Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nanako Kawaguchi | + 2034 word(s) | 2034 | 2022-01-17 07:45:00 | | | |
| 2 | Beatrix Zheng | Meta information modification | 2034 | 2022-01-23 04:51:50 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kawaguchi, N. Stem Cell Studies in Cardiovascular Biology and Medicine. Encyclopedia. Available online: https://encyclopedia.pub/entry/18647 (accessed on 07 February 2026).
Kawaguchi N. Stem Cell Studies in Cardiovascular Biology and Medicine. Encyclopedia. Available at: https://encyclopedia.pub/entry/18647. Accessed February 07, 2026.
Kawaguchi, Nanako. "Stem Cell Studies in Cardiovascular Biology and Medicine" Encyclopedia, https://encyclopedia.pub/entry/18647 (accessed February 07, 2026).
Kawaguchi, N. (2022, January 22). Stem Cell Studies in Cardiovascular Biology and Medicine. In Encyclopedia. https://encyclopedia.pub/entry/18647
Kawaguchi, Nanako. "Stem Cell Studies in Cardiovascular Biology and Medicine." Encyclopedia. Web. 22 January, 2022.
Copy Citation
Resident macrophages can trigger cell regeneration. As macrophages express chemokine receptors, chemokines are also important in the regulation of macrophages. Exosomes are used for cell-cell communication in macrophages and the surrounding cells. Therefore, macrophages may play a key role in regenerative medicine in the future.
induced pluripotent stem cell
iPSC
bone marrow stem cell
adipose-derived stem cell
exosome
macrophage
chemokine
CXCR4
inflammation
1. Somatic Stem Cells
1.1. Bone Marrow Stem Cells (BMSCs) and Mesenchymal Stem Cells (MSCs)
BMSCs were the first somatic stem cells to be identified as multipotent, with the ability to differentiate into mesenchymal cells such as adipocytes and osteoblasts [1]. Soon after this discovery, BMSCs were also reported to have the ability to differentiate into cardiomyocytes in vivo and in vitro and alleviate myocardial infarction [2]. Since then, these cells have been extensively studied for their clinical and preclinical applications. BMSCs also contain stem/progenitor cells of hematopoietic and mesenchymal stem cells that are associated with angiogenesis-producing paracrine factors. Recent research has focused on paracrine factors and vesicles released from these cells. MSCs are present in the bone marrow (BM) and various other organs. Endothelial progenitor cells release various cytokines and growth factors, including vascular endothelial growth factor (VEGF), which is found in the peripheral blood [3]. However, these cells do not originate from the BM but from resident niches identified following sex-mismatched transplantation [4].
The positive effect of BMSC transplantation on myocardial infarction is attributed to its paracrine effects. Efforts have been made to improve the ability of BMSCs to treat myocardial infarction through (1) isolation of specific cell types such as CD133 [5], CD271 [6], and CD117 (c-kit)-positive cells; (2) genetically engineered cells overexpressing VEGF [7], hepatocyte growth factor (HGF) [8], and insulin-like growth factor (IGF) [9]; (3) use of exosomes derived from BMCs; (4) using microRNAs such as miR-19a/19b [10] and miR-29a [11]; and (5) using 3D structures of BMCs or engineered BMCs and exosomes.
Extracellular vesicles (EVs) and exosomes (exos) from BMSCs have been well-characterized. MSC-EVs and MSC-exos are approximately 100 nm in diameter, contain micro-RNA and mRNA, and express CD9, CD63, and CD81, which act as surface markers of their extracellular domains. They have been shown to have a curative effect on myocardial infarction [12]. MSC-exos also attenuate cardiac hypertrophy and fibrosis [13]. Fu et al. showed that miR-338 in MSC-exos cured myocardial infarction by inhibiting cardiomyocyte apoptosis [14]. Moreover, MSCs from the umbilical cord (UC) have more positive effects [15]. Zhang et al. also found that UC-MSC-exos could rejuvenate aged BM-MSCs, most likely via miR-136, by targeting apoptotic protease activating factor-1 (Apaf1) [15]. MSC-exos are eventually internalized by neighboring cells. Overall, UC-derived MSCs were more potent than BM-derived MSCs. The efficacy of MSCs derived from adipose tissue has also been studied and is discussed in the next section.
