Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nabeel Mahdi Althabhawi | + 3301 word(s) | 3301 | 2022-01-18 04:55:03 | | | |
| 2 | Rita Xu | Meta information modification | 3301 | 2022-01-24 05:11:59 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Althabhawi, N. The Patent Eligibility of 3D Bioprinting. Encyclopedia. Available online: https://encyclopedia.pub/entry/18590 (accessed on 07 February 2026).
Althabhawi N. The Patent Eligibility of 3D Bioprinting. Encyclopedia. Available at: https://encyclopedia.pub/entry/18590. Accessed February 07, 2026.
Althabhawi, Nabeel. "The Patent Eligibility of 3D Bioprinting" Encyclopedia, https://encyclopedia.pub/entry/18590 (accessed February 07, 2026).
Althabhawi, N. (2022, January 21). The Patent Eligibility of 3D Bioprinting. In Encyclopedia. https://encyclopedia.pub/entry/18590
Althabhawi, Nabeel. "The Patent Eligibility of 3D Bioprinting." Encyclopedia. Web. 21 January, 2022.
Copy Citation
A combination of 3D printing techniques and synthetic biology, 3D bioprinting is a promising field. It is expected that 3D bioprinting technologies will have applications across an array of fields, spanning biotechnology, medical surgery and the pharmaceutical industry. Nonetheless, the progress of these technologies could be hindered, unless there is adequate and effective protection for related applications.
3D bioprinting
products of nature
patentable subject matters
1. Introduction
The novel technology of 3D bioprinting aims to synthetically produce tissues and other biological constructs by using 3D bioprinters [1]. Presently, this technology has application mainly in the area of medical surgery, where it is used to transplant synthetically printed tissues into patients [2]. Transplanted tissues are usually printed according to a patient’s cells [3]. Sometimes, they are printed according to donors’ cells [4]. According to Bicudo and others, it is hard to pinpoint the precise beginning of bioprinting technologies, although there were some experiments in this area in the middle of the 1980s. It seems that the real emergence of these technologies was in the 1990s, while the vast majority of bioprinting companies were established in the present century [1]. Today, bioprinting is still in an embryonic stage and only simple tissues have been printed. However, future research in this field is expected to enhance the ability to print whole organs and the value of 3D bioprinting will eventually rise to an estimated USD 3 billion in the near future [5].
There are three common methods in 3D bioprinting technologies [6][7][8]. First, bioprinting can be by way of the so-called inkjet-based printing. This is a low-cost method. However, it is constrained by the fact that it cannot be used to produce high-viscosity tissues. The second method is micro-extrusion printing, which is a method commonly used to print 3D biological tissues [9]. It overcomes the limitations of the inkjet printing method. Its drawback is that it is a low-resolution form of printing. Third, is the laser-assisted method (LAB), which is less in use than the micro-extrusion technique [10]. However, LAB has some drawbacks, such as the small number of biomaterials that can be transferred, as well as the low speed and high costs, which render LAB uncompetitive in respect to other methods [11].
The first step in 3D bioprinting is the creation of a digital blueprint of the targeted object, that is, a three-dimensional scan of the real object. Where the real object is not available or cannot be scanned, computer-aided design software (CAD) is used to model it [5][12]. The second step is the translation of this blueprint into a path that the printer can follow. This translation is conducted by computer-aided manufacturing software (CAM). The last stage is the printing of the targeted object layer by layer using bioprinters [13]. These bioprinters are robotic devices which function by receiving instructions from software [1]. Nonetheless, it is most significant to understand the microenvironment of the copy tissue before commencing the 3D bioprinting process [5].
There are a variety of biomaterials used in 3D bioprinting that can be transplanted from the natural environment without rejection. Hydrogels and sugar are used as a scaffold in the printing process [13]. The scaffold enables 3D-printed cells to replicate [5]. More significantly, bio-ink is a crucial factor in 3D bioprinting technologies. It is essentially made from cells of the same individual (autologous cells) or another individual from the same species (allogeneic cells). Bio-ink can also be made from different species (xenogeneic cells). Furthermore, bio-ink consists of pre-polymer solution hydrogels [9].
