358 (SG-A), and mutant strain (SG-N) were analyzed. The diffraction patterns for SG-A and SG-N showed broad peaks in the range of 2θ = 20–22. The crystallinities were calculated by integration XRD pattern of SG-A and SG-N. The crystallinity of SG-A and SG-N was 21.96% and 16.09%, respectively. On the other hand, the succinoglycan from Sinorhizobium meliloti 1021 strain displayed a broad peak at 2θ = 18.7. Because of poor crystallinity, agarose/succinoglycan hydrogels (AG/SG) showed lower and shifted diffraction peaks at angles of 17.5, 17.4, and 18.0 on the 2θ scale compared to pure agarose [1]. In addition, succinoglycan metallohydrogel using trivalent chromium (Cr3+) was verified via XRD measurements [2]. When the concentration of Cr3+ was increased from 6.6 mM to 52.8 mM, the crystallinity decreased. This is because succinoglycan changed from a crystalline state to an amorphous state, indicating that intermolecular hydrogen bonding between the hydroxyl/carboxyl group and the metal cation of succinoglycan occurred in the metal hydrogen gel. The results indicated that the addition of Cr3+ changed the intrinsic crystallinity of succinoglycan and produced another regular molecular arrangement.
The circular dichroism (CD) spectrum of polysaccharides was used as a tool to suggest the indicator of a secondary structure [3]. In the case of native succinoglycan, a characteristic spectrum with a negative band centered at approximately 200 nm was observed. This band corresponded to the n → π* transition by the carboxyl and carboxylates of pyruvate and succinate. Succinoglycan has a backbone with regular side groups formed by four-O-linked glycopyranose residues, wherein the backbone contains two consecutive β-(1,6) glycosidic bonds, one of which is connects the side chain to the main chain. This linkage can give flexibility to the lateral arms of succinoglycans. The fixed charge of pyruvate and succinate attached to this side chain enhance it, and the uncharged backbone is reinforced by the entangled residues of glycofuranose and galtopyranose, creating a regular helix structure [4][5]. Due to the spatial demands of charged bulky side chains, succinoglycans are likely single helices with partial lateral aggregation. Coordination of ferric cations (Fe3+) with succinoglycan forms a hydrogel. Conformation of Fe3+-coordinated succinoglycan (Fe3+-SG) hydrogels were investigated by CD spectropolarimetry. As Fe2+ was added to succinoglycan, the n → π* negative transition band due to the carboxyl and carboxylate of succinoglycan weakened. This decrease in the intensity of transition may have been due to the Fe2+ complexation of succinoglycan by the binding of Fe2+ to the carboxyl groups responsible for the transition. Contrarily, the CD spectra of Fe3+-SG showed sharp both positive bands at ~195 and 202–208 nm, and sharp negative bands at ~198 nm. The appearance of these new bands could be attributed to the charge transfer interactions between Fe3+ cations and the carboxyl group.
The conformation of succinoglycan macromolecules using atomic force microscopy (AFM) was determined and the obtained data were compared to the measurements obtained in solution [6]. Individual chains and dimers were found in succinoglycan precipitated from pure water, whereas only individual chains were found in 0.01 M KCl. At 0.5 M KCl, succinoglycan formed a gel-like structure on the mica surface [7][8]. Analysis of persistence lengths from the AFM images indicated that succinoglycan became more rigid with increasing ionic strength. Flexible chains corresponding to disordered conformations were observed in water, whereas single-helix chains were imaged at 0.01 M KCl.
