Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ahmed M. El-Zohry | + 1535 word(s) | 1535 | 2022-01-12 10:58:09 | | | |
| 2 | Vicky Zhou | -8 word(s) | 1527 | 2022-01-21 09:04:47 | | | | |
| 3 | Vicky Zhou | Meta information modification | 1527 | 2022-01-24 04:09:49 | | | | |
| 4 | Vicky Zhou | Meta information modification | 1527 | 2022-01-28 09:26:58 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
El-Zohry, A.M. CO Molecules Detection on Metal Surfaces. Encyclopedia. Available online: https://encyclopedia.pub/entry/18603 (accessed on 16 January 2026).
El-Zohry AM. CO Molecules Detection on Metal Surfaces. Encyclopedia. Available at: https://encyclopedia.pub/entry/18603. Accessed January 16, 2026.
El-Zohry, Ahmed M.. "CO Molecules Detection on Metal Surfaces" Encyclopedia, https://encyclopedia.pub/entry/18603 (accessed January 16, 2026).
El-Zohry, A.M. (2022, January 21). CO Molecules Detection on Metal Surfaces. In Encyclopedia. https://encyclopedia.pub/entry/18603
El-Zohry, Ahmed M.. "CO Molecules Detection on Metal Surfaces." Encyclopedia. Web. 21 January, 2022.
Copy Citation
Detection of intermediates during the catalytic process by infrared techniques has been widely implemented for many important reactions. For the reduction of CO2 into hydrocarbons on metal surfaces, CO molecule is one of the most important transient species to be followed due to its involvement in several products’ pathways, and its distinct vibrational features.
vibrational oscillation
carbon monoxide
interfaces
1. Introduction
Successful reduction of CO2 gas into hydrocarbons “reverse fuel” will contribute to solve two overlapping global problems related to the global warming caused by the excess of CO2 in the atmosphere, and the high demand of hydrocarbons to be utilized in the expanding urbanization [1][2]. However, attaining an efficient route to do this reduction process is still in demand, since many parameters are not well under control. Knowing these parameters will happen by understanding key intermediates for such reduction process, especially under operational conditions such as the presence of external bias [3][4][5][6][7].
One of these important intermediates during the CO2 reduction process is the CO (carbon monoxide). CO is an important surface intermediate for many catalytic reactions including methanol oxidation, and CO2 reduction process [8]. Depending on the nature of the catalyst and the working conditions with respect to electrolyte, pH, temperature, and other diverse ions, several reactions of CO2 reduction can take place. Each of the following reactions has required redox energy (versus SHE at pH = 7) (Some literature uses NHE instead; however, the relation between them is (ESHE = ENHE+0.059 pH)), and certain amount of moles for electrons and protons are needed. The lowest kinetic parameter needed to reduce CO2 is by using two moles of electrons as the following equation [3][9][10].
Mixing the resultant CO and H2 gases on metal surfaces under different conditions can produce varieties of hydrocarbons such as CH3CH2OH, CH3OH, and CH4 [11].
In addition, CO is one of the most transient species (COOH*, CO*, CHO*, and CH2O*) that show strong vibrational bands in the infrared (IR) region, allowing for better detection and analysis, see Figure 1 [6][12]. Moreover, due to the adsorption of CO2 and its transient species like CO on material surfaces, the concentration of such molecules is extremely low, and special spectroscopic techniques are needed to access such low concentrations with high accuracy. IR surface sensitive techniques can, however, be more sensitive than other techniques such as gas chromatography [2].