The 3D bioprinting process faces some problems that are not encountered in conventional 3D printing. For instance, the sensitivity of living cells precludes acceptance by the living organism, that is, the selection of bio-ink components. These problems must be overcome by integrating multiple fields, such as engineering, cell biology, physics and medicine. Until recent years, only a few 3D bioprinting processes have been successful (the result of a patent search conducted by authors on Espatcenet and based on the key phrases “3D bioprinted tissue”, “3D bioprinted organism” and “3D bioprinted organ” showed only 22 issued patents. This is mainly because of some non-natural characteristics, which render the copy organ distinguishable from the original version. For instance, blood vessels, when copied, may create a network of vessels that clearly does not match the network created by the original vessels. Moreover, the thickness of copy tissues must not exceed 200 micros, to allow oxygen to spread between the original organs and the transplanted ones [13].
Technological and biological obstacles apart, 3D bioprinting technologies question the adequacy of the current patent system in protecting inventions arising from this revolutionary field. The issue of patent eligibility has profound implications for emerging technologies as the scene remains unclear [14]. More specifically, patent protection is a key determinant of the progress of 3D bioprinting technologies. With patent protection, more investments and resources would be committed to enhance the state of these technologies. Impliedly, without patent rights, the progress of 3D bioprinting may be hindered [15]. Indeed, the most concerning debate in the patent eligibility of 3D bioprinting applications resonates with the patentability of nature-derived or nature-duplicated breakthroughs and the products of nature doctrine [16].
2. The Patentability of Nature-Related Inventions
There has been a debate about the patent eligibility of 3D bioprinting products, whether they are pre-printing materials, printed tissues, or organs, as they are nature-based [14]. Bioprinting products, such as bioprinted tissues and organs, as well as materials used in the printing process, that is, pre-printing materials, would face a challenge regarding their novelty vis-à-vis “natural” biomaterials. Their patentability depends on the level of human ingenuity involved and the extent of their distinction from natural equivalents [9]. Hsiao argues that 3D bioprinting inventions will not pass the eligibility test because their success will depend on the degree to which they resemble naturally occurring ones. It is believed that 3D bioprinting is nothing but a duplication of natural organs, without any “markedly different characteristics” [13].
On the other hand, Minssen, Mimler and Boucher contend that bioprinting products are still essentially different from their natural counterparts, thus patent-eligible [9][17]. Xin [18] takes a nuanced position, claiming that perfect 3D bioprinting resembles their original versions, although some of its products may be patent-eligible because they have multiple characteristics that are essentially distinct, such as being constituted by genetically engineered cells. He argues that, although all components of 3D bioprinting are naturally occurring, they may exhibit markedly different characteristics that render 3D bioprinting technologies patent-eligible. He adds that some new qualities can be produced, such as novel and inventive organs. In concluding, he acknowledges that 3D bioprinting products, which are indistinguishable, would not fit within the realm of patent protection based on more recent jurisprudence articulated by the U.S. Supreme Court in cases, such as Myriad and Roslin. Nevertheless, Xin proposes some means to have such products patentable. First, he suggests that 3D bioprinting products can be derived from non-natural “intermediate precursors”. Second, products which are a combination of natural and non-natural materials would not be caught by the nature-related exclusions [18].
3. The U.S. “Product of Nature” Doctrine
The U.S. Patent Act provides that patentable subject matters encompass any new and useful process, machine, manufacture, or composition of matter. According to Section 101 thereof, “whoever invents or discovers any new and useful process, machine, manufacture, or composition of matter, or any new and useful improvement thereof, may obtain a patent therefor, subject to the conditions and requirements of this title”. Under the U.S. patent law, eligible subject matters are expansive [19], without any exclusion of patentable inventions, as Congress had intended to protect anything under the sun [20]. Nonetheless, the U.S. courts have espoused that “the scope of the patentable subject matters under that system is broad but it is not endless” [19]. In line with this, current U.S. cases hold, under the so-called “product of nature” doctrine, that phenomena of nature, inter alia, are not patentable.