2. Thermal Analysis
The thermogram (TGA) of succinoglycan showed two-stage weight loss [9]. The initial weight loss accounts for the loss of absorbed water molecules attached to the carboxyl groups present in the succinoglycan. The first phase in weight loss is due to the high carboxyl group content, which explains the excellent water affinity and water retention capacity of succinoglycan [10][11]. The second phase in polysaccharide degradation involves the degradation of thermally stable structures formed by crosslinking and strong bonds of succinoglycans. The TGA and differential thermogravimetry (DTG) of succinoglycan obtained under a nitrogen atmosphere with heating rate of 10 °C/min shows a mass loss of about 8.05% at 95 °C in Figure 1a. In the second phase, succinoglycan exhibited a mass loss of 60.64% in the range of 246–370 °C. Succinoglycan solution (1–2%) has non-Newtonian shear-thinning fluid behavior under 25–55 °C. With increasing solution concentrations, viscosity and pseudoplasticity proportionally increase while a temperature increase is inversely proportional to viscosity and pseudoplasticity.
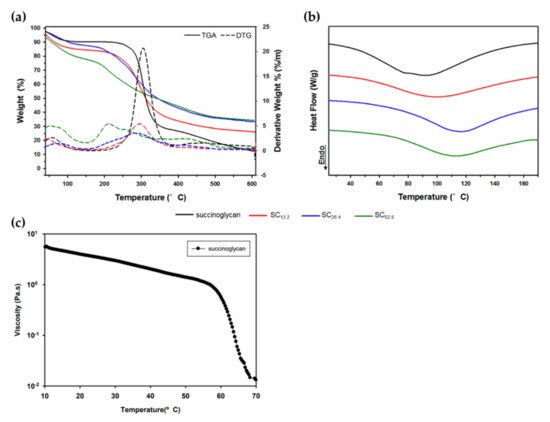
Figure 1. Comparison of the thermal analysis for succinoglycan with different Cr3+ concentrations: (a) thermogravimetric analysis (TGA) and (b) differential scanning calorimetry (DSC). (c) Viscosity change during the first heating cycle of 1 wt.% solution of succinoglycan. Copyright © 2022 by the authors. Licensed MDPI, Basel, Switzerland.
A typical differential scanning calorimetry (DSC) thermogram of a succinoglycan solution is representatively described. The endothermic peak of DSC is generally due to the breakdown of hydrogen bonds and loss of hydroxyl groups when the sample is heated. Thus, the appearance of the endothermic peak is due to the disorder of the structure [12]. The melting temperature (Tm) typically refers to the transition of the material from a crystalline state to an amorphous state, so the transition temperature corresponds to the melting temperature Tm. As shown in Figure 1b,c, the Tm of succinoglycan is observed as 92.17 °C and the viscosity of succinoglycan decreased rapidly at temperatures above 60 °C. During heating, hydrogen bonds break and the double helix changes its structure, causing the fusion of aggregates and disruption of the network [13]. Thermal properties obtained by DSC support the view that succinoglycans are double helix at 25 °C and semi-flexible in their structural arrangement by melting into single strands above 65 °C.
3. Rheological Properties
Succinoglycan may have different chemical substituent ratios (pyruvyl, succinyl, acetyl groups) and molecular weight (Mw) depending on the type of strain and the culture conditions for each strain. Rheological properties, such as viscosity and storage/loss modulus, of succinoglycan can be adjusted depending on the measurement conditions (concentration, temperature, pH, etc.). High viscosity in aqueous solution is one of the main characteristics of this water-soluble polymer [14]. It exhibits an order-disorder transition at a characteristic temperature which depends on the ionic strength, counterion, and polymer concentration [15][16][17][18][19][20].
Succinoglycan has been compared to xanthan, a bacterial polysaccharide with a different molecular weight [21][22]. Although their rheological properties show many similarities, the expansion of the ordered conformation of succinoglycan is greater than that of xanthan [23][24]. The main differences in the behavior of the two polymers can be found in the structural transitions and the rheology of solutions at temperatures above Tm. Succinoglycan exhibits more abrupt and temperature-dependent conformational transitions, is a more randomly coiling molecule than xanthan above the Tm, and slowly loses stiffness as it passes through the Tm. In addition, rheological behavior of a succinoglycan was reported in dilute and semi-dilute solutions as a function of the shear rate, temperature, ionic strength, counterion, succinate content, and conformational structure. Viscosity dependence as a function of molecular weight and lacking succinate substituents was compared with that of the native polymer xanthan.
