Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Emanuele Giurisato | + 3687 word(s) | 3687 | 2022-01-18 10:49:45 |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Giurisato, E. ERK5 Expression and Function in Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/18570 (accessed on 08 February 2026).
Giurisato E. ERK5 Expression and Function in Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/18570. Accessed February 08, 2026.
Giurisato, Emanuele. "ERK5 Expression and Function in Cancer" Encyclopedia, https://encyclopedia.pub/entry/18570 (accessed February 08, 2026).
Giurisato, E. (2022, January 20). ERK5 Expression and Function in Cancer. In Encyclopedia. https://encyclopedia.pub/entry/18570
Giurisato, Emanuele. "ERK5 Expression and Function in Cancer." Encyclopedia. Web. 20 January, 2022.
Copy Citation
Extracellular signal-regulated kinase 5 (ERK5) is a unique kinase among MAPKs family members, given its large structure characterized by the presence of a unique C-terminal domain. Despite increasing data demonstrating the relevance of the ERK5 pathway in the growth, survival, and differentiation of normal cells, ERK5 has recently attracted the attention of several research groups given its relevance in inflammatory disorders and cancer.
ERK5
cancer
cancer-stem cells
prognostic markers
tumor microenvironment
proliferation
metastasis
metabolism
1. ERK5 Structure
ERK5 is encoded by the Mitogen-Activated Protein Kinase 7 (MAPK7) gene (Figure 1).
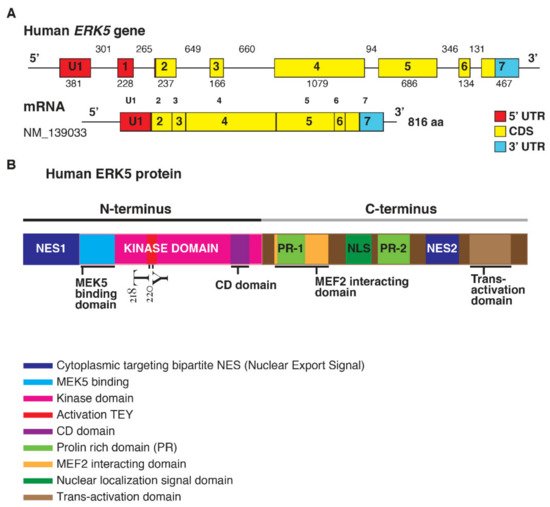
Figure 1. (A). Structure of the human ERK5 gene (MAPK7) and the mRNA transcript (NM_139033) encoding for the full-length ERK5 protein of 816 aa; length of the exons (below) and introns (upper) are also shown (size not to scale). The exon-intron scheme is depicted with 5′UTR in red, the coding region (CDS) in yellow, and 3′UTR in light blue. (B). Human ERK5 protein and its functional domains: at the N-terminal there is a cytoplasmic targeting signal and a kinase domain, which comprises the region of binding for MEK5 and the common docking (CD) domain. Residues Thr218/Tyr220 are underlined in red as the site of MEK5 phosphorylation. The C-terminal comprises two proline-rich (PR) domains, a MEF2-interacting region, the nuclear localization signal (NLS) domain, and a transcriptional activation domain. ERK5 contains a bipartite nuclear exportation signal (NES1 and NES2). The N- and C-terminal halves of ERK5 interact, producing a nuclear export signal that retains ERK5 in the cytoplasm of resting cells.
The elevated molecular weight of ERK5 (110 kDa), which is almost double compared to other family members, also explains the name of Big Mitogen-Activated Protein Kinase 1 (BMK1). Structurally, ERK5 is constituted by an N-terminal half (1–406 aa) and a unique C-terminal tail (410–816 aa), which exerts an autoinhibitory function [1]. The N-terminus includes a region required for cytoplasmic targeting (1–77 aa) and a kinase domain (78–406 aa), which shares 66% of sequence identity to the one of ERK2 [2]. Of note, a Mitogen-Activated Protein Kinase 5 (MEK5) interacting sequence (78–139 aa) and an oligomerization domain (140–406 aa) have been identified within the kinase domain of ERK5 [3]. The C-terminus is constituted by two proline-rich (PR) domains, termed PR1 (434–465 aa) and PR2 (578–701 aa), which are considered to be potential binding sites for Src-homology 3 (SH3)-domain-containing proteins [2][3], a MEF-2-interacting region (440–501aa) [2], a nuclear localization signal (NLS) (505–539 aa), and a transcriptional activation domain (TAD) (664–789 aa) [4]. The latter is associated with the activation of several transcription factors, an ability unique to ERK5 with respect to the other MAPKs [5].
2. Regulation of ERK5 Activation
The MEK5-ERK5 signaling axis is triggered by several growth factors such as Vascular-Endothelial Growth Factor (VEGF), Epidermal Growth Factor (EGF), Fibroblast Growth Factor-2 (FGF-2), Platelet-Derived Growth Factor (PDGF) [6][7][8], and inflammatory cytokines like Interleukin-6 (IL-6) and Interleukin-8 (IL-8) [9], as well as osmotic and oxidative stress [10], which, in particular, can activate the MEK/ERK Kinases 2/3 (MEKK2/3) determining the phosphorylation of MEK5 within the activation motif (Ser311-Thr315). The activated MEK5 eventually mediates the phosphorylation of the TEY (Thr218-Glu-Tyr220) motif and determines the terminal activation of ERK5 [11]. Notably, the presence of PB1 domains organizes MEKK3, MEK5, and ERK5 into one signaling competent complex, via PB1 domain-mediated heterodimerization [12][13].
Moreover, RAS, the Proto-oncogene tyrosine-protein kinase Src (SRC), TPL/COT, and Protein kinase B (Akt) have all been reported to be MEKK2/3 and/or MEK5 upstream activators [14][15]. Particularly, it is known that the PhosphatidylInositol 3-Kinase (PI3K/Akt) pathway can trigger the activation of MEK5-ERK5 in neuroblastoma and malignant mesothelioma [16][17]. Furthermore, the Signal Transducer and Activator Of Transcription 3 (STAT3) upregulation has been reported to significantly increase the transcription of MEK5 in breast cancer (BC) [18]. It has been also demonstrated that TGF-β1 can mediate the association between MEK5, ERK5, and their downstream target MEF2C, via Anaplastic Lymphoma Kinase (ALK) receptor and the p38 mitogen-activated protein kinase, in HKC-8 transformed cells and human primary renal proximal tubule epithelial cells (PTECs) [19].
The overexpression of MEKK2/3 has been observed in different tumors such as colorectal, prostate, esophageal, breast, cervical, kidney, and lung cancer (LC) [20][21][22][23]; the latter one is also characterized by an increased MEK5/ERK5 signaling axis phosphorylation, and it has been recently demonstrated that pharmacological and genetic inhibition of both proteins, reduces LC cell proliferation [24].
MEKK2 and MEKK3 can activate distinct MAPK signaling pathways, but the specificity of MEK5 is restricted uniquely to ERK5 [2]. Opposed to the positive regulation of these protein kinases, protein kinase C (PKC) has been reported to inhibit ERK5 activation [25]. Furthermore, different phosphatases, such as the Phosphotyrosine-specific phosphatase (PTP-SL) and Dual-specificity phosphatases (DUSPs) family members, are involved in the downregulation of the ERK5 signaling axis. As a matter of fact, PTP-SL is thought to dephosphorylate the TEY microdomain, hindering at the same time the nuclear translocation of ERK5 [26]. Interestingly, ERK1/2-driven upregulation of DUSP1, DUSP5, and DUSP6 has been reported to inhibit ERK5 activation, suggesting the existence of a negative-feedback regulation [27]. Consistent with this idea, pharmacological inhibition of ERK1/2 in melanoma cell lines resulted in a significant ERK5 upregulation [28]. Furthermore, ERK1/2 abrogation in Colorectal cancer (CRC) has been reported to trigger ERK5 compensatory activation [29]. Notably, miR-211-mediated DUSP6 downregulation resulted in an increased ERK5 phosphorylation and BRAF inhibitors resistance in melanoma [30]. Similar results have also been documented in Non-small-cell lung carcinoma (NSCLC) cell lines, where the downregulation of DUSP6 resulted in increased ERK5 activation and epithelial–mesenchymal transition (EMT), which was reversed by inducing DUSP6 re-activation [31]. On the other hand, the Ser/Thr phosphatases PP1 and PP2A are reported to act as positive regulators of ERK5 phosphorylation [32].