Sprott asserts that there is no practical guidance as to what constitutes a “product of nature”, nor clarity of its origin [21]. He traced the inception of the “product of nature” doctrine to the U.S. Supreme Court case of American Wood Paper Co. v. Fiber Disintegrating Co., which is believed to be the first case that addressed a nature-related invention. The Court restricted patent protection to the process of extraction, rather than the product itself [22]. Subsequently, it was held by the U.S. Patent Commissioner that discovering a new method to produce a material does not entitle the discoverer to seek patent protection for it [23]. The U.S. Seventh Circuit Court of Appeals endorsed the eligibility of a medicine purified with a useful difference, which makes the compound “therapeutically available” [24]. This case adopted the “therapeutic value test”, which bases patent eligibility, inter alia, on medical utility [21].
The therapeutic value test was cited by judge Learned Hand in the case of Parke-Davis & Co. v. H.K. Mulford Co., where he applied the purification rule to a biologically purified adrenaline that was lifted out from its natural environment to produce “a new thing commercially and therapeutically” [25]. The therapeutic value test was reaffirmed by the Seventh Circuit in Dennis v. Pitner. The plaintiff brought this case alleging infringement of a patent issued to protect an insecticide that was extracted from the roots of the cube plant. In reply, the defendant argued that the patent was incompatible with the law, as the purported invention was a mere revelation of nature. However, the Court rejected this proposition, relying on the patent’s “value to mankind”. Remarkably, the Court stressed that the application of the “laws of nature” principle has been confusing and devoid of clarity [26].
The therapeutic value test survived, even after the enactment of the U.S. Patent Act of 1952. The District Court of Columbia had an opportunity to examine the patentability of a purified arterenol compound, which the Commissioner of Patent had declared patent-ineligible. After close examination of the matter, the Court concluded that the rejection ignored the fact that isolation and purification are necessary to attain therapeutic value [27]. Subsequently, it was clarified, in Merck & Co. v. Olin Mathieson Chemical Corporation, that the U.S. Patent Act does not “preclude the issuance of a patent upon a “product of nature”, when it is a “new and useful composition of matter” and there is compliance with the specified conditions for patentability. All the tangible things with which man deals and for which patent protection is granted are products of nature in the sense that nature provides the basic source materials” [28].
The patent eligibility of materials isolated from their natural environment was again on the table of discussion in the U.S. Court of Customs and Patent Appeals (CCPA) in Application of Bergstrom [29]. The case came to the Court after the Patent Office rejected an application to patent two compounds that had the effect of simulating smooth muscles and decrease blood pressure. The justification for the rejection by the Patent Office was the lack of new properties, compared with the non-purified forms of the compounds. Taking issues with the attorney’s position, which deemed the compound as “naturally occurring”, the Court concluded that the claimed compounds did not exist. It held that the pure materials were “new” within the meaning of the statutory requirement of novelty [29].
The patentability of isolated materials has proved controversial. As Sprott argues, the patentability of isolated and purified substances was a significant loophole in the “product of nature” doctrine. Multiple patents were issued, despite their nature-originated claims [21]. The problems associated with isolation and purification became more perplexing with advancements and breakthroughs in the field of biotechnology. The borderlines between products of nature and products of man became increasingly vague. This strengthened the position of those opposed to the exclusion of products of nature. The inclusion of “living products” within the meaning of statutory patentable subject matters was first initiated by the U.S. Court of Customs and Patent Appeals in Application of Bergy [30]. The Court held that there was no ground in Section 101 of the U.S. Patent act, even if strictly construed, to exclude a manufacture or composition of matter because it was alive.