The fate of stem cells depends on the ECM, which determines substrate stiffness [16][17]. Computation is a useful tool for regulating this parameter. Urdeitx and Doneider used a piezoelectric fibered extracellular matrix in a 3D computational model [18] to calculate the intracellular force affected by the fiber. Computational models can be powerful tools for estimating precise cellular changes that connect differentiation.
1.2. Adipose-Derived MSCs
Similar characteristics have been identified in MSCs derived from adipose tissue and BM. For example, both cells have the capacity to differentiate into multiple cell types. They also release growth factors and cytokines, although not the same factors [19]. MSCs circulate in or exist in tissue niches surrounded by low oxygen levels. Cell populations and characteristics also differ between adult and neonatal tissues [20]. Adolfsson et al. compared MSCs derived from the BM with those derived from adipose tissue. They found that adipose-derived MSCs proliferated more than BM-derived MSCs, and adipose-derived MSCs had higher angiopoetin 1 (angpt1), Leukemia inhibitory factor (LIF), and Transforming growth factor (TGF)-β1 expression levels, but equal VEGF-A and HGF expression levels compared with BMSCs [21]. Conditioned medium from adipose-derived stromal cells has also been studied because of its positive ameliorating effect on various damaged tissues, including the infarcted heart, which occurs through paracrine factors [22]. Lu et al. fractionated conditioned media based on molecular weight and found that fractions over 50 kD protected the endothelium from barrier dysfunction caused by H2O2 and fractions less than 3 kD protected against apoptosis induced by tumor necrosis factor (TNF)-α [23]. Lai et al. reported that exosomes from conditioned media from adipose-derived MSCs contained high levels of miR-221/222 and attenuated myocardial infarction in a mouse model. Knockout of miR-221/222 in mice increased apoptosis and fibrosis; however, treatment with conditioned medium from adipose-derived MSCs decreased apoptosis and fibrosis [24]. Lee et al. found that intramuscular injection of conditioned media from adipose-derived MSCs attenuates ischemia in mice [25]. Taken together, exosomes from adipose-derived stromal cells ameliorated ischemia through the action of miR-221/222.
Attempts have been made to differentiate adipose-derived stem and stromal cells into cardiomyocytes. Seheli et al. reported that 5-azacytidine, a DNA methyltransferase inhibitor, played a role in this differentiation [26]. Darche et al. reported that adipose-derived stem/stromal cells can function as pacemaker cells [27]. However, Stepniewski et al. compared the abilities of iPSC-CMs and adipose-derived stem/stromal cells (derived CMs) to cure myocardial infarction and demonstrated better outcomes with iPSC-CMs [28].
1.3. Cardiac Stem Cells (CSCs)/Cardiac Progenitor Cells (CPCs)
Cardiac stem cells (CSCs) were originally found to be lineage (-) c-kit-positive cells in adult rat hearts [29]. CSCs have been reported to differentiate into small cardiomyocytes when cultured in a differentiation medium. Other cell markers, such as Sca-1 and abcg2, have also been found in adult rat hearts. The formation of the cardiosphere was also found to be a characteristic of CSCs, and clinical studies for post-myocardial infarction treatment using cardiosphere-derived cells have been performed and improvement was observed [30][31]. Cardiosphere-derived cells were found to grow as self-adherent clusters from subcultures of postnatal atrial or ventricular human biopsy specimens and from murine hearts [32][33]. These cardiac stem cell studies were reviewed by Matsa et al. [34]. Islet-1 has been found to be a distinct cardiac lineage cell progenitor in embryonic and neonatal mice and human hearts [35][36]. Interestingly, a recent study suggested that Islet-1 leads Gcn5 to bind to the GATA4/Nkx2.5 promoter region, which promotes cardiomyocyte differentiation in BMSCs [37]. Therefore, the introduction of Islet-1 into somatic stem cells has the potential to produce cardiomyocytes.