Several studies on the rheological effect on the difference in functional group and molecular weight of succinoglycan isolated from genetically modified strains have been conducted. The rheological effect of the selective removal of acetyl or succinyl substituents on the function of succinoglycan isolated in genetically modified Rhizobium meliloti 1021 was studied [25][26][27][28]. Removal of the acetyl substituent led to a decrease in the order-disorder transition temperature, whereas removal of the succinyl group led to an increase [29][30][31]. Consequently, it was found that removal of the succinyl group dramatically improved the pseudoplasticity of aqueous succinoglycan and increased the cooperativity of the order-disorder transition exhibited by polysaccharides [32][33][34][35]. Recently, a high yield of succinoglycan was obtained from the mutant strain Rhizobium radiobacter ATCC 19358 by NTG mutagenesis. Succinoglycan from the wild-type strain (SG-A) has two molecular weights of 1.55 × 107 Da and 1.26 × 106 Da depending on the culture conditions. On the other hand, the mutant succinoglycan (SG-N) was a homogeneous polysaccharide and had a molecular weight of 1.01 × 107 Da. Dynamic frequency sweep tests of SG-A and SG-N showed that the G′ and G″ curves crossed over at 65 °C, indicating a thermal-induced order-disordered transition conformation. From the results for the effect of concentration (2.5–15%) and temperature (25–75 °C) on the apparent viscosity of SG-A and SG-N, succinoglycan solution exhibited non-Newtonian and shear thinning behavior.
Afterwards, in order to increase the production yield of succinoglycan, a study was conducted using a sucrose-based carbon source in Rhizobium radiobactor (Agrobacterium tumefaciens) strain, and the physical properties of the thus-produced succinoglycan were studied. To investigate possible succinoglycan degradation at elevated temperatures, succinoglycan was dissolved in water at a concentration of 4 g/L and heated in the range of 25 to 90 °C for 5 h. The chemical identity and molecular integrity of the polymers were then confirmed by NMR analysis, rheological measurements, and MW measurements [36][37]. Other studies have reported the structural and rheological studies of succinoglycan prepared by fermentation of sucrose or date syrup under various conditions at concentrations (0.5, 1.0, 1.5, and 2.0% w/w), temperature (5, 25 and 40 °C), and pH (2.5, 4.0, 7.0, and 10.0). The results exhibited that shear thinning (pseudoplasticity) behavior and the viscosity of succinoglycan of date syrup medium was higher than that of sucrose medium at all tested concentrations. Succinoglycan solution (0.1–1.0%, w/v) also exhibited non-Newtonian and shear thinning behavior at shear rates that ranged from 0.01 s−1 to 1000 s−1. A weak gel with a concentration of 0.75% was obtained at room temperature (25 °C). The changes in the storage (G′) and loss (G″) modulus during the heating and cooling cycles indicated that succinoglycan can form a thermo-reversible gel. On the other hand, succinoglycan produced from sucrose (EPS-S) showed lower levels of succinylation and acetylation compared to succinoglycan produced from molasses (EPS-M). According to the TGA thermogram, not only was the melting temperature (Tm) of EPS-M much higher than that of EPS-S, but EPS-M was more thermally stable than EPS-S. In addition, the succinoglycan showed non-Newtonian and shear-thinning behavior, and the viscosity of EPS-M was higher than that of EPS-S. Production of the succinoglycan using the sugar cane molasses, sucrose, glucose, and lactose as carbon source was analyzed. The molecular weights of succinoglycan from lactose and sugar cane molasses were 2.734 × 106 g/mol and 2.326 × 106 g/mol, respectively. Rheological analysis of the succinoglycan at concentration (0.5–2.0%), temperature (5–75 °C), and pH (2.5–10.0) revealed non-Newtonian and shear thinning behavior.