Notwithstanding, there can be no satisfactory discussion on the “product of nature” doctrine without referring to the landmark case of Diamond v. Chakrabarty [20]. In Chakrabarty, the U.S. Supreme Court parted with the general understanding that living microorganisms could not be patented and opened the gate to the patenting of many genetically modified living organisms [26]. The Court referred to the wide interpretation of the term “manufacture”, which was given, in American Fruit Growers, Inc. v. Brogdex Co. [31], as “the production of articles for use from raw or prepared materials by giving to these materials new forms, qualities, properties, or combinations, whether by hand-labour or by machinery”.
Hsiao points out that a two-phase test was conducted by the Court in Chakrabarty to render the invention a “product of man”. First, the invention shall be the result of human ingenuity. Second, the invention shall bear different characteristics from its natural counterparts [13]. Agarwal and Agarwal argue that 3D bioprinting inventions have a good prospect of passing the second prong of the Chakrabarty test. As they explain, there are substantial differences between 3D bioprinting products and their natural counterparts. First, some essential features, such as innervation, are not printable. Second, the aggregation of printed cells is not analogous to the aggregation of natural ones. Third, some 3D bioprinting products are the results of the combination of natural and artificial materials [16].
After Chakrabarty, many patents were granted for modified and isolated genes. Approximately, 47,000 of such patents were issued [32]. However, the debate led US Patents and Trademark Office (USPTO) to elucidate the foggy scene in 1989 by stating that it would consider “non-naturally occurring, non-human multicellular living organisms, including animal”. The position taken in Chakrabarty was reaffirmed in the leading case of Amgen Inc. v. Chugai Pharmaceutical Co., Ltd. [33]. In Amgen, the U.S. Court of Appeals for the Federal Circuit upheld the decision of the District Court of Massachusetts, which found that claims directed to a purified and isolated DNA sequence were patentable. According to Sprott, Amgen paved the way to the filing of many applications of purified and isolated DNA [21]. However, the USPTO, in a 1989 statement, excluded claims directed to or including human beings from the above rule. Cho [32] connects the USPTO’s statement with the Leahy–Smith America Invents Act (AIA) of 2011, which states that “no patent may issue on a claim directed to or encompassing a human organism”. However, Ebrahim points out that, whereas the terms “directed to” and “encompassing” are well-known, the phrase “human organism” is undefined; understanding of it is lacking in both the U.S. courts and the USPTO [14].
The turning point in the modern history of the patentability of genetic inventions came in 2012, when the U.S. Supreme Court heard the case of Mayo Collaborative v. Prometheus Labs. Discussing a patent directed to a process aimed at assisting medical personnel to use thiopurine drugs to confront autoimmune diseases by the determination of the dosage level, the Court concluded that the process had not transformed the laws of nature into a patent-eligible application [34]. However, it did not reject the inclusion of laws of nature in an invention, if it contained what the Court called an “inventive concept”. The long story about the patentability of genes came to its end in 2013, when the U.S. Supreme Court decided the renowned case of Ass’n for Molecular Pathology v. Myriad. In Myriad, the Court held that isolated genomic DNAs are “products of nature” as they identical to naturally occurring ones, without any human ingenuity which should have intervened to create or alter the DNAs. The mere isolation of genes does not qualify for patent protection [35].
In 2014, the USPTO issued an interim guidance for the determination of the patent eligibility of nature-derived inventions. The guidance provides that, first, to test the patentability of an invention, the claim must relate to a process, manufacture, machine or composition of matter. The guidance then proceeds to the “judicially recognized exceptions” test, which has two prongs. In the first prong (2A), it shall be determined whether the claim is directed to a “product of nature” or a natural phenomenon. If so, the second prong (2B) shall be conducted to identify any potential additional elements that vest the claim with “markedly different characteristics” [36].