In the inactive state, ERK5 is retained in the cytosol in a closed conformation, determined by the interaction between the N-terminus, the C-terminal half [33], and the chaperones Hsp90 and Cdc37, which inhibits its catalytic activity and generates a nuclear export signal [34]. Since the inhibition of Hsp90 or Cdc37 results in a rapid ERK5 ubiquitylation and proteasomal degradation, these interactions can contribute to ERK5 stability [34]. MEK5-dependent phosphorylation of the TEY motif determines the activation of the kinase domain, which in turn mediates the phosphorylation of the C-terminal half at multiple residues and the subsequent dissociation from Hsp90, exposition of the NLS, and nuclear translocation. In addition, it has been recently reported that Small Ubiquitin-related Modifier (SUMO) modification represents a necessary event for HSP90 dissociation in response to MEK5 activation and nuclear shuttling of ERK5 [35].
Once in the nucleus, ERK5 can activate either through direct target phosphorylation or through the TAD located in the C-terminal tail, a variety of genes and transcription factors such as MEF2 (A, B, and C), Activator Protein 1 (AP-1), c-Fos, c-Myc, Fos-related antigen 1 (Fra-1), Promyelocytic Leukemia Protein (PML) and Nuclear Factor kappa-light-chain-enhancer of activated B cells (NF-κB) [36][37][38][39][40][41][42]. Notably, ERK5 can phosphorylate PML and inhibits its tumor-suppressor function through activation of p21 [43]. Furthermore, it has been observed that MEK5/ERK5 hyperactivation contributes to NF-κB mediated human colon cancer (CC) progression [44] and that MEKK3 hyperactivation in ovarian cancer and glioma cells resulted in increased NF-κB activation and resistance to chemotherapeutic drugs [22][45].
Similarly to other MAPK family members, ERK5 can dock and phosphorylate its substrates by recognizing amino acids Ser or Thr followed by a Pro residue (-X-Ser/Thr-Pro-X-sequence), though Mody et al. [46] demonstrated that ERK5 can also recognize Ser/Thr sites not directly preceding Pro residues, as in the case of ERK5 C-terminus transactivation and ERK5-dependent MEK5 phosphorylation.
The phosphorylation of the C-terminus has been described as a key step for nuclear shuttling, and in particular, it has been recently observed that Thr732 acts as a functional gatekeeper residue controlling C-terminal-mediated nuclear translocation and transcriptional enhancement [47]. Furthermore, it has been reported that the C-terminus truncated construct of ERK5 (1–490 aa) is retained in the cytosolic complex with Hsp90 and Cdc37 even after ERK5 activation, since the autophosphorylation of the C-terminal tail represents a crucial event for the release of Hsp90 and the nuclear translocation of the protein [34].
Interestingly, it has been observed that the phosphorylation of the Ser730-Glu-Thr732-Pro motif could occur independently from the phosphorylation state of the TEY microdomain in the activation loop. In fact, in recent years, other mechanisms of translocation, independent from the canonical MEK5-mediated phosphorylation of the N-terminus, have been identified. During mitosis, ERK5 is phosphorylated at multiple residues within the C-terminal region [48]; these events represent a non-canonical pathway controlling ERK5 C-terminal phosphorylation. In mitosis, which involves kinase activities distinct from MEK5, Cyclin Dependent Kinase 1 (CDK1) is considered to be responsible for these phosphorylation events since its inhibition reverses mitotic phosphorylation of ERK5 [48]. ERK1/2 and CDK5 can also mediate the C-terminal domain phosphorylation in a similar manner, and subsequently induce ERK5 shuttling into the nucleus [41][49]. In particular, it has been observed that CDK5 upregulation in CRC is able to promote carcinogenesis by modulating the ERK5-AP-1 axis [41]. Besides kinase activities, the overexpression of Cdc37 can induce nuclear translocation of ERK5 independently from the phosphorylation state of the TEY motif, which could represent a mechanism exploited by many tumors; indeed, ERK5 and Cdc37 seem to cooperate to promote PC3 adenocarcinoma cell proliferation [34].
Interestingly, ERK5 mRNA expression is also subject to micro RNA-mediated post-transcriptional regulation. In particular, several reports have underlined an inverse correlation between the levels of ERK5 and the expression of miR-143. As a matter of fact, the downregulation of miR-143 usually results in a significant overexpression of ERK5, as in the case of different types of malignancies such as prostate cancer (PCa) [50][51], bladder cancer [52], BC [53][54], esophageal squamous cell carcinoma [55], nasopharyngeal carcinoma [56], acute myeloid leukemia [57], T-cell leukemia Jurkat cells [58], and B-cell malignancies [59]. In addition, it was recently demonstrated that restoring the expression of miR-200b-3p in glioma cells determined a drastic reduction of cell proliferation and EMT as a result of ERK5 suppression [60].
3. ERK5 and Cancer Proliferation
The involvement of ERK5 in tumor cell proliferation and cell cycle regulation has been widely supported by several works over the last years [61][62]. The first evidence that ERK5 was involved in regulating cell proliferation was provided through discovering that serum was a potent inducer of proto-oncogene c-JUN gene transcription via ERK5-induced MEF2C transcriptional activation [63]. Consistent with this study, it has been demonstrated that mitogens, including EGF, granulocyte colony-stimulating factor (G-CSF) and macrophages colony-stimulating factor (M-CSF), transmit their growth promoting signals via ERK5 [6][25][64]. ERK5 signaling cascade is considered an important player of cell proliferation via the expression of several mitogenic signals such as cyclin D1, SGK c-MYC, RSK2, n-MYC, and NF-κB [65][66][67][68][69][16][70]. Besides regulating the expression of proto-oncogenes required for cell growth, ERK5 is involved in sustaining the activation of survival signaling pathways [71]. Emerging data highlighted the regulative role of ERK5 in different phases of the cell cycle. In fact, by the expression of cyclin D1 and the suppression of CDK inhibitor p21, ERK5 mediates G1/S transition [66][72]. Moreover, ERK5 regulates G2/M transition and M phase by inducing the transcription factor NF-kB, which enhances the expression of mitosis-related genes, including cyclins B1 and B2 and phosphatase 2 (CDC25B) [69][48]. Recently, ERK5 has become an interesting molecule to study as a promising therapeutic target given its role in tumorigenesis and tumor malignancy [62]. The inhibition of ERK5 has been reported to decrease the proliferation rate and to intensify the number of G0/G1 cells in hepatocellular carcinoma via a mechanism involving the upregulation of p27 and p15 [73]. In addition, Tusa et al. [74] also observed that the Hedgehog (HH)-GLI signaling axis can enhance the transcription and the activation of ERK5, and that shRNA-mediated suppression of ERK5, reversed the HH-GLI-dependent proliferation of melanoma cells. In LC, characterized by an increased MEK5/ERK5 signaling axis phosphorylation, was recently demonstrated that pharmacological and genetic inhibition of both proteins reduced LC cell proliferation [24]. Shukla et al. [75] reported a link between asbestos-related ERK5 upregulation and proliferation of mesothelioma cells, as well as the inhibition of proliferative-related genes after ERK5 blockade. These findings strongly involves ERK5 as an innovative target for anti-cancer therapy. Furthermore, knockdown experiments indicated that ERK5 silencing negatively affects the proliferation of triple negative breast cancer (TNBC) cells, revealing a relevance for ERK5 in TNBC cell growth [76]. In PCa, overexpression of ERK5 enhanced the proliferation index of tumor cells, and interestingly the blockade of ERK1/2 was not sufficient to reduce the proliferation rate of tumor cells compared with ERK5 inhibition [77]. Studies on intestinal organoids demonstrated that the pharmacological inhibition of both ERK1/2 and ERK5 pathways better decreased tumor growth and the proliferation of human CRC cells [29]. Other previous evidence demonstrated that MEK5/ERK5 overexpression elicits the proliferation of CC cells and that the activation of the CDK5-ERK5 axis is required for the growth of CRC cells [41][78]. The requirement of RAS for mediating ERK5 activation downstream of growth factor stimulation remains controversial [79]. It has been reported that in a set of CRC cell lines with oncogenic KRAS-BRAF or ERK5 amplification, ERK5 did not contribute to tumor proliferation and no evidence about the regulation of ERK5 by the mutated KRAS/BRAF signaling was observed [80]. However, recent findings showed that, in melanoma samples, mutated BRAF positively regulates activation of ERK5 that was highly correlated with melanoma proliferation by in vitro and in vivo studies [81]. Lately, Tubita et al. [82] observed a blockade in the cell cycle progression in ERK5 knockdown in BRAF-mutated melanoma cells linked to the activation of cellular senescence mechanisms which includes the involvement of CDK inhibitors. Additionally, NRAS-mutant melanoma cells showed a proliferative advantage when, in response to MEK inhibitors treatment, expressed high levels of active ERK5 and the high rate of ERK5 was correlated with nuclear localization of the stem-like factor KLF2 [28]. Furthermore, the presence of MAPK7 gene amplification has been considered as a driver of proliferation in different tumor cell lines [83].