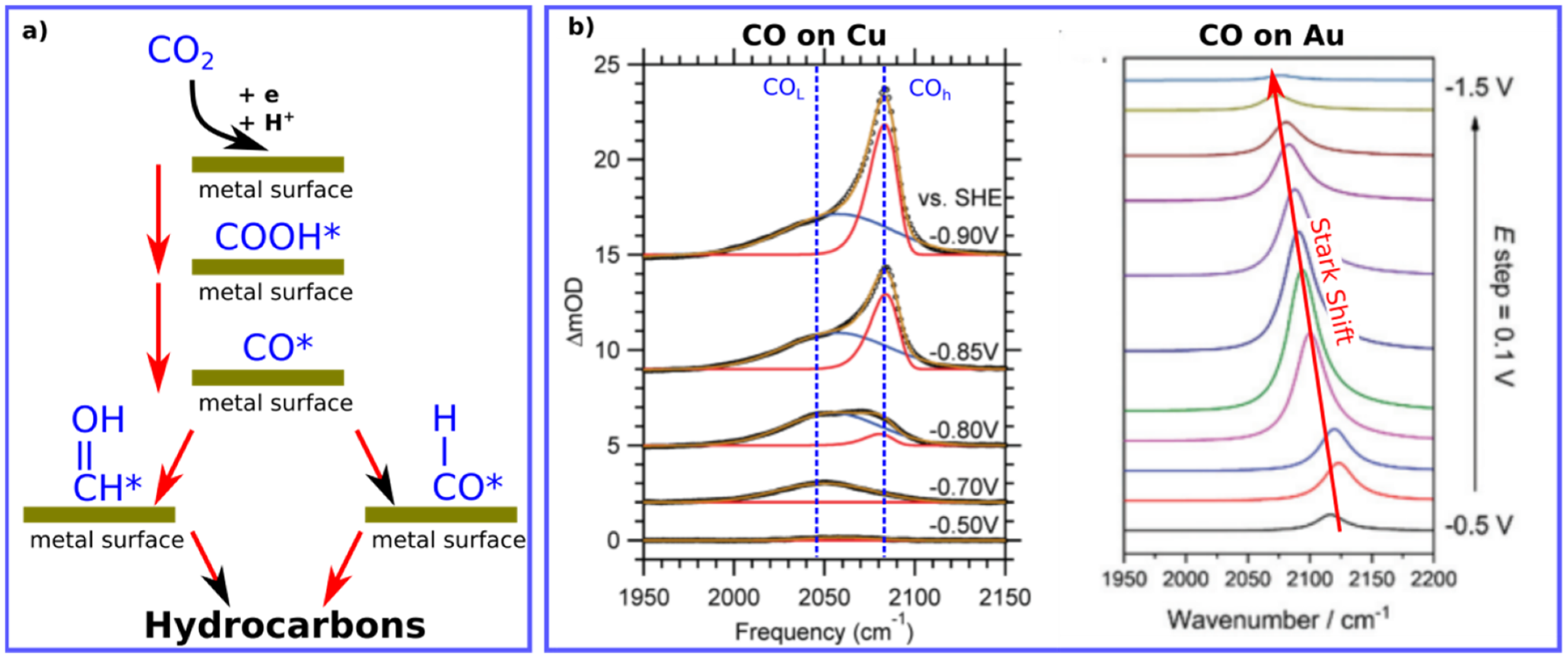
Figure 1. (a) Illustration for the important of CO intermediate during the reduction process of CO2 into hydrocarbons. (b) Typical positions of adsorbed CO molecules on Cu (ATR-SEIRAS) and Au (VSFG) surfaces found by IR surface-sensitive techniques. Data are adapted with permissions from references [7][13].
Various spectroscopic techniques at different spectral range can generally be utilized to investigate the dynamics of reactants, CO herein, at surfaces and interfaces such X-ray spectroscopic techniques [14][15]. However, utilizing the IR is more applicable for many groups due to its relative simplicity and accessibility in comparison to other methods based on X-rays. Moreover, IR energy is considered as a non-destructive source of energy that doesn’t generally cause any sample degradation or sample ionization; however, sample heating is expected. The IR source has to be utilized in a specific manner to be surface sensitive such as in ATR-SEIRAS (Attenuated total reflectance−surface enhanced infrared absorption spectroscopy), and (Vibrational sum frequency generation) VSFG. Figure 1 shows typical IR signatures for CO molecules adsorbed on metal surfaces during electrochemical processes by both techniques. The position of CO can vary depending on the utilized metal, applied voltage and the IR technique as well. For instance, CO on Cu measured by ATR-SEIRAS shows two vibrational bands, while change of on CO vibrational position on Au is shown using VSFG, see Figure 1.