The Court of Appeals for the Federal Circuit held, in the 2014 case of Roslin, that it was not correct to argue that the copies (clones) of a sheep were patentable as the results of human ingenuity. The copy, according to the Court, was “an exact genetic replica of another sheep” and did not possess “markedly different characteristics from any [farm animals] found in nature” [37]. The Court rejected patent protection for environment-generated characteristics, insisting that patentability shall only be extended to protect human ingenuity. In response to the decision in Roslin, the USPTO released the Revised Patent Subject Matter Eligibility Guidelines (RPEG) [38]. The central point of the guidelines is the case law-created exclusions. The guidelines elaborate step 2A in the interim guidance, which excludes claims “directed to a judicial exception”. The RPEG sets two prongs for that test. First, if an abstract idea, a law of nature or natural phenomenon is recited in a claim, then, according to the RPEG, the mere inclusion is a recitation. If there is a recitation, then a patent examiner must proceed to the second prong of the test. The question, here, would be whether there is any practical application that lifts a claim out of the exclusions (see Figure 1)
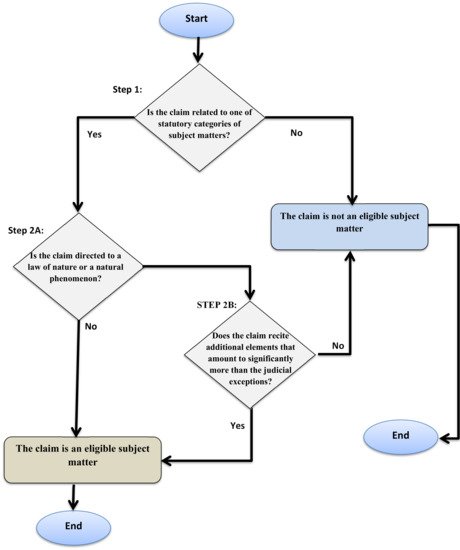
Figure 1. The USPTO’s nature exclusion test.
On the other hand, a congressional draft bill was released in May 2019. The draft stated that “no implicit or other judicially created exceptions to subject matter eligibility, including “abstract ideas”, “laws of nature”, or “natural phenomena”, shall be used to determine patent eligibility under Section 101 and all cases establishing or interpreting those exceptions to eligibility were thereby abrogated. The eligibility of a claimed invention under Section 101 shall be determined without regard to the manner in which the claimed invention was made; whether individual limitations of a claim are well known, conventional or routine; the state of the art at the time of the invention; or any other considerations relating to Sections 102, 103, or 112”.
4. The EU Exclusion of Discovery
In Europe, the issue of nature-derived patents is less complicated. This is because statutory exclusions exist that put some breakthroughs out of the patent realm. While, in the U.S., the issue of exclusions is two-fold—what is the law and how it is applied—the debate, in Europe, relates only to the second fold. Article 52(2) of European Patent Convention (EPC) provides that “(2) the following, in particular, shall not be regarded as inventions within the meaning of paragraph 1: (a) discoveries, scientific theories and mathematical methods…” Moreover, Article 53 of this instrument excludes “(b) plant or animal varieties or essentially biological processes for the production of plants or animals—this provision shall not apply to microbiological processes or the products thereof; and (c) methods for the treatment of the human or animal body by surgery or therapy and diagnostic methods practiced on the human or animal body—this provision shall not apply to products, in particular substances or compositions, for use in any of these methods.”
Nevertheless, patent applications, which consist of any excluded subject matters, would be, theoretically, patent-eligible, if they introduce an industrial application based on the exclusion. In Aerotel v. Telco Holdings, the UK Court of Appeal held that “[a] physical embodiment, such as a cloning vector employing knowledge of the discovery of a DNA sequence is not discovery as such” [39]. The implementation regulations of the EPC require the invention to have technical features related to a technical field that are a concern to a technical problem. On this basis, it has been argued that elements of technicality are present in 3D bioprinting, thus rendering it patentable [9].
References
- Bicudo, E.; Faulkner, A.; Li, P. Online Survey with Bioprinting Companies Preliminary Findings; University of Sussex: Brighton, UK, 2020.
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2020, 226, 119536.
- Mahfouzi, S.H.; Safiabadi Tali, S.H.; Amoabediny, G. 3D bioprinting for lung and tracheal tissue engineering: Criteria, advances, challenges, and future directions. Bioprinting 2021, 21, e00124.