The existence of CSCs, which have recently been termed cardiac progenitor cells (CPCs), has been discussed since their discovery. As previously confirmed by us, c-Kit positive cells exist in adult rat hearts [38], and we isolated and characterized them after long-term culture and observed cardiac progenitor and BMSC characteristics [1]. Ellison et al. reported a modified method for the isolation of CSCs and showed that sufficient amounts of endogenous CSCs can be isolated from rats with heart injury using high-dose isoproterenol [39]. They also found that c-kit was not sufficient to enhance myogenesis, but other selection markers could [40]. Although these findings facilitate this area of research, discussions are ongoing. Recently, Vegnozzi et al. suggested that the positive repair effect of cardiac stem cell implantation for repairing damaged tissues could be induced by resident macrophages [41], as described in the next section. Hoving et al. isolated human CSCs (hCSCs) from the cultured tissue debris in a CSC medium and characterized their migration behavior in human serum. They found that CSC migration caused by human serum was inhibited by a p-38 MAPK inhibitor [42]. We isolated these cells in a similar manner, but by using a c-kit antibody after collecting the cells which were migrated from the tissue debris, and obtained multipotent stem cells [43], which were used for differentiation analysis [44]. Thus, the isolation methodology can be continuously modified and improved.
2. Stem Cell Microenvironment and Macrophage Involvement
The microenvironment that maintains stem cell quiescence in the BM is facilitated by niches consisting of CXC chemokine ligand (CXCL)12-abundant reticular (CAR) cells. CAR cells express high levels of CXCL12/SDF-1, stem cell factor (SCF), forkhead box C1 (FOXC1), and early B cell factor 3 (EBF3) in the murine BM. CAR cells have been identified in humans, and patients with chronic myeloid leukemia have reduced levels of these factors [45]. Chemokines such as CXCR4 play important roles in the migration and maintenance of these niches [46]. We previously observed the proliferation of CXCR4+ inflammatory cells in cultured BMCs using silibinin (CXCR4 antagonists), particularly when inflammation was activated [47]. Chemokines can function either positively or negatively during wound healing and tissue repair [48]. Interestingly, silibinin increases macrophage and neutrophil counts in cultured BM cells [47]. We first hypothesized that silibinin ameliorated PAH because it may bind CXCR4 positive inflammatory cells and inhibit these cells. However, since there are anti-inflammatory resident macrophages as we describe below, we now consider that silibinin may affect resident macrophages during damage healing.
Accumulating evidence has suggested that macrophages play an important role in stem cell regulation. In skeletal muscles, pax3 expressing muscle stem cells (MuSCs) differentiate into muscle cells following injury. Macrophages transiently migrate to the wound site, and dwelling macrophages are associated with MuSCs. Ablation of dwelling macrophages leads to a reduction in MuSCs [49]. Dwelling macrophages secrete nicotinamide phosphoribosyltransferase (Nampt), which stimulates myoblast proliferation. Interestingly, the C-C motif chemokine receptor 5 (Ccr5), a receptor for Nampt, is expressed by MuSCs. Thus, Nampt is hypothesized to function in muscle regeneration and is a potential therapeutic target. Furthermore, Vagnozzi et al. suggested that macrophages are key regulators in the healing of damage caused by an infarcted myocardium [41]. Attenuation of the infarcted heart was limited to the absence of CCR2+ and CX3CR1+ macrophages. Tissue-specific macrophages have also been identified [50]. Macrophages in the heart are heterogeneous and contain CCR2+ and CX3CR1+ subpopulations [51]. Different macrophage subpopulations can express different cell surface proteins and may have different functions [51], as is the case in the human system [52]. They can act either positively or negatively during the healing of damaged tissues.
Macrophages in the Heart
Resident cardiac macrophages that originate from the yolk sac or fetal liver during embryonic development are characterized by Ccr2− and MHC II lo/hi, whereas those that originate from the bone marrow during postnatal development are characterized by Ccr2+ and MHC II lhi [53][54]. Resident macrophages exert anti-inflammatory and antifibrotic effects in injured hearts. Inflammation causes fibrosis in the heart, resulting in arrhythmia. Bajpai et al. showed that tissue-resident Ccr2− macrophage-deficient mice had larger infarct sizes than control mice, while tissue-resident Ccr2+ macrophages could cause inflammation by promoting monocyte recruitment [55]. Monocytes can develop into macrophages, particularly during inflammation. Interestingly, they postulated that the population of tissue-resident Ccr2+ macrophages increases with age, causing further inflammation in the heart. Exosomes can also contribute to recovery from myocardial infarction and inhibit fibrosis [56]. Myocardial infarction biomarkers include specific miRNAs for the early diagnosis of hypertrophic cardiomyopathy (miR-21, miR425, and miR-744) and heart failure (miR34a, miR192, and miR-194), which are released by exosomes [56]. Intracellular communication has also recently been considered as a factor [57]. Extracellular vesicles from cardiac-derived adherent proliferating (CardAP) cells enhance angiogenesis in human umbilical vein endothelial cells (HUVECs) [58]. Angiogenesis can also play a role in recovery from myocardial infarction through the supplementation of oxygen and nutrients to the infarcted area.