4. Role of ERK5 in Cancer Migration and Metastasis
Although most ERK5 targets are nuclear substrates, ERK5 is also a crucial regulator of cytosolic targets involved in cytoskeleton remodelling, like Akt, p90RSK, SGK, and the focal adhesion kinase (FAK) [65][84][85][86]. Interestingly, it has been demonstrated that ERK5-signaling suppression in TNBC and PC-3 cell lines lead to a drastic decrease in FAK phosphorylation, motility, and cell adhesion [87]. In accordance, recent studies have further underlined the critical linkage between the MEK5/ERK5 axis and the maintenance of the invasive capability of TNBC [88][89], LC, and melanoma [90] through FAK activation. Additionally, it has been found that ERK5 mainly localizes in the cytoplasm and membrane of ERα-negative BC cells, promoting actin remodeling, cell mobility, and invasion [91]. Moreover, suppressing the MEK5/ERK5 pathway completely prevented the TGFβ-induced EMT in murine BC cells, forcing at the same time highly metastatic tumor cells into a differentiated epithelial state [92]. In addition, ERK5 contributes to BC cell migration as an effector of the Breast tumor kinase [93] Cdc42 [94] and SRC associated in mitosis of 68 kDa signaling pathways [95]. Different reports have also highlighted the requirement for the upstream ERK5 activator MEKK2 in the migration, motility, and focal adhesion stability of invasive BC cell lines [96][97]. It is worth noting that MEK5 overexpression in BC cells can also determine a TNF-α chemotherapy-resistant phenotype characterized by the upregulation of several distinct EMT-associated genes [98]. Furthermore, STAT3 upregulation has been reported to significantly increase the transcription of MEK5, consequently enhancing BC invasiveness and metastasis formation [18]. Moreover, ERK5 has been reported to sustain the mesenchymal and migratory phenotype of TNBC cells by modulating FRA-1 expression [99] and regulating matrix-associated genes, integrins, and pro-angiogenic factors [100]. Strikingly, as revealed by in vivo studies on an orthotopic mouse model, MAPK7 silencing consistently reduced the number of circulating tumor cells and the insurgence of lung metastases [101]. Other than BC, ERK5 was also reported to be a crucial mediator of migration and invasion in osteosarcoma cell lines, regulating the expression of Slug and MMP-9 [102][103][104]. Furthermore, it has been documented that ERK5 activation is required for Src-mediated transformation and actin cytoskeleton disruption in NIH3T3 cells [14]. In addition, a series of in vitro experiments revealed that ERK5 pharmacological inhibition significantly decreased the invasiveness of HER-2-overexpressed meningioma cell lines [105]. ERK5 and its molecular target AP-1 have also been recently associated with the benzidine-induced EMT mechanism among bladder cancer cells [106]. Furthermore, ERK5 is implicated in the mechanism of podosomes formation of Src-transformed fibroblasts, inducing the Rho GTPase-activating protein 7 and thereby limiting Rho activation [107]. Moreover, pcDNA-MAPK7-transfected ovarian cancer cell lines displayed a stark increase in invasiveness and migration due to type II collagen upregulation, which was subsequently reversed through a MAPK7 silencing approach [108]. Similarly, the pharmacological inhibition of MEK5 using the specific kinase inhibitor BIX02189 resulted in a drastic reduction of cell migration in LC cells due to the suppression of the TGF-β1-induced EMT [109]. A significant association between tumor stage, presence of lymph-node metastasis and ERK5 expression was also identified by using a whole-transcriptome chip to a set of 35 primary oral squamous cell carcinomas (OSCC) [90]. In line with these observations, ERK5 expression was found to be increased in almost 60% of patients affected by clear cell renal cell carcinoma (RCC), and associated with the presence of metastasis and more advanced tumor stages [110]. Interestingly, it has been reported that triggering the EGF/ERK5/MEF2/DNA damage induced apoptosis suppressor (DDIAS) pathway enhanced cancer cells invasion through the expression of β-catenin target genes [111]. In addition ERK5 has also been identified as a downstream effector of the Protein-tyrosine kinase 6-p130 CRK-associated substrate axis, playing an important role in cancer cells migration and invasion [112]. Moreover, it has been recently demonstrated that restoring the expression of miR-200b-3p [60] and miR-429 [113] in glioma cells determined a drastic reduction of cell migration and EMT as a result of ERK5 suppression. In a similar manner, the upregulation of miR-143 in BC induced a marked reduction of ERK5 expression, proliferation, and migration among tumor cells [114]. Although a great part of the current literature describes ERK5 as a promoter of EMT and cell migration, Chen et al. [115] identified an opposite function of ERK5 in BC cell line 4T1, which could act as a metastasis suppressor by regulating the mTOR/Akt signaling.
5. ERK5 and Cancer Stemness
Compelling evidence about the role of ERK5 in the regulation of staminality has been emerging since the beginning of the last decade. By using small-molecule inhibitors and a CRISPR/Cas9 silencing approach, Williams et al. [116] demonstrated that ERK5 is involved in the activation of several pluripotency factors, including the Kruppel Like Factor 2 (Klf2), the Estrogen Related Receptor Beta (Esrrb), and Rex1. Interestingly, it was also observed that the TAD of ERK5, which is not essential for the kinase activity of the protein, is required for the maintenance of staminality through the activation of Mef2- and/or Sp-family transcription factors. Strikingly, recent studies have revealed a novel function of ERK5 in the process of stem cells rejuvenation. In particular, ERK5 is able to induce the transcription of the rejuvenation factor Zinc finger and SCAN domain containing 4 (ZSCAN4) at the early embryonic 2-cell stage (2C), consequently increasing telomere length and prolonging the viability of the cell culture [117]. In addition, ERK5 activity has been found strictly associated with the self-renewal of stem cells. Overexpression of Akt with angiopoietin-1 (Ang-1) determined the upregulation of ERK5, which drove transcriptional activation of cyclin D1 and Cdk4 and enhanced stem cell proliferation [118]. In line with these observations, Tusa and colleagues demonstrated that targeting the MEK5/ERK5 pathway in chronic myeloid leukemia (CML) led to a drastic decrease in progenitor/stem cells number due to a cell cycle block in G0/G1 [119]. Furthermore, the combined effect of ERK5 inhibitors with imatinib significantly reduced the expression of stemness-related genes such as c-MYC, SOX2, and NANOG [119]. Moreover, it was recently documented that the MEK5/ERK5 signaling axis is also responsible for maintaining a stem-like phenotype in CRC cells. Blocking ERK5 not only reduced the expression of pluripotency markers such as SOX2, NANOG, and OCT, but also hindered multicellular sphere formation and increased the sensitivity of CRC cells to 5-fluorouracil-based chemotherapy [120]. At last, a bivalent ERK5 inhibitor blocking the MEK5/ERK5 interaction, as well as the binding of ATP, has been developed in recent years. Strikingly, the compound was able to inhibit different cancer stem cells (CSCs) activities, such as colony formation, proliferation, and migration [121].
6. ERK5 and Cancer Cell Metabolism
The connection between the MEK5–ERK5 axis and cell metabolism has been reported [122][123][124]. In particular, it has been documented that ERK5 is essential for cell survival in oxidative phosphorylation (OXPHOS) conditions [123][125][126][127] and the MEF2 family of transcription factors mediates most of the effects of ERK5 on metabolism. Furthermore, transcriptome analysis showed that the ERK5 pathway regulates the expression of several genes involved in tumor cell metabolic remodeling [128] by controlling hypoxia-responsive genes. A recent study in pancreatic ductal adenocarcinoma identified a link between MEK5/ERK5 and the stability of MYC, a regulator of cell metabolism and growth [129]. More recently, transcriptomics analyses identified a role for the MEK5/ERK5 axis in the metabolism of Small-cell lung cancer (SCLC) cells, including lipid metabolism [130]. In-depth lipidomics analyses showed that in the absence of MEK5/ERK5, several lipid metabolism pathways are perturbed, including the mevalonate pathway that controls cholesterol synthesis. In addition, a connection between MEK5-ERK5 signaling and pyruvate metabolism and glyoxylate and dicarboxylate metabolism, as well as the citrate cycle and glycolysis, was observed. Notably, depletion of MEK5/ERK5 sensitized SCLC cells to pharmacological inhibition of the mevalonate pathway by statins [130], suggesting possible future therapeutic avenues for SCLC treatment.