Succinoglycan has been studied with the aim of developing a mucoadhesive agent. The investigation of the mucoadhesive properties of a series of polymers and their association was carried out by a rheological synergistic approach. The combination of succinoglycan or xanthan with guar hydroxypropyl trimonium chloride and lambda carrageenan is characterized by the highest mucosal adhesion. The mucosal adhesion of succinoglycan was adequate through various approaches, such as rheological synergy, tensile, and washout tests.
In addition, the effect of different extraction methods on production, rheological, and structural properties of succinoglycan were investigated [38]. Eleven different chemical and physical methods were tested for the extraction of succinoglycans from Rhizobium radiobacter CAS. Comparing the succinoglycan yields of all methods, the acetone method (3014 mg/L) proved the highest, followed by the cetyl-trimethyl-ammonium-bromide (CTAB 2939 mg/L) and vacuum evaporation (2804 mg/L) methods. Comparing the rheological properties of succinoglycan, succinoglycan recovered by acetone and CTAB method with a shear rate of 50 s−1 showed a tendency to make the solution highly viscous with viscosities of 150 and 146 mPa·s, respectively. The results showed that the physicochemical method for EPS extraction had a significant effect on the physical properties.
Recently, it was investigated whether hydrogels could be formed by mixing aqueous solutions of various metal ions, such as K+, Na+, Ca2+, Mg2+, Cu2+, Zn2+, Al3+, Fe3+, and Cr3+, with an aqueous succinoglycan solution. As shown in Figure 2a,b, physical gel formation occurred only when Cr3+ and Fe3+ solutions were added to the aqueous succinoglycan solution, and this was confirmed by measuring the difference between the storage modulus (G′) and the loss modulus (G″) through a rheological experiment. In addition, Figure 2c shows the results of the reverse vial test for each mixed solution corresponding to a different metal ion. Except for Fe3+ and Cr3, most metal ions did not induce physical gels. These results suggest that an appropriate amount of trivalent metal ion can coordinate effectively with the carboxyl group of succinoglycan, resulting in a denser and harder hydrogel network.
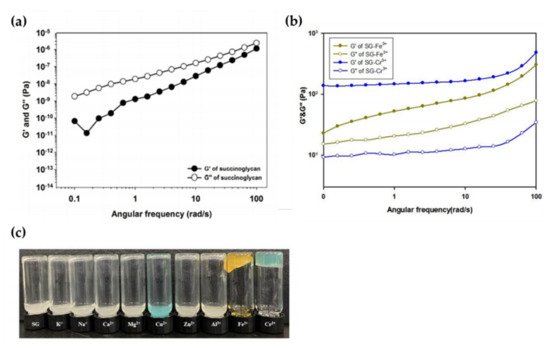
Figure 2. (a) Frequency sweep test of succinoglycan (1 wt.%), (b) Frequency sweep of aqueous succinoglycan solution containing metal ions Fe3+ and Cr3+, and (c) Gelling performance of succinoglycan with respect to the addition of various metal ions: Inverted vial test after mixing various metal ion solutions (0.25 M) with aqueous succinoglycan solution (2 wt.%). Copyright © 2022 by the authors. Licensed MDPI, Basel, Switzerland.
References
- Hu, Y.; Kim, Y.; Hong, I.; Kim, M.; Jung, S. Fabrication of Flexible pH-Responsive Agarose/Succinoglycan Hydrogels for Controlled Drug Release. Polymers 2021, 13, 2049.
- Labille, J.; Thomas, F.; Bihannic, I.; Santaella, C. Destabilization of montmorillonite suspensions by Ca2+ and succinoglycan. Clay Miner. 2003, 38, 173–185.
- Fidanza, M.; Dentini, M.; Crescenzi, V.; Del Vecchio, P. Influence of charged groups on the conformational stability of succinoglycan in dilute aqueous solution. Int. J. Biol. Macromol. 1989, 11, 372–376.
- Yamakawa, H. Helical Wormlike Chains in Polymer Solutions; Springer: Berlin/Heidelberg, Germany, 1997; Volume 1.