Macrophages are classified as M1 or M2; M1 macrophages are inflammatory, whereas M2 macrophages are anti-inflammatory in nature. Exosomes from ESCs reduce the inflammation caused by doxorubicin (DOX)-induced cardiotoxicity, which can lead to heart failure [59] and an increase in M2 macrophages. DOX is an effective antineoplastic agent with adverse cardiotoxic effects. Macrophages secrete exosomes containing miR-155, which promote inflammation during cardiac injury [60]. Wang et al. found that miR-155 in cardiac fibroblasts was derived from exosomes secreted by macrophages [60]. In contrast, exosomes derived from M1-like macrophages are often secreted after myocardial infarction and promote cardiac dysfunction. Regenerative medicines that inhibit M1-like macrophages or enhance M2-like macrophages can be developed as potential treatments. Taken together, macrophages can function as key regulators, receiving signals from exosomes or cytokines secreted by myogenic or non-myogenic cells (Figure 1). Therefore, macrophages are likely to be receiving increased attention in regenerative medicine.
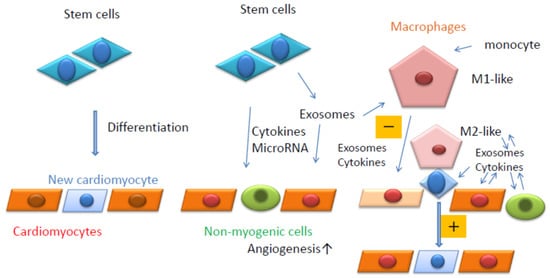
Figure 1. Summary of the current review. Stem cells can differentiate into cardiomyocytes (Left). Stem cells can release cytokines, microRNAs, and exosomes. Exosomes also contain cytokines and microRNAs (Middle). Resident macrophages can contact stem cells in close proximity to cardiomyocytes and induce their differentiation into cardiomyocytes (Right). Resident macrophages and monocyte-derived macrophages are affected by exosomes secreted by surrounding cells and can affect the surrounding cells positively (M2-like, +) or negatively (M1-like, −) (Right).
References
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147.
- Orlic, D.; Kajstura, J.; Chimenti, S.; Jakoniuk, I.; Anderson, S.M.; Li, B.; Pickel, J.; McKay, R.; Nadal-Ginard, B.; Bodine, D.M.; et al. Bone marrow cells regenerate infarcted myocardium. Nature 2001, 410, 701–705.
- Asahara, T.; Murohara, T.; Sullivan, A.; Silver, M.; van der Zee, R.; Li, T.; Witzenbichler, B.; Schatteman, G.; Isner, J.M. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997, 275, 964–967.
- Fujisawa, T.; Tura-Ceide, O.; Hunter, A.; Mitchell, A.; Vesey, A.; Medine, C.; Gallogly, S.; Hadoke, P.W.F.; Keith, C.; Sproul, A.; et al. Endothelial Progenitor Cells Do Not Originate From the Bone Marrow. Circulation 2019, 140, 1524–1526.
- Soetisna, T.W.; Sukmawan, R.; Setianto, B.; Mansyur, M.; Murni, T.W.; Listiyaningsih, E.; Santoso, A. Combined transepicardial and transseptal implantation of autologous CD 133+ bone marrow cells during bypass grafting improves cardiac function in patients with low ejection fraction. J. Card. Surg. 2020, 35, 740–746.
- Sasse, S.; Skorska, A.; Lux, C.A.; Steinhoff, G.; David, R.; Gaebel, R. Angiogenic Potential of Bone Marrow Derived CD133+ and CD271+ Intramyocardial Stem Cell Trans-Plantation Post MI. Cells 2019, 9, 78.