The metabolic switch to OXPHOS controls the expression of ATP-binding cassette (ABC) transporters in wild-type p53-expressing cells. ABC transporters control the export of drugs from cancer cells and render tumors resistant to chemotherapy, playing an important role in multiple drug resistance (MDR). In this context, Belkahla et al. [131] found that OXPHOS increased the expression of ABC transporters in mutated (mut) or null p53-expressing cells and OXPHOS induced expression of the ERK5. Since ABC transporter promoters contain binding sites for the transcription factors MEF2, NRF1, and NRF2, which are targets of ERK5, the authors observed that decreasing ERK5 levels in mutp53 cells inhibited ABC expression. These results showed that the ERK5/MEF2 pathway controlled ABC expression depending on p53 status, suggesting a link between ERK5 signature, cancer cell metabolism, and MDR. Although all these data identify a new MEK5/ERK5–lipid metabolism axis that promotes cancer growth, the molecular mechanisms underlying the role of ERK5 on cell metabolism in the tumor microenvironment (TME) remain to be investigated.
References
- Yang, S.-H.; Sharrocks, A.D.; Whitmarsh, A.J. Transcriptional regulation by the MAP kinase signaling cascades. Gene 2003, 320, 3–21.
- Zhou, G.; Bao, Z.Q.; Dixon, J.E. Components of a new human protein kinase signal transduction pathway. J. Biol. Chem. 1995, 270, 12665–12669.
- Yan, C.; Luo, H.; Lee, J.D.; Abe, J.; Berk, B.C. Molecular cloning of mouse ERK5/BMK1 splice variants and characterization of ERK5 functional domains. J. Biol. Chem. 2001, 276, 10870–10878.
- Chang, L.; Karin, M. Mammalian MAP kinase signalling cascades. Nature 2001, 410, 37–40.
- Mu, D.-W.; Guo, H.-Q.; Zhou, G.-B.; Li, J.-Y.; Su, B. Oleanolic acid suppresses the proliferation of human bladder cancer by Akt/mTOR/S6K and ERK1/2 signaling. Int. J. Clin. Exp. Pathol. 2015, 8, 13864–13870.
- Kato, Y.; Tapping, R.I.; Huang, S.; Watson, M.H.; Ulevitch, R.J.; Lee, J.D. Bmk1/Erk5 is required for cell proliferation induced by epidermal growth factor. Nature 1998, 395, 713–716.
- Kesavan, K.; Lobel-Rice, K.; Sun, W.; Lapadat, R.; Webb, S.; Johnson, G.L.; Garrington, T.P. MEKK2 regulates the coordinate activation of ERK5 and JNK in response to FGF-2 in fibroblasts. J. Cell Physiol. 2004, 199, 140–148.
- Hayashi, M.; Kim, S.-W.; Imanaka-Yoshida, K.; Yoshida, T.; Abel, E.D.; Eliceiri, B.; Yang, Y.; Ulevitch, R.J.; Lee, J.-D. Targeted deletion of BMK1/ERK5 in adult mice perturbs vascular integrity and leads to endothelial failure. J. Clin. Investig. 2004, 113, 1138–1148.
- Carvajal-Vergara, X.; Tabera, S.; Montero, J.C.; Esparís-Ogando, A.; López-Pérez, R.; Mateo, G.; Gutiérrez, N.; Parmo-Cabañas, M.; Teixidó, J.; San Miguel, J.F.; et al. Multifunctional role of Erk5 in multiple myeloma. Blood 2005, 105, 4492–4499.
- Abe, J.; Kusuhara, M.; Ulevitch, R.J.; Berk, B.C.; Lee, J.D. Big mitogen-activated protein kinase 1 (BMK1) is a redox-sensitive kinase. J. Biol. Chem. 1996, 271, 16586–16590.
- Chao, T.H.; Hayashi, M.; Tapping, R.I.; Kato, Y.; Lee, J.D. MEKK3 directly regulates MEK5 activity as part of the big mitogen-activated protein kinase 1 (BMK1) signaling pathway. J. Biol. Chem. 1999, 274, 36035–36038.
- Nakamura, K.; Johnson, G.L. PB1 domains of MEKK2 and MEKK3 interact with the MEK5 PB1 domain for activation of the ERK5 pathway. J. Biol. Chem. 2003, 278, 36989–36992.
- Nakamura, K.; Johnson, G.L. Noncanonical function of MEKK2 and MEK5 PB1 domains for coordinated extracellular signal-regulated kinase 5 and c-Jun N-terminal kinase signaling. Mol. Cell. Biol. 2007, 27, 4566–4577.
- Barros, J.C.; Marshall, C.J. Activation of either ERK1/2 or ERK5 MAP kinase pathways can lead to disruption of the actin cytoskeleton. J. Cell Sci. 2005, 118, 1663–1671.
- Nithianandarajah-Jones, G.N.; Wilm, B.; Goldring, C.E.P.; Müller, J.; Cross, M.J. ERK5: Structure, regulation and function. Cell. Signal. 2012, 24, 2187–2196.
- Umapathy, G.; El Wakil, A.; Witek, B.; Chesler, L.; Danielson, L.; Deng, X.; Gray, N.S.; Johansson, M.; Kvarnbrink, S.; Ruuth, K.; et al. The kinase ALK stimulates the kinase ERK5 to promote the expression of the oncogene MYCN in neuroblastoma. Sci. Signal. 2014, 7, ra102.
- Ramos-Nino, M.E.; Blumen, S.R.; Sabo-Attwood, T.; Pass, H.; Carbone, M.; Testa, J.R.; Altomare, D.A.; Mossman, B.T. HGF mediates cell proliferation of human mesothelioma cells through a PI3K/MEK5/Fra-1 pathway. Am. J. Respir. Cell Mol. Biol. 2008, 38, 209–217.
- Liu, F.; Zhang, H.; Song, H. Upregulation of MEK5 by Stat3 promotes breast cancer cell invasion and metastasis. Oncol. Rep. 2017, 37, 83–90.
- Browne, J.A.; Pearson, A.L.; Zahr, R.A.; Niculescu-Duvaz, I.; Baines, D.L.; Dockrell, M.E.C. TGF-beta activates ERK5 in human renal epithelial cells. Biochem. Biophys. Res. Commun. 2008, 373, 440–444.
- Jiang, L.; Huang, M.; Wang, L.; Fan, X.; Wang, P.; Wang, D.; Fu, X.; Wang, J. Overexpression of MEKK2 is associated with colorectal carcinogenesis. Oncol. Lett. 2013, 6, 1333–1337.
- Lu, H.; Cao, X.; Chen, Q.; Chen, L.; Chen, L.; Gan, M. The expression and role of MEKK3 in renal clear cell carcinoma. Anat. Rec. 2015, 298, 727–734.
- Samanta, A.K.; Huang, H.J.; Bast, R.C.; Liao, W.S.-L. Overexpression of MEKK3 confers resistance to apoptosis through activation of NFkappaB. J. Biol. Chem. 2004, 279, 7576–7583.
- Hasan, R.; Sharma, R.; Saraya, A.; Chattopadhyay, T.K.; DattaGupta, S.; Walfish, P.G.; Chauhan, S.S.; Ralhan, R. Mitogen activated protein kinase kinase kinase 3 (MAP3K3/MEKK3) overexpression is an early event in esophageal tumorigenesis and is a predictor of poor disease prognosis. BMC Cancer 2014, 14, 2.
- Sánchez-Fdez, A.; Re-Louhau, M.F.; Rodríguez-Núñez, P.; Ludeña, D.; Matilla-Almazán, S.; Pandiella, A.; Esparís-Ogando, A. Clinical, genetic and pharmacological data support targeting the MEK5/ERK5 module in lung cancer. NPJ Precis. Oncol. 2021, 5, 78.
- Dong, F.; Gutkind, J.S.; Larner, A.C. Granulocyte colony-stimulating factor induces ERK5 activation, which is differentially regulated by protein-tyrosine kinases and protein kinase C. Regulation of cell proliferation and survival. J. Biol. Chem. 2001, 276, 10811–10816.