- Norisuye, T. Semiflexible polymers in dilute solution. Prog. Polym. Sci. 1993, 18, 543–584.
- Hugel, T.; Grosholz, M.; Clausen-Schaumann, H.; Pfau, A.; Gaub, H.; Seitz, M. Elasticity of single polyelectrolyte chains and their desorption from solid supports studied by AFM based single molecule force spectroscopy. Macromolecules 2001, 34, 1039–1047.
- Tricot, M. Comparison of experimental and theoretical persistence length of some polyelectrolytes at various ionic strengths. Macromolecules 1984, 17, 1698–1704.
- Takahashi, R.; Tokunou, H.; Kubota, K.; Ogawa, E.; Oida, T.; Kawase, T.; Nishinari, K. Solution properties of gellan gum: Change in chain stiffness between single-and double-stranded chains. Biomacromolecules 2004, 5, 516–523.
- Halder, U.; Banerjee, A.; Bandopadhyay, R. Structural and functional properties, biosynthesis, and patenting trends of Bacterial succinoglycan: A review. Indian J. Microbiol. 2017, 57, 278–284.
- Burova, T.V.; Golubeva, I.A.; Grinberg, N.V.; Mashkevich, A.Y.; Grinberg, V.Y.; Usov, A.I.; Navarini, L.; Cesàro, A. Calorimetric study of the order-disorder conformational transition in succinoglycan. Biopolymers 1996, 39, 517–529.
- Kim, S.; Jeong, D.; Lee, H.; Kim, D.; Jung, S. Succinoglycan dialdehyde-reinforced gelatin hydrogels with toughness and thermal stability. Int. J. Biol. Macromol. 2020, 149, 281–289.
- Choi, J.-M.; Kim, K.-T.; Cho, E.; Jung, S.-H. Solubility enhancement of salicylic acid by complexation with succinoglycan monomers isolated from Sinorhizobium meliloti. Bull. Korean Chem. Soc. 2012, 33, 2091–2094.
- Kim, K.; Cho, E.; Choi, J.M.; Kim, H.; Jang, A.; Choi, Y.; Yu, J.-H.; Jung, S. Intermolecular complexation of low-molecular-weight succinoglycans directs solubility enhancement of pindolol. Carbohydr. Polym. 2014, 106, 101–108.
- Milas, M.; Rinaudo, M. Properties of xanthan gum in aqueous solutions: Role of the conformational transition. Carbohydr. Res. 1986, 158, 191–204.
- Harada, T.; Amemura, A.; Jansson, P.-E.; Lindberg, B. Comparative studies of polysaccharides elaborated by Rhizobium, Alcaligenes, and Agrobacterium. Carbohydr. Res. 1979, 77, 285–288.
- Rinaudo, M.; Mils, M. Polyelectroyte behavior of a bacterial polysaccharide from Xanthomonas campestris: Comparison with carboxymethylcellulose. Biopolym. Orig. Res. Biomol. 1978, 17, 2663–2678.
- Milas, M.; Rinaudo, M.; Tinland, B. Comparative depolymerization of xanthan gum by ultrasonic and enzymic treatments. Rheological and structural properties. Carbohydr. Polym. 1986, 6, 95–107.
- Boutebba, A.; Milas, M.; Rinaudo, M. On the interchain associations in aqueous solutions of a succinoglycan polysaccharide. Int. J. Biol. Macromol. 1999, 24, 319–327.
- Manning, G.S. Counterion binding in polyelectrolyte theory. Acc. Chem. Res. 1979, 12, 443–449.
- Manning, G.S. Limiting laws and counterion condensation in polyelectrolyte solutions I. Colligative properties. J. Chem. Phys. 1969, 51, 924–933.
- Gravanis, G.; Milas, M.; Rinaudo, M.; Tinland, B. Comparative behavior of the bacterial polysaccharides xanthan and succinoglycan. Carbohydr. Res. 1987, 160, 259–265.