- Yang, J.; Zhou, W.; Zheng, W.; Ma, Y.; Lin, L.; Tang, T.; Liu, J.; Yu, J.; Zhou, X.; Hu, J. Effects of myocardial transplantation of marrow mesenchymal stem cells transfected with vascular endothelial growth factor for the improvement of heart function and angiogenesis after myocardial infarction. Cardiology 2007, 107, 17–29.
- Park, B.W.; Jung, S.H.; Das, S.; Lee, S.M.; Park, J.H.; Kim, H.; Hwang, J.W.; Lee, S.; Kim, H.J.; Kim, H.Y.; et al. In vivo priming of human mesenchymal stem cells with hepatocyte growth factor-engineered mesenchymal stem cells promotes therapeutic potential for cardiac repair. Sci. Adv. 2020, 6, eaay6994.
- Haider, H.K.; Jiang, S.; Idris, N.M.; Ashraf, M. IGF-1-overexpressing mesenchymal stem cells accelerate bone marrow stem cell mobilization via paracrine activation of SDF-1alpha/CXCR4 signaling to promote myocardial repair. Circ. Res. 2008, 103, 1300–1308.
- Yang, W.; Han, Y.; Yang, C.; Chen, Y.; Zhao, W.; Su, X.; Yang, K.; Jin, W. MicroRNA-19b-1 reverses ischaemia-induced heart failure by inhibiting cardiomyocyte apoptosis and targeting Bcl2 l11/BIM. Heart Vessel. 2019, 34, 1221–1229.
- Li, K.S.; Jiang, W.P.; Li, Q.C.; Zhang, H.W.; Bai, Y.; Zhang, X.; Li, H.Y. MiR-29a in mesenchymal stem cells inhibits FSTL1 secretion and promotes cardiac myocyte apoptosis in hypoxia-reoxygenation injury. Cardiovasc. Pathol. 2020, 46, 107180.
- Firoozi, S.; Pahlavan, S.; Ghanian, M.H.; Rabbani, S.; Barekat, M.; Nazari, A.; Pakzad, M.; Shekari, F.; Hassani, S.N.; Moslem, F.; et al. Mesenchymal stem cell-derived extracellular vesicles alone or in conjunction with a SDKP-conjugated self-assembling peptide improve a rat model of myocardial infarction. Biochem. Biophys. Res. Commun. 2020, 524, 903–909.
- Chen, F.; Li, X.; Zhao, J.; Geng, J.; Xie, J.; Xu, B. Bone marrow mesenchymal stem cell-derived exosomes attenuate cardiac hypertrophy and fibrosis in pressure overload induced remodeling. In Vitro Cell Dev. Biol. Anim. 2020, 56, 567–576.
- Fu, D.L.; Jiang, H.; Li, C.Y.; Gao, T.; Liu, M.R.; Li, H.W. MicroRNA-338 in MSCs-derived exosomes inhibits cardiomyocyte apoptosis in myocardial infarction. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 10107–10117.
- Zhang, N.; Zhu, J.; Ma, Q.; Zhao, Y.; Wang, Y.; Hu, X.; Chen, J.; Zhu, W.; Han, Z.; Yu, H. Exosomes derived from human umbilical cord MSCs rejuvenate aged MSCs and enhance their functions for myocardial repair. Stem Cell Res. Ther. 2020, 11, 273.
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689.
- Gumbiner, B.M. Cell adhesion: The molecular basis of tissue architecture and morphogenesis. Cell 1996, 84, 345–357.
- Urdeitx, P.; Doweidar, M.H. Enhanced Piezoelectric Fibered Extracellular Matrix to Promote Cardiomyocyte Maturation and Tissue Formation: A 3D Computational Model. Biology 2021, 10, 135.
- Strioga, M.; Viswanathan, S.; Darinskas, A.; Slaby, O.; Michalek, J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012, 21, 2724–2752.
- Hass, R.; Kasper, C.; Böhm, S.; Jacobs, R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal. 2011, 9, 12.
- Adolfsson, E.; Helenius, G.; Friberg, Ö.; Samano, N.; Frøbert, O.; Johansson, K. Bone marrow- and adipose tissue-derived mesenchymal stem cells from donors with coronary artery disease; growth, yield, gene expression and the effect of oxygen concentration. Scand. J. Clin. Lab. Investig. 2020, 80, 318–326.