- Buschbeck, M.; Eickhoff, J.; Sommer, M.N.; Ullrich, A. Phosphotyrosine-specific phosphatase PTP-SL regulates the ERK5 signaling pathway. J. Biol. Chem. 2002, 277, 29503–29509.
- Sarközi, R.; Miller, B.; Pollack, V.; Feifel, E.; Mayer, G.; Sorokin, A.; Schramek, H. ERK1/2-driven and MKP-mediated inhibition of EGF-induced ERK5 signaling in human proximal tubular cells. J. Cell Physiol. 2007, 211, 88–100.
- Adam, C.; Fusi, L.; Weiss, N.; Goller, S.G.; Meder, K.; Frings, V.G.; Kneitz, H.; Goebeler, M.; Houben, R.; Schrama, D.; et al. Efficient Suppression of NRAS-Driven Melanoma by Co-Inhibition of ERK1/2 and ERK5 MAPK Pathways. J. Investig. Dermatol. 2020, 140, 2455–2465.e10.
- De Jong, P.R.; Taniguchi, K.; Harris, A.R.; Bertin, S.; Takahashi, N.; Duong, J.; Campos, A.D.; Powis, G.; Corr, M.; Karin, M.; et al. ERK5 signalling rescues intestinal epithelial turnover and tumour cell proliferation upon ERK1/2 abrogation. Nat. Commun. 2016, 7, 11551.
- Lee, B.; Sahoo, A.; Sawada, J.; Marchica, J.; Sahoo, S.; Layng, F.I.A.L.; Finlay, D.; Mazar, J.; Joshi, P.; Komatsu, M.; et al. MicroRNA-211 Modulates the DUSP6-ERK5 Signaling Axis to Promote BRAFV600E-Driven Melanoma Growth In Vivo and BRAF/MEK Inhibitor Resistance. J. Investig. Dermatol. 2021, 141, 385–394.
- Moncho-Amor, V.; Pintado-Berninches, L.; Ibañez de Cáceres, I.; Martín-Villar, E.; Quintanilla, M.; Chakravarty, P.; Cortes-Sempere, M.; Fernández-Varas, B.; Rodriguez-Antolín, C.; de Castro, J.; et al. Role of Dusp6 Phosphatase as a Tumor Suppressor in Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2019, 20, 2036.
- Garcia, L.; Garcia, F.; Llorens, F.; Unzeta, M.; Itarte, E.; Gómez, N. PP1/PP2A phosphatases inhibitors okadaic acid and calyculin A block ERK5 activation by growth factors and oxidative stress. FEBS Lett. 2002, 523, 90–94.
- Buschbeck, M.; Ullrich, A. The unique C-terminal tail of the mitogen-activated protein kinase ERK5 regulates its activation and nuclear shuttling. J. Biol. Chem. 2005, 280, 2659–2667.
- Erazo, T.; Moreno, A.; Ruiz-Babot, G.; Rodríguez-Asiain, A.; Morrice, N.A.; Espadamala, J.; Bayascas, J.R.; Gómez, N.; Lizcano, J.M. Canonical and kinase activity-independent mechanisms for extracellular signal-regulated kinase 5 (ERK5) nuclear translocation require dissociation of Hsp90 from the ERK5-Cdc37 complex. Mol. Cell. Biol. 2013, 33, 1671–1686.
- Erazo, T.; Espinosa-Gil, S.; Diéguez-Martínez, N.; Gómez, N.; Lizcano, J.M. SUMOylation Is Required for ERK5 Nuclear Translocation and ERK5-Mediated Cancer Cell Proliferation. Int. J. Mol. Sci. 2020, 21, 2203.
- Kamakura, S.; Moriguchi, T.; Nishida, E. Activation of the protein kinase ERK5/BMK1 by receptor tyrosine kinases. Identification and characterization of a signaling pathway to the nucleus. J. Biol. Chem. 1999, 274, 26563–26571.
- Kato, Y.; Chao, T.H.; Hayashi, M.; Tapping, R.I.; Lee, J.D. Role of BMK1 in regulation of growth factor-induced cellular responses. Immunol. Res. 2000, 21, 233–237.
- Terasawa, K.; Okazaki, K.; Nishida, E. Regulation of c-Fos and Fra-1 by the MEK5-ERK5 pathway. Genes Cells 2003, 8, 263–273.
- Yang, S.H.; Whitmarsh, A.J.; Davis, R.J.; Sharrocks, A.D. Differential targeting of MAP kinases to the ETS-domain transcription factor Elk-1. EMBO J. 1998, 17, 1740–1749.
- Kasler, H.G.; Victoria, J.; Duramad, O.; Winoto, A. ERK5 is a novel type of mitogen-activated protein kinase containing a transcriptional activation domain. Mol. Cell. Biol. 2000, 20, 8382–8389.
- Zhuang, K.; Zhang, J.; Xiong, M.; Wang, X.; Luo, X.; Han, L.; Meng, Y.; Zhang, Y.; Liao, W.; Liu, S. CDK5 functions as a tumor promoter in human colorectal cancer via modulating the ERK5-AP-1 axis. Cell Death Dis. 2016, 7, e2415.
- Luiz, J.P.M.; Toller-Kawahisa, J.E.; Viacava, P.R.; Nascimento, D.C.; Pereira, P.T.; Saraiva, A.L.; Prado, D.S.; LeBert, M.; Giurisato, E.; Tournier, C.; et al. MEK5/ERK5 signaling mediates IL-4-induced M2 macrophage differentiation through regulation of c-Myc expression. J. Leukoc. Biol. 2020, 108, 1215–1223.
- Yang, Q.; Deng, X.; Lu, B.; Cameron, M.; Fearns, C.; Patricelli, M.P.; Yates, J.R.; Gray, N.S.; Lee, J.-D. Pharmacological inhibition of BMK1 suppresses tumor growth through promyelocytic leukemia protein. Cancer Cell 2010, 18, 258–267.
- Simões, A.E.S.; Pereira, D.M.; Gomes, S.E.; Brito, H.; Carvalho, T.; French, A.; Castro, R.E.; Steer, C.J.; Thibodeau, S.N.; Rodrigues, C.M.P.; et al. Aberrant MEK5/ERK5 signalling contributes to human colon cancer progression via NF-κB activation. Cell Death Dis. 2015, 6, e1718.
- Samanta, A.K.; Huang, H.J.; Le, X.-F.; Mao, W.; Lu, K.H.; Bast, R.C.; Liao, W.S.-L. MEKK3 expression correlates with nuclear factor kappa B activity and with expression of antiapoptotic genes in serous ovarian carcinoma. Cancer 2009, 115, 3897–3908.
- Mody, N.; Campbell, D.G.; Morrice, N.; Peggie, M.; Cohen, P. An analysis of the phosphorylation and activation of extracellular-signal-regulated protein kinase 5 (ERK5) by mitogen-activated protein kinase kinase 5 (MKK5) in vitro. Biochem. J. 2003, 372, 567–575.
- Pearson, A.J.; Fullwood, P.; Toro Tapia, G.; Prise, I.; Smith, M.P.; Xu, Q.; Jordan, A.; Giurisato, E.; Whitmarsh, A.J.; Francavilla, C.; et al. Discovery of a Gatekeeper Residue in the C-Terminal Tail of the Extracellular Signal-Regulated Protein Kinase 5 (ERK5). Int. J. Mol. Sci. 2020, 21, 929.
- Díaz-Rodríguez, E.; Pandiella, A. Multisite phosphorylation of Erk5 in mitosis. J. Cell Sci. 2010, 123, 3146–3156.
- Honda, T.; Obara, Y.; Yamauchi, A.; Couvillon, A.D.; Mason, J.J.; Ishii, K.; Nakahata, N. Phosphorylation of ERK5 on Thr732 is associated with ERK5 nuclear localization and ERK5-dependent transcription. PLoS ONE 2015, 10, e0117914.
- Clapé, C.; Fritz, V.; Henriquet, C.; Apparailly, F.; Fernandez, P.L.; Iborra, F.; Avancès, C.; Villalba, M.; Culine, S.; Fajas, L. miR-143 interferes with ERK5 signaling, and abrogates prostate cancer progression in mice. PLoS ONE 2009, 4, e7542.
- Ahmad, I.; Singh, L.B.; Yang, Z.H.; Kalna, G.; Fleming, J.; Fisher, G.; Cooper, C.; Cuzick, J.; Berney, D.M.; Møller, H.; et al. Mir143 expression inversely correlates with nuclear ERK5 immunoreactivity in clinical prostate cancer. Br. J. Cancer 2013, 108, 149–154.