- Barry, S.I.; Gowman, L.M.; Ethier, C.R. Obtaining the concentration-dependent diffusion coefficient from ultrafiltration experiments: Application to hyaluronate. Biopolymers 1996, 39, 1–11.
- Kido, S.; Nakanishi, T.; Norisuye, T.; Kaneda, I.; Yanaki, T. Ordered conformation of succinoglycan in aqueous sodium chloride. Biomacromolecules 2001, 2, 952–957.
- Kaneda, I.; Kobayashi, A.; Miyazaw, K.; Yanaki, T. Double helix of Agrobacterium tumefaciens succinoglycan in dilute solution. Polymer 2002, 43, 1301–1305.
- York, G.M.; Walker, G.C. The succinyl and acetyl modifications of succinoglycan influence susceptibility of succinoglycan to cleavage by the Rhizobium meliloti glycanases ExoK and ExsH. J. Bacteriol. 1998, 180, 4184–4191.
- Leigh, J.A.; Reed, J.W.; Hanks, J.F.; Hirsch, A.M.; Walker, G.C. Rhizobium meliloti mutants that fail to succinylate their calcofluor-binding exopolysaccharide are defective in nodule invasion. Cell 1987, 51, 579–587.
- Reuber, T.L.; Walker, G.C. The acetyl substituent of succinoglycan is not necessary for alfalfa nodule invasion by Rhizobium meliloti Rm1021. J. Bacteriol. 1993, 175, 3653–3655.
- Reuber, T.L.; Walker, G.C. Biosynthesis of succinoglycan, a symbiotically important exopolysaccharide of Rhizobium meliloti. Cell 1993, 74, 269–280.
- Morris, V.; Brownsey, G.; Gunning, A.; Harris, J. Gelation of the extracellular polysaccharide produced by Agrobacterium rhizogenes. Carbohydr. Polym. 1990, 13, 221–225.
- Dentini, M.; Crescenzi, V.; Blasi, D. Conformational properties of xanthan derivatives in dilute aqueous solution. Int. J. Biol. Macromol. 1984, 6, 93–98.
- Rinaudo, M. Role of substituents on the properties of some polysaccharides. Biomacromolecules 2004, 5, 1155–1165.
- Morfin, I.; Reed, W.F.; Rinaudo, M.; Borsali, R. Further evidence of liquid-like correlations in polyelectrolyte solutions. J. Phys. II 1994, 4, 1001–1019.
- Boutebba, A.; Milas, M.; Rinaudo, M. Order—Disorder conformational transition in succinoglycan: Calorimetric measurements. Biopolym. Orig. Res. Biomol. 1997, 42, 811–819.
- Milas, M.; Rinaudo, M.; Knipper, M.; Schuppiser, J.L. Flow and viscoelastic properties of xanthan gum solutions. Macromolecules 1990, 23, 2506–2511.
- Robinson, G.; Manning, C.E.; Morris, E.R. Conformation and physical properties of the bacterial polysaccharides gellan, welan, and rhamsan. In Food Polymers, Gels and Colloids; Elsevier: Amsterdam, The Netherlands, 1991; pp. 22–33.
- Rinaudo, M.; Milas, M.; Lambert, F.; Vincendon, M. Proton and carbon-13 NMR investigation of xanthan gum. Macromolecules 1983, 16, 816–819.
- Chouly, C.; Colquhoun, I.J.; Jodelet, A.; York, G.; Walker, G.C. NMR studies of succinoglycan repeating-unit octasaccharides from Rhizobium meliloti and Agrobacterium radiobacter. Int. J. Biol. Macromol. 1995, 17, 357–363.
- Andhare, P.; Goswami, D.; Delattre, C.; Pierre, G.; Michaud, P.; Pathak, H. Edifying the strategy for the finest extraction of succinoglycan from Rhizobium radiobacter strain CAS. Appl. Biol. Chem. 2017, 60, 339–348.
More
Information
Subjects:
Biochemical Research Methods
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
24 Jan 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