- Liang, X.; Ding, Y.; Zhang, Y.; Tse, H.F.; Lian, Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: Current status and perspectives. Cell Transplant. 2014, 23, 1045–1059.
- Lu, H.; Merfeld-Clauss, S.; Jawed, Y.; March, K.L.; Coleman, M.E.; Bogatcheva, N.V. Distinct Factors Secreted by Adipose Stromal Cells Protect the Endothelium From Barrier Dysfunction and Apoptosis. Front. Cell Dev. Biol. 2020, 8, 584653.
- Lai, T.C.; Lee, T.L.; Chang, Y.C.; Chen, Y.C.; Lin, S.R.; Lin, S.W.; Pu, C.M.; Tsai, J.S.; Chen, Y.L. MicroRNA-221/222 Mediates ADSC-Exosome-Induced Cardioprotection Against Ischemia/Reperfusion by Targeting PUMA and ETS-1. Front. Cell Dev. Biol. 2020, 8, 569150.
- Lee, T.L.; Lai, T.C.; Lin, S.R.; Lin, S.W.; Chen, Y.C.; Pu, C.M.; Lee, I.T.; Tsai, J.S.; Lee, C.W.; Chen, Y.L. Conditioned medium from adipose-derived stem cells attenuates ischemia/reperfusion-induced cardiac injury through the microRNA-221/222/PUMA/ETS-1 pathway. Theranostics 2021, 11, 3131–3149.
- Saheli, M.; Pirhajati Mahabadi, V.; Mesbah-Namin, S.A.; Seifalian, A.; Bagheri-Hosseinabadi, Z. DNA methyltransferase inhibitor 5-azacytidine in high dose promotes ultrastructural maturation of cardiomyocyte. Stem Cell Investig. 2020, 7, 22.
- Darche, F.F.; Rivinius, R.; Rahm, A.K.; Köllensperger, E.; Leimer, U.; Germann, G.; Reiss, M.; Koenen, M.; Katus, H.A.; Thomas, D.; et al. In vivo cardiac pacemaker function of differentiated human mesenchymal stem cells from adipose tissue transplanted into porcine hearts. World J. Stem Cells 2020, 12, 1133–1151.
- Stępniewski, J.; Tomczyk, M.; Andrysiak, K.; Kraszewska, I.; Martyniak, A.; Langrzyk, A.; Kulik, K.; Wiśniewska, E.; Jeż, M.; Florczyk-Soluch, U.; et al. Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes, in Contrast to Adipose Tissue-Derived Stromal Cells, Efficiently Improve Heart Function in Murine Model of Myocardial Infarction. Biomedicines 2020, 8, 578.
- Beltrami, A.P.; Barlucchi, L.; Torella, D.; Baker, M.; Limana, F.; Chimenti, S.; Kasahara, H.; Rota, M.; Musso, E.; Urbanek, K.; et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 2003, 114, 763–776.
- Ostovaneh, M.R.; Makkar, R.R.; Ambale-Venkatesh, B.; Ascheim, D.; Chakravarty, T.; Henry, T.D.; Kowalchuk, G.; Aguirre, F.V.; Kereiakes, D.J.; Povsic, T.J.; et al. Effect of cardiosphere-derived cells on segmental myocardial function after myocardial infarction: ALLSTAR randomised clinical trial. Open Heart 2021, 8, e001614.
- Malliaras, K.; Makkar, R.R.; Smith, R.R.; Cheng, K.; Wu, E.; Bonow, R.O.; Marbán, L.; Mendizabal, A.; Cingolani, E.; Johnston, P.V.; et al. Intracoronary cardiosphere-derived cells after myocardial infarction: Evidence of therapeutic regeneration in the final 1-year results of the CADUCEUS trial (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction). J. Am. Coll Cardiol. 2014, 63, 110–122.
- Messina, E.; De Angelis, L.; Frati, G.; Morrone, S.; Chimenti, S.; Fiordaliso, F.; Salio, M.; Battaglia, M.; Latronico, M.V.; Coletta, M.; et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ. Res. 2004, 95, 911–921.