- Noguchi, S.; Mori, T.; Hoshino, Y.; Maruo, K.; Yamada, N.; Kitade, Y.; Naoe, T.; Akao, Y. MicroRNA-143 functions as a tumor suppressor in human bladder cancer T24 cells. Cancer Lett. 2011, 307, 211–220.
- Zhou, L.L.; Dong, J.L.; Huang, G.; Sun, Z.L.; Wu, J. MicroRNA-143 inhibits cell growth by targeting ERK5 and MAP3K7 in breast cancer. Braz. J. Med. Biol. Res. 2017, 50, e5891.
- Zhai, L.; Ma, C.; Li, W.; Yang, S.; Liu, Z. miR-143 suppresses epithelial-mesenchymal transition and inhibits tumor growth of breast cancer through down-regulation of ERK5. Mol. Carcinog. 2016, 55, 1990–2000.
- Ni, Y.; Meng, L.; Wang, L.; Dong, W.; Shen, H.; Wang, G.; Liu, Q.; Du, J. MicroRNA-143 functions as a tumor suppressor in human esophageal squamous cell carcinoma. Gene 2013, 517, 197–204.
- Chen, J.-H.; Yang, R.; Zhang, W.; Wang, Y.-P. Functions of microRNA-143 in the apoptosis, invasion and migration of nasopharyngeal carcinoma. Exp. Ther. Med. 2016, 12, 3749–3755.
- Hartmann, J.-U.; Bräuer-Hartmann, D.; Kardosova, M.; Wurm, A.A.; Wilke, F.; Schödel, C.; Gerloff, D.; Katzerke, C.; Krakowsky, R.; Namasu, C.Y.; et al. MicroRNA-143 targets ERK5 in granulopoiesis and predicts outcome of patients with acute myeloid leukemia. Cell Death Dis. 2018, 9, 814.
- Akao, Y.; Nakagawa, Y.; Iio, A.; Naoe, T. Role of microRNA-143 in Fas-mediated apoptosis in human T-cell leukemia Jurkat cells. Leuk. Res. 2009, 33, 1530–1538.
- Akao, Y.; Nakagawa, Y.; Kitade, Y.; Kinoshita, T.; Naoe, T. Downregulation of microRNAs-143 and -145 in B-cell malignancies. Cancer Sci. 2007, 98, 1914–1920.
- Wu, J.; Cui, H.; Zhu, Z.; Wang, L. MicroRNA-200b-3p suppresses epithelial-mesenchymal transition and inhibits tumor growth of glioma through down-regulation of ERK5. Biochem. Biophys. Res. Commun. 2016, 478, 1158–1164.
- Hoang, V.T.; Yan, T.J.; Cavanaugh, J.E.; Flaherty, P.T.; Beckman, B.S.; Burow, M.E. Oncogenic signaling of MEK5-ERK5. Cancer Lett. 2017, 392, 51–59.
- Paudel, R.; Fusi, L.; Schmidt, M. The MEK5/ERK5 pathway in health and disease. Int. J. Mol. Sci. 2021, 22, 7594.
- Kato, Y.; Kravchenko, V.V.; Tapping, R.I.; Han, J.; Ulevitch, R.J.; Lee, J.D. BMK1/ERK5 regulates serum-induced early gene expression through transcription factor MEF2C. EMBO J. 1997, 16, 7054–7066.
- Rovida, E.; Spinelli, E.; Sdelci, S.; Barbetti, V.; Morandi, A.; Giuntoli, S.; Dello Sbarba, P. ERK5/BMK1 is indispensable for optimal colony-stimulating factor 1 (CSF-1)-induced proliferation in macrophages in a Src-dependent fashion. J. Immunol. 2008, 180, 4166–4172.
- Hayashi, M.; Tapping, R.I.; Chao, T.H.; Lo, J.F.; King, C.C.; Yang, Y.; Lee, J.D. BMK1 mediates growth factor-induced cell proliferation through direct cellular activation of serum and glucocorticoid-inducible kinase. J. Biol. Chem. 2001, 276, 8631–8634.
- Mulloy, R.; Salinas, S.; Philips, A.; Hipskind, R.A. Activation of cyclin D1 expression by the ERK5 cascade. Oncogene 2003, 22, 5387–5398.
- Hayashi, M.; Fearns, C.; Eliceiri, B.; Yang, Y.; Lee, J.-D. Big mitogen-activated protein kinase 1/extracellular signal-regulated kinase 5 signaling pathway is essential for tumor-associated angiogenesis. Cancer Res. 2005, 65, 7699–7706.
- Garaude, J.; Cherni, S.; Kaminski, S.; Delepine, E.; Chable-Bessia, C.; Benkirane, M.; Borges, J.; Pandiella, A.; Iñiguez, M.A.; Fresno, M.; et al. ERK5 activates NF-kappaB in leukemic T cells and is essential for their growth in vivo. J. Immunol. 2006, 177, 7607–7617.
- Cude, K.; Wang, Y.; Choi, H.-J.; Hsuan, S.-L.; Zhang, H.; Wang, C.-Y.; Xia, Z. Regulation of the G2-M cell cycle progression by the ERK5-NFkappaB signaling pathway. J. Cell Biol. 2007, 177, 253–264.
- Buse, P.; Tran, S.H.; Luther, E.; Phu, P.T.; Aponte, G.W.; Firestone, G.L. Cell cycle and hormonal control of nuclear-cytoplasmic localization of the serum- and glucocorticoid-inducible protein kinase, Sgk, in mammary tumor cells. A novel convergence point of anti-proliferative and proliferative cell signaling pathways. J. Biol. Chem. 1999, 274, 7253–7263.
- Liu, L.; Cavanaugh, J.E.; Wang, Y.; Sakagami, H.; Mao, Z.; Xia, Z. ERK5 activation of MEF2-mediated gene expression plays a critical role in BDNF-promoted survival of developing but not mature cortical neurons. Proc. Natl. Acad. Sci. USA 2003, 100, 8532–8537.
- Perez-Madrigal, D.; Finegan, K.G.; Paramo, B.; Tournier, C. The extracellular-regulated protein kinase 5 (ERK5) promotes cell proliferation through the down-regulation of inhibitors of cyclin dependent protein kinases (CDKs). Cell. Signal. 2012, 24, 2360–2368.
- Rovida, E.; Di Maira, G.; Tusa, I.; Cannito, S.; Paternostro, C.; Navari, N.; Vivoli, E.; Deng, X.; Gray, N.S.; Esparís-Ogando, A.; et al. The mitogen-activated protein kinase ERK5 regulates the development and growth of hepatocellular carcinoma. Gut 2015, 64, 1454–1465.
- Tusa, I.; Gagliardi, S.; Tubita, A.; Pandolfi, S.; Menconi, A.; Lulli, M.; Dello Sbarba, P.; Stecca, B.; Rovida, E. The Hedgehog-GLI Pathway Regulates MEK5-ERK5 Expression and Activation in Melanoma Cells. Int. J. Mol. Sci. 2021, 22, 11259.
- Shukla, A.; Miller, J.M.; Cason, C.; Sayan, M.; MacPherson, M.B.; Beuschel, S.L.; Hillegass, J.; Vacek, P.M.; Pass, H.I.; Mossman, B.T. Extracellular signal-regulated kinase 5: A potential therapeutic target for malignant mesotheliomas. Clin. Cancer Res. 2013, 19, 2071–2083.
- Ortiz-Ruiz, M.J.; Álvarez-Fernández, S.; Parrott, T.; Zaknoen, S.; Burrows, F.J.; Ocaña, A.; Pandiella, A.; Esparís-Ogando, A. Therapeutic potential of ERK5 targeting in triple negative breast cancer. Oncotarget 2014, 5, 11308–11318.
- McCracken, S.R.C.; Ramsay, A.; Heer, R.; Mathers, M.E.; Jenkins, B.L.; Edwards, J.; Robson, C.N.; Marquez, R.; Cohen, P.; Leung, H.Y. Aberrant expression of extracellular signal-regulated kinase 5 in human prostate cancer. Oncogene 2008, 27, 2978–2988.
- Pereira, D.M.; Simões, A.E.S.; Gomes, S.E.; Castro, R.E.; Carvalho, T.; Rodrigues, C.M.P.; Borralho, P.M. MEK5/ERK5 signaling inhibition increases colon cancer cell sensitivity to 5-fluorouracil through a p53-dependent mechanism. Oncotarget 2016, 7, 34322–34340.