Herein, general overview of the IR spectroscopy along with main IR sensitive techniques utilized (SEIRAS and VSFG) for detecting CO on metal surfaces along with literature values will be discussed, aiming for better understanding of the implications for utilizing such methods for following reaction dynamics of CO2 reduction on metal surfaces.
2. IR Spectral Signatures for CO on Metal Surfaces
Table 1 shows a list of IR signatures for CO detected on various metal surfaces using common IR techniques (SEIRAS, VSFG). Clearly, copper and gold are one of the most studied metal surfaces compared to other metals [12]. Generally Ag, Au, and Cu have moderate binding energies towards CO species, allowing them to produce hydrocarbons more than other metals that strongly stick CO molecules such Ir [16], see Figure 2. Among various metals, copper is known by efficient conversion of CO2 into hydrocarbons due to its intermediate binding energies towards various intermediate species (COOH, CO, CHO, and CH2O) [12][16][17]. On the other hand, copper is known by its weak stability towards corrosion and weak selectivity for specific products. However, gold is a robust metal that provide stable and steady surface property, making it attractive to study primary steps for the reduction of CO2 molecules. Still, gold is not an efficient surface to produce hydrocarbons, due to the higher energy required to stick species such as CHO and CH2O, see Figure 2. Clearly from Table 1, more studies using SEIRAS more than VSFG are found, reflecting the ease of utilizing SEIRAS technique over VSFG as mentioned before.
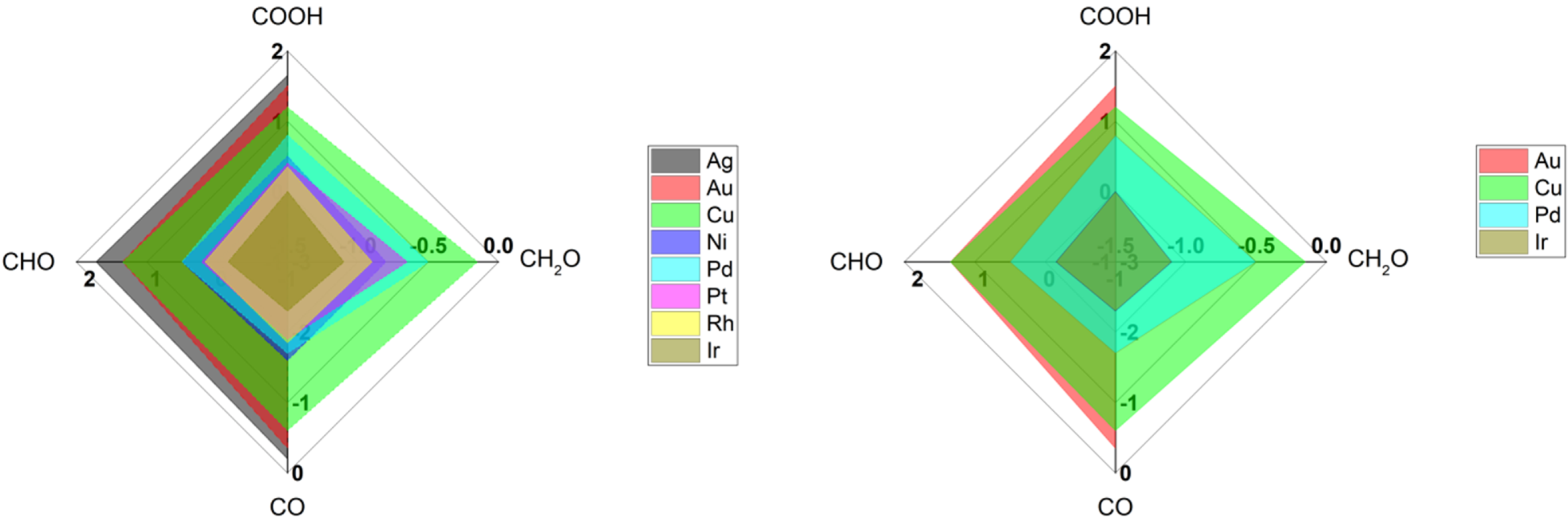
Figure 2. Radar plot for the binding energies for various key intermediate species for reduction of CO2 into hydrocarbons on various metal surfaces. The extracted values are found in Nørskov’s paper [16].