- Smith, R.R.; Barile, L.; Cho, H.C.; Leppo, M.K.; Hare, J.M.; Messina, E.; Giacomello, A.; Abraham, M.R.; Marbán, E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation 2007, 115, 896–908.
- Matsa, E.; Sallam, K.; Wu, J.C. Cardiac stem cell biology: Glimpse of the past, present, and future. Circ. Res. 2014, 114, 21–27.
- Laugwitz, K.L.; Moretti, A.; Lam, J.; Gruber, P.; Chen, Y.; Woodard, S.; Lin, L.Z.; Cai, C.L.; Lu, M.M.; Reth, M.; et al. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature 2005, 433, 647–653.
- Moretti, A.; Caron, L.; Nakano, A.; Lam, J.T.; Bernshausen, A.; Chen, Y.; Qyang, Y.; Bu, L.; Sasaki, M.; Martin-Puig, S.; et al. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell 2006, 127, 1151–1165.
- Xu, H.; Zhou, Q.; Yi, Q.; Tan, B.; Tian, J.; Chen, X.; Wang, Y.; Yu, X.; Zhu, J. Islet-1 synergizes with Gcn5 to promote MSC differentiation into cardiomyocytes. Sci. Rep. 2020, 10, 1817.
- Kawaguchi, N. Stem cells for cardiac regeneration and possible roles of the transforming growth factor-β superfamily. Biomol. Concepts 2012, 3, 99–106.
- Ellison, G.M.; Vicinanza, C.; Smith, A.J.; Aquila, I.; Leone, A.; Waring, C.D.; Henning, B.J.; Stirparo, G.G.; Papait, R.; Scarfò, M.; et al. Adult c-kit(pos) cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair. Cell 2013, 154, 827–842.
- Vicinanza, C.; Aquila, I.; Scalise, M.; Cristiano, F.; Marino, F.; Cianflone, E.; Mancuso, T.; Marotta, P.; Sacco, W.; Lewis, F.C.; et al. Adult cardiac stem cells are multipotent and robustly myogenic: C-kit expression is necessary but not sufficient for their identification. Cell Death Differ. 2017, 24, 2101–2116.
- Vagnozzi, R.J.; Maillet, M.; Sargent, M.A.; Khalil, H.; Johansen, A.K.Z.; Schwanekamp, J.A.; York, A.J.; Huang, V.; Nahrendorf, M.; Sadayappan, S.; et al. An acute immune response underlies the benefit of cardiac stem cell therapy. Nature 2020, 577, 405–409.
- Höving, A.L.; Schmitz, J.; Schmidt, K.E.; Greiner, J.F.W.; Knabbe, C.; Kaltschmidt, B.; Grünberger, A.; Kaltschmidt, C. Human Blood Serum Induces p38-MAPK- and Hsp27-Dependent Migration Dynamics of Adult Human Cardiac Stem Cells: Single-Cell Analysis via a Microfluidic-Based Cultivation Platform. Biology 2021, 10, 708.
- Kamrul Hasan, M.; Komoike, Y.; Tsunesumi, S.; Nakao, R.; Nagao, H.; Matsuoka, R.; Kawaguchi, N. Myogenic differentiation in atrium-derived adult cardiac pluripotent cells and the transcriptional regulation of GATA4 and myogenin on ANP promoter. Genes Cells 2010, 15, 439–454.
- Kawaguchi, N.; Nakao, R.; Yamaguchi, M.; Ogawa, D.; Matsuoka, R. TGF-beta superfamily regulates a switch that mediates differentiation either into adipocytes or myocytes in left atrium derived pluripotent cells (LA-PCS). Biochem. Biophys Res. Commun. 2010, 396, 619–625.
- Aoki, K.; Kurashige, M.; Ichii, M.; Higaki, K.; Sugiyama, T.; Kaito, T.; Ando, W.; Sugano, N.; Sakai, T.; Shibayama, H.; et al. Identification of CXCL12-abundant reticular cells in human adult bone marrow. Br. J. Haematol. 2021, 193, 659–668.
- Singh, P.; Mohammad, K.S.; Pelus, L.M. CXCR4 expression in the bone marrow microenvironment is required for hematopoietic stem and progenitor cell maintenance and early hematopoietic regeneration after myeloablation. Stem Cells 2020, 38, 849–859.