- Giurisato, E.; Tournier, C. Can tumor cells proliferate without ERK5? Cell Cycle 2016, 15, 619–620.
- Lochhead, P.A.; Clark, J.; Wang, L.-Z.; Gilmour, L.; Squires, M.; Gilley, R.; Foxton, C.; Newell, D.R.; Wedge, S.R.; Cook, S.J. Tumor cells with KRAS or BRAF mutations or ERK5/MAPK7 amplification are not addicted to ERK5 activity for cell proliferation. Cell Cycle 2016, 15, 506–518.
- Tusa, I.; Gagliardi, S.; Tubita, A.; Pandolfi, S.; Urso, C.; Borgognoni, L.; Wang, J.; Deng, X.; Gray, N.S.; Stecca, B.; et al. ERK5 is activated by oncogenic BRAF and promotes melanoma growth. Oncogene 2018, 37, 2601–2614.
- Tubita, A.; Lombardi, Z.; Tusa, I.; Lazzeretti, A.; Sgrignani, G.; Papini, D.; Menconi, A.; Gagliardi, S.; Lulli, M.; Dello Sbarba, P.; et al. Inhibition of ERK5 elicits cellular senescence in melanoma via the cyclin-dependent kinase inhibitor p21. Cancer Res. 2021.
- Gavine, P.R.; Wang, M.; Yu, D.; Hu, E.; Huang, C.; Xia, J.; Su, X.; Fan, J.; Zhang, T.; Ye, Q.; et al. Identification and validation of dysregulated MAPK7 (ERK5) as a novel oncogenic target in squamous cell lung and esophageal carcinoma. BMC Cancer 2015, 15, 454.
- Ranganathan, A.; Pearson, G.W.; Chrestensen, C.A.; Sturgill, T.W.; Cobb, M.H. The MAP kinase ERK5 binds to and phosphorylates p90 RSK. Arch. Biochem. Biophys. 2006, 449, 8–16.
- Lennartsson, J.; Burovic, F.; Witek, B.; Jurek, A.; Heldin, C.-H. Erk 5 is necessary for sustained PDGF-induced Akt phosphorylation and inhibition of apoptosis. Cell. Signal. 2010, 22, 955–960.
- Villa-Moruzzi, E. Targeting of FAK Ser910 by ERK5 and PP1delta in non-stimulated and phorbol ester-stimulated cells. Biochem. J. 2007, 408, 7–18.
- Sawhney, R.S.; Liu, W.; Brattain, M.G. A novel role of ERK5 in integrin-mediated cell adhesion and motility in cancer cells via Fak signaling. J. Cell. Physiol. 2009, 219, 152–161.
- Ali, M.; Mutahir, Z.; Riaz, A. CRISPR/Cas9 engineering of ERK5 identifies its FAK/PYK2 dependent role in adhesion-mediated cell survival. Biochem. Biophys. Res. Commun. 2019, 513, 179–185.
- Xu, Q.; Zhang, J.; Telfer, B.A.; Zhang, H.; Ali, N.; Chen, F.; Risa, B.; Pearson, A.J.; Zhang, W.; Finegan, K.G.; et al. The extracellular-regulated protein kinase 5 (ERK5) enhances metastatic burden in triple-negative breast cancer through focal adhesion protein kinase (FAK)-mediated regulation of cell adhesion. Oncogene 2021, 40, 3929–3941.
- Sticht, C.; Freier, K.; Knöpfle, K.; Flechtenmacher, C.; Pungs, S.; Hofele, C.; Hahn, M.; Joos, S.; Lichter, P. Activation of MAP kinase signaling through ERK5 but not ERK1 expression is associated with lymph node metastases in oral squamous cell carcinoma (OSCC). Neoplasia 2008, 10, 462–470.
- Madak-Erdogan, Z.; Ventrella, R.; Petry, L.; Katzenellenbogen, B.S. Novel roles for ERK5 and cofilin as critical mediators linking ERα-driven transcription, actin reorganization, and invasiveness in breast cancer. Mol. Cancer Res. 2014, 12, 714–727.
- Pavan, S.; Meyer-Schaller, N.; Diepenbruck, M.; Kalathur, R.K.R.; Saxena, M.; Christofori, G. A kinome-wide high-content siRNA screen identifies MEK5-ERK5 signaling as critical for breast cancer cell EMT and metastasis. Oncogene 2018, 37, 4197–4213.
- Castro, N.E.; Lange, C.A. Breast tumor kinase and extracellular signal-regulated kinase 5 mediate Met receptor signaling to cell migration in breast cancer cells. Breast Cancer Res. 2010, 12, R60.
- Zuo, Y.; Wu, Y.; Wehrli, B.; Chakrabarti, S.; Chakraborty, C. Modulation of ERK5 is a novel mechanism by which Cdc42 regulates migration of breast cancer cells. J. Cell. Biochem. 2015, 116, 124–132.
- Locatelli, A.; Lange, C.A. Met receptors induce Sam68-dependent cell migration by activation of alternate extracellular signal-regulated kinase family members. J. Biol. Chem. 2011, 286, 21062–21072.
- Mirza, A.A.; Kahle, M.P.; Ameka, M.; Campbell, E.M.; Cuevas, B.D. MEKK2 regulates focal adhesion stability and motility in invasive breast cancer cells. Biochim. Biophys. Acta 2014, 1843, 945–954.
- Cronan, M.R.; Nakamura, K.; Johnson, N.L.; Granger, D.A.; Cuevas, B.D.; Wang, J.G.; Mackman, N.; Scott, J.E.; Dohlman, H.G.; Johnson, G.L. Defining MAP3 kinases required for MDA-MB-231 cell tumor growth and metastasis. Oncogene 2012, 31, 3889–3900.
- Zhou, C.; Nitschke, A.M.; Xiong, W.; Zhang, Q.; Tang, Y.; Bloch, M.; Elliott, S.; Zhu, Y.; Bazzone, L.; Yu, D.; et al. Proteomic analysis of tumor necrosis factor-alpha resistant human breast cancer cells reveals a MEK5/Erk5-mediated epithelial-mesenchymal transition phenotype. Breast Cancer Res. 2008, 10, R105.
- Matossian, M.D.; Hoang, V.T.; Burks, H.E.; La, J.; Elliott, S.; Brock, C.; Rusch, D.B.; Buechlein, A.; Nephew, K.P.; Bhatt, A.; et al. Constitutive activation of MEK5 promotes a mesenchymal and migratory cell phenotype in triple negative breast cancer. Oncoscience 2021, 8, 64–71.
- Hoang, V.T.; Matossian, M.D.; Ucar, D.A.; Elliott, S.; La, J.; Wright, M.K.; Burks, H.E.; Perles, A.; Hossain, F.; King, C.T.; et al. ERK5 is required for tumor growth and maintenance through regulation of the extracellular matrix in triple negative breast cancer. Front. Oncol. 2020, 10, 1164.
- Javaid, S.; Zhang, J.; Smolen, G.A.; Yu, M.; Wittner, B.S.; Singh, A.; Arora, K.S.; Madden, M.W.; Desai, R.; Zubrowski, M.J.; et al. MAPK7 regulates EMT features and modulates the generation of ctcs. Mol. Cancer Res. 2015, 13, 934–943.
- Yue, B.; Ren, Q.X.; Su, T.; Wang, L.N.; Zhang, L. ERK5 silencing inhibits invasion of human osteosarcoma cell via modulating the Slug/MMP-9 pathway. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2640–2647.
- Huang, Y.; Yao, J.; Zhu, B.; Zhang, J.; Sun, T. Mitogen-activated protein kinase 7 promotes cell proliferation, migration and invasion in SOSP-M human osteosarcoma cell line. Tumori 2017, 103, 483–488.
- Tesser-Gamba, F.; da Silva Lopes, L.J.; Petrilli, A.S.; Toledo, S.R.C. MAPK7 gene controls proliferation, migration and cell invasion in osteosarcoma. Mol. Carcinog. 2016, 55, 1700–1713.
- Wang, Z.; Wang, W.; Xu, S.; Wang, S.; Tu, Y.; Xiong, Y.; Mei, J.; Wang, C. The role of MAPK signaling pathway in the Her-2-positive meningiomas. Oncol. Rep. 2016, 36, 685–695.
- Sun, X.; Zhang, T.; Deng, Q.; Zhou, Q.; Sun, X.; Li, E.; Yu, D.; Zhong, C. Benzidine Induces Epithelial-Mesenchymal Transition of Human Bladder Cancer Cells through Activation of ERK5 Pathway. Mol. Cells 2018, 41, 188–197.