- Kent, C. The Future of Bioprinting: A New Frontier in Regenerative Healthcare. Med. Device Netw. 2019. Available online: https://www.borderless.net/news/life-sciences/the- (accessed on 29 November 2021).
- Ammar, J. Defective Computer-Aided Design Software Liability In 3d Bioprinted Human Organ Equivalents Recommended Citation Defective Computer-Aided Design Software Liability In 3d Bioprinted Human Organ Equivalents. High Technol. Law J. 2019, 35, 4–6.
- Gudapati, H.; Dey, M.; Ozbolat, I. A comprehensive review on droplet-based bioprinting: Past, present and future. Biomaterials 2016, 102, 20–42.
- Ozbolat, I.T.; Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 2016, 76, 321–343.
- Ng, W.L.; Lee, J.M.; Zhou, M.; Chen, Y.-W.; Lee, K.-X.A.; Yeong, W.Y.; Shen, Y.-F. Vat polymerization-based bioprinting—Process, materials, applications and regulatory challenges. Biofabrication 2020, 12, 22001.
- Minssen, T.; Mimler, M. Chapter 7: Patenting Bioprinting-Technologies in the US and Europe—The 5th Element in the 3rd Dimension. In 3D Printing, Intellectual Property and Innovation—Insights from Law and Technology; Wolters Kluwer: Alphen aan den Rijn, The Netherlands, 2017; pp. 117–148.
- Fisch, P.; Holub, M.; Zenobi-Wong, M. Improved accuracy and precision of bioprinting through progressive cavity pump-controlled extrusion. Biofabrication 2021, 13, 15012.
- Dou, C.; Perez, V.; Qu, J.; Tsin, A.; Xu, B.; Li, J. A State-of-the-Art Review of Laser-Assisted Bioprinting and its Future Research Trends. ChemBioEng Rev. 2021, 8, 517–534.
- Weinberg, M. It Will Be Awesome if They Don’t Screw It Up: 3D Printing, Intellectual Property and the Fight Over the Next Great Disruptive Technology; Public Knowledge: Washington, DC, USA, 2010; Volume 2.
- Hsiao, J.-H. Patent Eligibility of 3d Bioprinted Organs in Taiwan. Albany Law J. Sci. Technol. 2018, 28, 1–22.
- Ebrahim, T.Y. 3D Bioprinting Patentable Subject Matter Boundaries. Seattle Univ. Law Rev. 2017, 41, 1–59.
- Ammar, J. The Medical Mile Gearing toward 3D-Bespoke Healthcare: A Comparison of United States and European Union Patent Regimes. Gonzaga Law Rev. 2016, 52, 279–326.
- Agarwal, R.; Agarwal, P. 3D Bio-Printing: Addressing the Conundrum of Patent Eligibility. Ex Gratia Law J. 2020, 1. Available online: https://exgratialawjournal.com/journal/volume-1/vol1-issue3-dec2020/3d-bio-printing-addressing-the-conundrum-of-patent-eligibility-by-riya-agarwal-and-priya-agarwal/ (accessed on 3 March 2021).
- Boucher, P. 3D Bio-Printing for Medical and Enhancement Purposes In-Depth Analysis Science and Technology Options Assessment; European Parliament: Strasbourg, France, 2018.
- Xin, X. Patent Eligibility of 3D-Printed Organs. AIPLA Q. J. 2016, 44, 143–170.
- Bilski v. Kappos, 561 US—Supreme Court. 2010, p. 593. Available online: https://supreme.justia.com/cases/federal/us/561/593/ (accessed on 29 November 2021).
- Diamond v. Chakrabarty, 447 US 303—Supreme Court. 1980, p. 303. Available online: https://supreme.justia.com/cases/federal/us/447/303/ (accessed on 29 November 2021).
- Sprott, W.D. From Pine Straw to CDNA: The History of the Product of Nature Doctrine. Houst. Bus. Tax Law J. 2013, 14, 290–322.