Interestingly, the average frequency positions of CO on Cu and Au surfaces are ca. 2075 cm−1. and 2100 cm−1, respectively (see Table 1). The higher frequency difference for CO on Cu, compared to CO vibration in gas phase, reflects the higher the bond force present between CO and Cu surface in comparison to CO on Au surfaces, matching with the binding energies reported for CO on Cu and Au surfaces [16], see Figure 2. Such a small difference in vibrational frequencies can correlate with the binding energies for CO on Cu vs Au, highlighting the ability of CO on Cu to interact with water (or protons) to form hydrocarbons but not on Au surfaces [6][16].
Interestingly, most of the found peak-positions of CO on metal surfaces indicate towards the preference of atop position for CO molecules on metals. The bridge position of CO is a matter of debate and it has been attributed to some contaminations in recent studies [18].
Apparently, there are no significant differences between extracted values for CO on metals using SEIRAS or VSFG. On one hand, this is an advantage for the SEIRAS technique as it is easier to operate and utilize. However, VSFG has lots of potential to extract more information like capturing short-lived intermediates, orientation and phase shifts during for the adsorbed CO molecules.
Table 1. Summarized data for the detection of adsorbed CO on metal surfaces using infrared techniques under applied external fields.
| Metal | IR Technique | Peak Position (cm−1) | Notes | References |
|---|---|---|---|---|
| Cu | VSFG | not available | assigned to fast CO conversion into products | [6] |
| Cu | SEIRAS | 2075–2065 | peak positions depend on applied voltage | [19] |
| Cu | SEIRAS | 2083–2052 | peak positions depend on applied voltage | [17] |
| Cu | SEIRAS | 2075–2025 | peak shifts with time after CO insertion at specific potential | [20] |
| Cu | SEIRAS | 2085 (2050) | high frequency CO and (low frequency CO) | [13] |
| Cu | SEIRAS | 2090–2060 | depends on copper surface properties | [21] |
| Cu | SEIRAS | 2100–2050 (1900–1800) |
atop CO shifts with voltage (bridge CO shifts with voltage) |
[22] |
| Ag | SEIRAS | 1900 | in ionic liquid | [23] |
| Pt | VSFG | 2060–2090 | peak positions depend on applied voltage | [24] |
| Au | VSFG | 2100–2125 | peak positions depend on applied voltage | [7] |
| Au | VSFG | 2110–2075 | peak positions depend on applied voltage | [4] |
| Au | VSFG | 2130–2080 | peak positions depend on applied voltage | [6] |
| Au | SEIRAS | 2100 | atop CO don’t shift with applied potential | [25] |
| Au | SEIRAS | 2100–2075 | peak positions depend on applied voltage | [19] |
| Au | SEIRAS | not available (2100–2075) |
under CO2 atmosphere due to adsorption instability of CO on Au but only under (CO atmosphere) | [18] |
| Au | ATR-SEIRAS | 2100–2120 | peak positions depend on the applied voltage | [18] |
3. Conclusions
The Electrocatalytic reduction of CO2 into hydrocarbons on metal surfaces is limited by several factors and to disassemble such process, IR surface sensitive techniques are excellent tools to achieve such approaches. Following the IR signature of CO during the catalysis process is very important due to the involvement of CO transient species in many reaction paths of CO2 reduction process, and the ability to capture the vibrational modes of CO on metal surfaces. As shown from this brief summary about the common IR techniques for detecting CO on metal surfaces, VSFG and SEIRAS are the common IR techniques to follow the electrochemical conversion of CO2 into hydrocarbons. The extracted data from IR measurements can be correlated with other theoretical approaches to understand such as the binding energies for various species. Both techniques have different geometrical configurations and can reveal different information for the catalytic process of CO2. From the current results present in the literature, there is no significant information that one technique can provide over the other one, despite the fact that VSFG can theoretically provide more information than SEIRAS, such as the contribution of surface response, the distinction between surface and double region dynamics, and the effect of dipole alignments on surfaces. Thus, clearly, due to its ease of utilization and low cost, SEIRAS is more common than VSFG to follow the electrochemical process of CO2 on metal surfaces. However, VSFG should be utilized under various conditions to explore more information that could not be shown by SEIRAS.