- Zhang, T.; Kawaguchi, N.; Tsuji, K.; Hayama, E.; Furutani, Y.; Sugiyama, H.; Nakanishi, T. Silibinin Upregulates CXCR4 Expression in Cultured Bone Marrow Cells (BMCs) Especially in Pulmonary Arterial Hypertension Rat Model. Cells 2020, 9, 1276.
- Kawaguchi, N.; Zhang, T.T.; Nakanishi, T. Involvement of CXCR4 in Normal and Abnormal Development. Cells 2019, 8, 185.
- Ratnayake, D.; Nguyen, P.D.; Rossello, F.J.; Wimmer, V.C.; Tan, J.L.; Galvis, L.A.; Julier, Z.; Wood, A.J.; Boudier, T.; Isiaku, A.I.; et al. Macrophages provide a transient muscle stem cell niche via NAMPT secretion. Nature 2021, 591, 281–287.
- Wu, Y.; Hirschi, K.K. Tissue-Resident Macrophage Development and Function. Front. Cell Dev. Biol. 2021, 8, 617879.
- Zaman, R.; Hamidzada, H.; Epelman, S. Exploring cardiac macrophage heterogeneity in the healthy and diseased myocardium. Curr. Opin. Immunol. 2021, 68, 54–63.
- Bajpai, G.; Schneider, C.; Wong, N.; Bredemeyer, A.; Hulsmans, M.; Nahrendorf, M.; Epelman, S.; Kreisel, D.; Liu, Y.; Itoh, A.; et al. The human heart contains distinct macrophage subsets with divergent origins and functions. Nat. Med. 2018, 24, 1234–1245.
- Alvarez-Argote, S.; O’Meara, C.C. The Evolving Roles of Cardiac Macrophages in Homeostasis, Regeneration, and Repair. Int. J. Mol. Sci. 2021, 22, 7923.
- Lavine, K.J.; Epelman, S.; Uchida, K.; Weber, K.J.; Nichols, C.G.; Schilling, J.D.; Ornitz, D.M.; Randolph, G.J.; Mann, D.L. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc. Natl. Acad. Sci. USA 2014, 111, 16029–16034.
- Bajpai, G.; Bredemeyer, A.; Li, W.; Zaitsev, K.; Koenig, A.L.; Lokshina, I.; Mohan, J.; Ivey, B.; Hsiao, H.M.; Weinheimer, C.; et al. Tissue Resident CCR2- and CCR2+ Cardiac Macrophages Differentially Orchestrate Monocyte Recruitment and Fate Specification Following Myocardial Injury. Circ. Res. 2019, 124, 263–278.
- Ranjan, P.; Kumari, R.; Verma, S.K. Cardiac Fibroblasts and Cardiac Fibrosis: Precise Role of Exosomes. Front. Cell Dev. Biol. 2019, 7, 318.
- Koohsarian, P.; Talebi, A.; Rahnama, M.A.; Zomorrod, M.S.; Kaviani, S.; Jalili, A. Reviewing the role of cardiac exosomes in myocardial repair at a glance. Cell Biol. Int. 2021, 45, 1352–1363.
- Singla, D.K.; Johnson, T.A.; Tavakoli Dargani, Z. Exosome Treatment Enhances Anti-Inflammatory M2 Macrophages and Reduces Inflammation-Induced Pyroptosis in Doxorubicin-Induced Cardiomyopathy. Cells 2019, 10, 1224.
- Beez, C.M.; Schneider, M.; Haag, M.; Pappritz, K.; Van Linthout, S.; Sittinger, M.; Seifert, M. Cardiac Extracellular Vesicles (EVs) Released in the Presence or Absence of Inflammatory Cues Support Angiogenesis in Different Manners. Int. J. Mol. Sci. 2019, 20, 6363.
- Wang, C.; Zhang, C.; Liu, L.A.X.; Chen, B.; Li, Y.; Du, J. Macrophage-Derived mir-155-Containing Exosomes Suppress Fibroblast Proliferation and Promote Fibroblast Inflammation during Cardiac Injury. Mol. Ther. 2017, 25, 192–204.
More
Information
Subjects:
Cardiac & Cardiovascular Systems
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
617
Revisions:
2 times
(View History)
Update Date:
23 Jan 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