- American Wood Paper Co. v. Fiber Disintegrating Co; 1874; Vol. 90 US, p. 566. Available online: https://www.law.cornell.edu/supremecourt/text/90/566 (accessed on 29 November 2021).
- Ex Parte Latimer; 1889; Vol. Dec, p. 123. Available online: https://brooklynworks.brooklaw.edu/cgi/viewcontent.cgi?referer=www.google.com/&httpsredir=1&article=1460&context=faculty (accessed on 29 November 2021).
- Kuehmsted v. Farbenfabriken of Elberfeld Co.; 1910; Vol. 179 F. 701, p. 701. Available online: https://cite.case.law/f/179/701/ (accessed on 29 November 2021).
- PARKE-DAVIS & CO. v. H. K. MULFORD CO; 1911; Vol. 189 F. 95. Available online: https://cite.case.law/f/189/95/ (accessed on 29 November 2021).
- Dennis v. Pitner; 1939; Vol. 106 F.2d 1. Available online: https://law.justia.com/cases/federal/appellate-courts/F2/106/142/1494472/ (accessed on 29 November 2021).
- Sterling Drug v. Watson, 135 F. Supp. 173—Dist. Court, Dist. of Columbia 1955. Available online: https://casetext.com/case/sterling-drug-v-watson (accessed on 29 November 2021).
- Merck & Co. v. Olin Mathieson Chemical Corporation, 253 F. 2d 156—Court of Appeals, 4th Circuit 1958. Available online: https://law.justia.com/cases/federal/appellate-courts/F2/253/156/145548/ (accessed on 29 November 2021).
- Application of Bergstrom, 427 F. 2d 1394—Court of Customs and Patent Appeals 1970. Available online: https://casetext.com/case/application-of-bergstrom (accessed on 29 November 2021).
- Application of Bergy, 596 F. 2d 952—Court of Customs and Patent Appeals 1979. Available online: https://casetext.com/case/application-of-bergy-2 (accessed on 29 November 2021).
- American Fruit Growers, Inc. v. Brogdex Co., 283 US 1—Supreme Court 1931. Available online: https://supreme.justia.com/cases/federal/us/283/1/ (accessed on 29 November 2021).
- Cho, S. The Current Application of the Myriad and Mayo/Alice Ruling on Patent Eligibility: Inconsistent Results and Contradistinguishing Biotechnology Products. HeinOnline 2020, 38, 183–218.
- Amgen, Inc. v. Chugai Pharmaceutical Co., Ltd., 927 F. 2d 1200—Court of Appeals, Federal Circuit 1991. Available online: https://casetext.com/case/amgen-inc-v-chugai-pharmaceutical-co-ltd (accessed on 29 November 2021).
- Mayo Collaborative v. Prometheus Labs., 132 S. Ct. 1289—Supreme Court; 2012. Available online: https://h2o.law.harvard.edu/cases/4410 (accessed on 29 November 2021).
- Ass’n for Molecular Pathology v. Myriad, 133 S. Ct. 2107—Supreme Court 2013. Available online: https://h2o.law.harvard.edu/collages/13891 (accessed on 29 November 2021).
- Federal Register: 2014 Interim Guidance on Patent Subject Matter Eligibility. Available online: https://www.federalregister.gov/documents/2014/12/16/2014-29414/2014-interim-guidance-on-patent-subject-matter-eligibility (accessed on 3 January 2021).
- In Re Roslin Institute (Edinburgh), 750 F. 3d 1333—Court of Appeals, Federal Circuit 2014. Available online: https://casetext.com/case/in-re-institution (accessed on 29 November 2021).
- Federal Register: 2019 Revised Patent Subject Matter Eligibility Guidance. Available online: https://www.federalregister.gov/documents/2019/01/07/2018-28282/2019-revised-patent-subject-matter-eligibility-guidance (accessed on 30 November 2020).
- Aerotel Ltd V Telco Holdings Ltd Macrossan’s Patent Application. Rep. Pat. Des. Trade Mark Cases 2007, 124, 117–161.
More
Information
Subjects:
Law
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
24 Jan 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