References
- Gernaat, D.E.H.J.; de Boer, H.S.; Daioglou, V.; Yalew, S.G.; Muller, C.; van Vuuren, D.P. Climate change impacts on renewable energy supply. Nat. Clim. Chang. 2021, 11, 119–125.
- De, S.; Dokania, A.; Ramirez, A.; Gascon, J. Advances in the Design of Heterogeneous Catalysts and Thermocatalytic Processes for CO2 Utilization. ACS Catal. 2020, 10, 14147–14185.
- Hori, Y. Electrochemical CO2 reduction on metal electrodes. In Modern Aspects of Electrochemistry; Springer: Berlin/Heidelberg, Germany, 2008; pp. 89–189.
- Wallentine, S.; Bandaranayake, S.; Biswas, S.; Baker, L.R. Direct Observation of Carbon Dioxide Electroreduction on Gold: Site Blocking by the Stern Layer Controls CO2 Adsorption Kinetics. J. Phys. Chem. Lett. 2020, 11, 8307–8313.
- Hiragond, C.B.; Kim, H.; Lee, J.; Sorcar, S.; Erkey, C.; In, S.I. Electrochemical CO2 Reduction to CO Catalyzed by 2D Nanostructures. Catalysts 2020, 10, 98.
- Zhu, S.Q.; Li, T.H.; Cai, W.B.; Shao, M.H. CO2 Electrochemical Reduction As Probed through Infrared Spectroscopy. ACS Energy Lett. 2019, 4, 682–689.
- Huang-Fu, Z.C.; Song, Q.T.; He, Y.H.; Wang, J.J.; Ye, J.Y.; Zhou, Z.Y.; Sun, S.G.; Wang, Z.H. Electrochemical CO2 reduction on Cu and Au electrodes studied using in situ sum frequency generation spectroscopy. Phys. Chem. Chem. Phys. 2019, 21, 25047–25053.
- Nakamura, J. Molecular Catalysts for Energy Conversion. Springer Ser. Mater. Sci. 2008, 111, 185.
- Sun, Z.Y.; Ma, T.; Tao, H.C.; Fan, Q.; Han, B.X. Fundamentals and Challenges of Electrochemical CO2 Reduction Using Two-Dimensional Materials. Chem 2017, 3, 560–587.
- Dean, J.A. Lange’s Handbook of Chemistry. Mater. Manuf. Processes 1990, 5, 687–688.
- Vannice, M. The catalytic synthesis of hydrocarbons from carbon monoxide and hydrogen. J. Catal. Rev.—Sci. Eng. 1976, 14, 153–191.
- Vasileff, A.; Xu, C.C.; Jiao, Y.; Zheng, Y.; Qiao, S.Z. Surface and Interface Engineering in Copper-Based Bimetallic Materials for Selective CO2 Electroreduction. Chem 2018, 4, 1809–1831.