- Schramp, M.; Ying, O.; Kim, T.Y.; Martin, G.S. ERK5 promotes Src-induced podosome formation by limiting Rho activation. J. Cell Biol. 2008, 181, 1195–1210.
- Dai, J.; Wang, T.; Wang, W.; Zhang, S.; Liao, Y.; Chen, J. Role of MAPK7 in cell proliferation and metastasis in ovarian cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 10444–10451.
- Park, S.J.; Choi, Y.S.; Lee, S.; Lee, Y.J.; Hong, S.; Han, S.; Kim, B.-C. BIX02189 inhibits TGF-β1-induced lung cancer cell metastasis by directly targeting TGF-β type I receptor. Cancer Lett. 2016, 381, 314–322.
- Serrano-Oviedo, L.; Giménez-Bachs, J.M.; Nam-Cha, S.Y.; Cimas, F.J.; García-Cano, J.; Sánchez-Prieto, R.; Salinas-Sánchez, A.S. Implication of VHL, ERK5, and HIF-1alpha in clear cell renal cell carcinoma: Molecular basis. Urol. Oncol. 2017, 35, 114.e15–114.e22.
- Im, J.-Y.; Yoon, S.-H.; Kim, B.-K.; Ban, H.S.; Won, K.-J.; Chung, K.-S.; Jung, K.E.; Won, M. DNA damage induced apoptosis suppressor (DDIAS) is upregulated via ERK5/MEF2B signaling and promotes β-catenin-mediated invasion. Biochim. Biophys. Acta 2016, 1859, 1449–1458.
- Zheng, Y.; Asara, J.M.; Tyner, A.L. Protein-tyrosine kinase 6 promotes peripheral adhesion complex formation and cell migration by phosphorylating p130 CRK-associated substrate. J. Biol. Chem. 2012, 287, 148–158.
- Chen, W.; Zhang, B.; Guo, W.; Gao, L.; Shi, L.; Li, H.; Lu, S.; Liu, Y.; Li, X. miR-429 inhibits glioma invasion through BMK1 suppression. J. Neurooncol. 2015, 125, 43–54.
- Xia, C.; Yang, Y.; Kong, F.; Kong, Q.; Shan, C. MiR-143-3p inhibits the proliferation, cell migration and invasion of human breast cancer cells by modulating the expression of MAPK7. Biochimie 2018, 147, 98–104.
- Chen, R.; Yang, Q.; Lee, J.-D. BMK1 kinase suppresses epithelial-mesenchymal transition through the Akt/GSK3β signaling pathway. Cancer Res. 2012, 72, 1579–1587.
- Williams, C.A.C.; Fernandez-Alonso, R.; Wang, J.; Toth, R.; Gray, N.S.; Findlay, G.M. Erk5 Is a Key Regulator of Naive-Primed Transition and Embryonic Stem Cell Identity. Cell Rep. 2016, 16, 1820–1828.
- Brown, H.A.; Williams, C.A.; Zhou, H.; Rios-Szwed, D.; Fernandez-Alonso, R.; Mansoor, S.; McMulkin, L.; Toth, R.; Gourlay, R.; Peltier, J.; et al. An ERK5-KLF2 signalling module regulates early embryonic gene expression and telomere rejuvenation in stem cells. Biochem. J. 2021, 478, 4119–4136.
- Lai, V.K.; Ashraf, M.; Jiang, S.; Haider, K. MicroRNA-143 is a critical regulator of cell cycle activity in stem cells with co-overexpression of Akt and angiopoietin-1 via transcriptional regulation of Erk5/cyclin D1 signaling. Cell Cycle 2012, 11, 767–777.
- Tusa, I.; Cheloni, G.; Poteti, M.; Gozzini, A.; DeSouza, N.H.; Shan, Y.; Deng, X.; Gray, N.S.; Li, S.; Rovida, E.; et al. Targeting the Extracellular Signal-Regulated Kinase 5 Pathway to Suppress Human Chronic Myeloid Leukemia Stem Cells. Stem Cell Rep. 2018, 11, 929–943.
- Pereira, D.M.; Gomes, S.E.; Borralho, P.M.; Rodrigues, C.M.P. MEK5/ERK5 activation regulates colon cancer stem-like cell properties. Cell Death Discov. 2019, 5, 68.
- Kedika, S.R.; Shukla, S.P.; Udugamasooriya, D.G. Design of a dual ERK5 kinase activation and autophosphorylation inhibitor to block cancer stem cell activity. Bioorg. Med. Chem. Lett. 2020, 30, 127552.
- Lopez-Royuela, N.; Rathore, M.G.; Allende-Vega, N.; Annicotte, J.-S.; Fajas, L.; Ramachandran, B.; Gulick, T.; Villalba, M. Extracellular-signal-regulated kinase 5 modulates the antioxidant response by transcriptionally controlling Sirtuin 1 expression in leukemic cells. Int. J. Biochem. Cell Biol. 2014, 53, 253–261.
- Khan, A.U.H.; Rathore, M.G.; Allende-Vega, N.; Vo, D.-N.; Belkhala, S.; Orecchioni, S.; Talarico, G.; Bertolini, F.; Cartron, G.; Lecellier, C.-H.; et al. Human Leukemic Cells performing Oxidative Phosphorylation (OXPHOS) Generate an Antioxidant Response Independently of Reactive Oxygen species (ROS) Production. EBioMedicine 2016, 3, 43–53.
- Khan, A.U.H.; Allende-Vega, N.; Gitenay, D.; Gerbal-Chaloin, S.; Gondeau, C.; Vo, D.-N.; Belkahla, S.; Orecchioni, S.; Talarico, G.; Bertolini, F.; et al. The PDK1 inhibitor dichloroacetate controls cholesterol homeostasis through the ERK5/MEF2 pathway. Sci. Rep. 2017, 7, 10654.
- Charni, S.; de Bettignies, G.; Rathore, M.G.; Aguiló, J.I.; van den Elsen, P.J.; Haouzi, D.; Hipskind, R.A.; Enriquez, J.A.; Sanchez-Beato, M.; Pardo, J.; et al. Oxidative phosphorylation induces de novo expression of the MHC class I in tumor cells through the ERK5 pathway. J. Immunol. 2010, 185, 3498–3503.
- Rathore, M.G.; Saumet, A.; Rossi, J.-F.; de Bettignies, C.; Tempé, D.; Lecellier, C.-H.; Villalba, M. The NF-κB member p65 controls glutamine metabolism through miR-23a. Int. J. Biochem. Cell Biol. 2012, 44, 1448–1456.
- Villalba, M.; Rathore, M.G.; Lopez-Royuela, N.; Krzywinska, E.; Garaude, J.; Allende-Vega, N. From tumor cell metabolism to tumor immune escape. Int. J. Biochem. Cell Biol. 2013, 45, 106–113.
- Schweppe, R.E.; Cheung, T.H.; Ahn, N.G. Global gene expression analysis of ERK5 and ERK1/2 signaling reveals a role for HIF-1 in ERK5-mediated responses. J. Biol. Chem. 2006, 281, 20993–21003.
- Vaseva, A.V.; Blake, D.R.; Gilbert, T.S.K.; Ng, S.; Hostetter, G.; Azam, S.H.; Ozkan-Dagliyan, I.; Gautam, P.; Bryant, K.L.; Pearce, K.H.; et al. KRAS Suppression-Induced Degradation of MYC Is Antagonized by a MEK5-ERK5 Compensatory Mechanism. Cancer Cell 2018, 34, 807–822.e7.
- Cristea, S.; Coles, G.L.; Hornburg, D.; Gershkovitz, M.; Arand, J.; Cao, S.; Sen, T.; Williamson, S.C.; Kim, J.W.; Drainas, A.P.; et al. The MEK5-ERK5 Kinase Axis Controls Lipid Metabolism in Small-Cell Lung Cancer. Cancer Res. 2020, 80, 1293–1303.
- Belkahla, S.; Haq Khan, A.U.; Gitenay, D.; Alexia, C.; Gondeau, C.; Vo, D.-N.; Orecchioni, S.; Talarico, G.; Bertolini, F.; Cartron, G.; et al. Changes in metabolism affect expression of ABC transporters through ERK5 and depending on p53 status. Oncotarget 2018, 9, 1114–1129.
More
Information
Subjects:
Health Care Sciences & Services
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
869
Revision:
1 time
(View History)
Update Date:
20 Jan 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