- Gunathunge, C.M.; Li, X.; Li, J.; Hicks, R.P.; Ovalle, V.J.; Waegele, M.M. Spectroscopic Observation of Reversible Surface Reconstruction of Copper Electrodes under CO2 Reduction. J. Phys. Chem. C 2017, 121, 12337–12344.
- Öström, H.; Öberg, H.; Xin, H.; LaRue, J.; Beye, M.; Dell’Angela, M.; Gladh, J.; Ng, M.L.; Sellberg, J.A.; Kaya, S.; et al. Probing the transition state region in catalytic CO oxidation on Ru. Science 2015, 347, 978–982.
- Gladh, J.; Öberg, H.; Pettersson, L.G.; Öström, H. Detection of adsorbate overlayer structural transitions using sum-frequency generation spectroscopy. Surf. Sci. 2015, 633, 77–81.
- Peterson, A.A.; Norskov, J.K. Activity Descriptors for CO2 Electroreduction to Methane on Transition-Metal Catalysts. J. Phys. Chem. Lett. 2012, 3, 251–258.
- Zhu, S.Q.; Jiang, B.; Cai, W.B.; Shao, M.H. Direct Observation on Reaction Intermediates and the Role of Bicarbonate Anions in CO2 Electrochemical Reduction Reaction on Cu Surfaces. J. Am. Chem. Soc. 2017, 139, 15664–15667.
- Dunwell, M.; Lu, Q.; Heyes, J.M.; Rosen, J.; Chen, J.G.G.; Yan, Y.S.; Jiao, F.; Xu, B.J. The Central Role of Bicarbonate in the Electrochemical Reduction of Carbon Dioxide on Gold. J. Am. Chem. Soc. 2017, 139, 3774–3783.
- Heyes, J.; Dunwell, M.; Xu, B.J. CO2 Reduction on Cu at Low Overpotentials with Surface-Enhanced in Situ Spectroscopy. J. Phys. Chem. C 2016, 120, 17334–17341.
- Wuttig, A.; Liu, C.; Peng, Q.; Yaguchi, M.; Hendon, C.H.; Motobayashi, K.; Ye, S.; Osawa, M.; Surendranath, Y. Tracking a Common Surface-Bound Intermediate during CO2-to-Fuels Catalysis. ACS Cent. Sci. 2016, 2, 522–528.
- Malkani, A.S.; Dunwell, M.; Xu, B. Operando Spectroscopic Investigations of Copper and Oxide-Derived Copper Catalysts for Electrochemical CO Reduction. ACS Catal. 2019, 9, 474–478.
- Gunathunge, C.M.; Ovalle, V.J.; Li, Y.; Janik, M.J.; Waegele, M.M. Existence of an Electrochemically Inert CO Population on Cu Electrodes in Alkaline pH. ACS Catal. 2018, 8, 7507–7516.
- Papasizza, M.; Cuesta, A. In Situ Monitoring Using ATR-SEIRAS of the Electrocatalytic Reduction of CO2 on Au in an Ionic Liquid/Water Mixture. ACS Catal. 2018, 8, 6345–6352.
- Chou, K.C.; Markovic, N.M.; Kim, J.; Ross, P.N.; Somorjai, G.A. An in situ time-dependent study of CO oxidation on Pt(111) in aqueous solution by voltammetry and sum frequency generation. J. Phys. Chem. B 2003, 107, 1840–1844.
- Wuttig, A.; Yaguchi, M.; Motobayashi, K.; Osawa, M.; Surendranath, Y. Inhibited proton transfer enhances Au-catalyzed CO2-to-fuels selectivity. Proc. Natl. Acad. Sci. USA 2016, 113, E4585–E4593.
More
Information
Subjects:
Chemistry, Physical; Spectroscopy
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
685
Revisions:
4 times
(View History)
Update Date:
28 Jan 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




