
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dragos Catalin Jianu | + 2334 word(s) | 2334 | 2021-12-14 07:38:28 | | | |
| 2 | Vicky Zhou | Meta information modification | 2334 | 2022-01-20 02:53:20 | | | | |
| 3 | Vicky Zhou | + 5 word(s) | 2339 | 2022-01-30 08:26:59 | | |
Video Upload Options
Giant cell arteritis (GCA) is a primary autoimmune vasculitis that specifically affects medium-sized extracranial arteries, like superficial temporal arteries (TAs). The most important data to be considered for the ultrasound (US) diagnosis of temporal arteritis are stenosis, acute occlusions and “dark halo” sign, which represent the edema of the vascular wall. The vessel wall thickening of large vessels in GCA can be recognized by the US, which has high sensitivity and is facile to use. Ocular complications of GCA are common and consist especially of anterior arterial ischemic optic neuropathies or central retinal artery occlusion with sudden, painless, and sharp loss of vision in the affected eye. Color Doppler imaging of the orbital vessels (showing low-end diastolic velocities and a high resistance index) is essential to quickly differentiate the mechanism of ocular involvement (arteritic versus non-arteritic), since the characteristics of TAs on US do not correspond with ocular involvement on GCA. GCA should be cured immediately with systemic corticosteroids to avoid further visual loss of the eyes.
1. Introduction-Giant Cell Arteritis (GCA)
-
An arteritis usually interesting the aorta and its major branches, especially the branches of the external carotid and vertebral arteries (the temporal artery being often affected);
-
Usually patients with an age greater than 50 years at the appearance of clinical disease;
-
Frequently associated with polymyalgia rheumatica, manifested by systemic symptoms, represented by fever, pain in the shoulders and hips, malaise, weight loss;
-
New onset of a medium temporal headache;
-
A clinically modified temporal artery (consisting in tenderness of the vessel or reduced temporal artery pulse), associated with scalp tenderness.
-
Claudication of the jaw on mastication or tongue on mastication and on deglutition.
-
An augmented erythrocyte sedimentation rate, more than 50 mm/h;
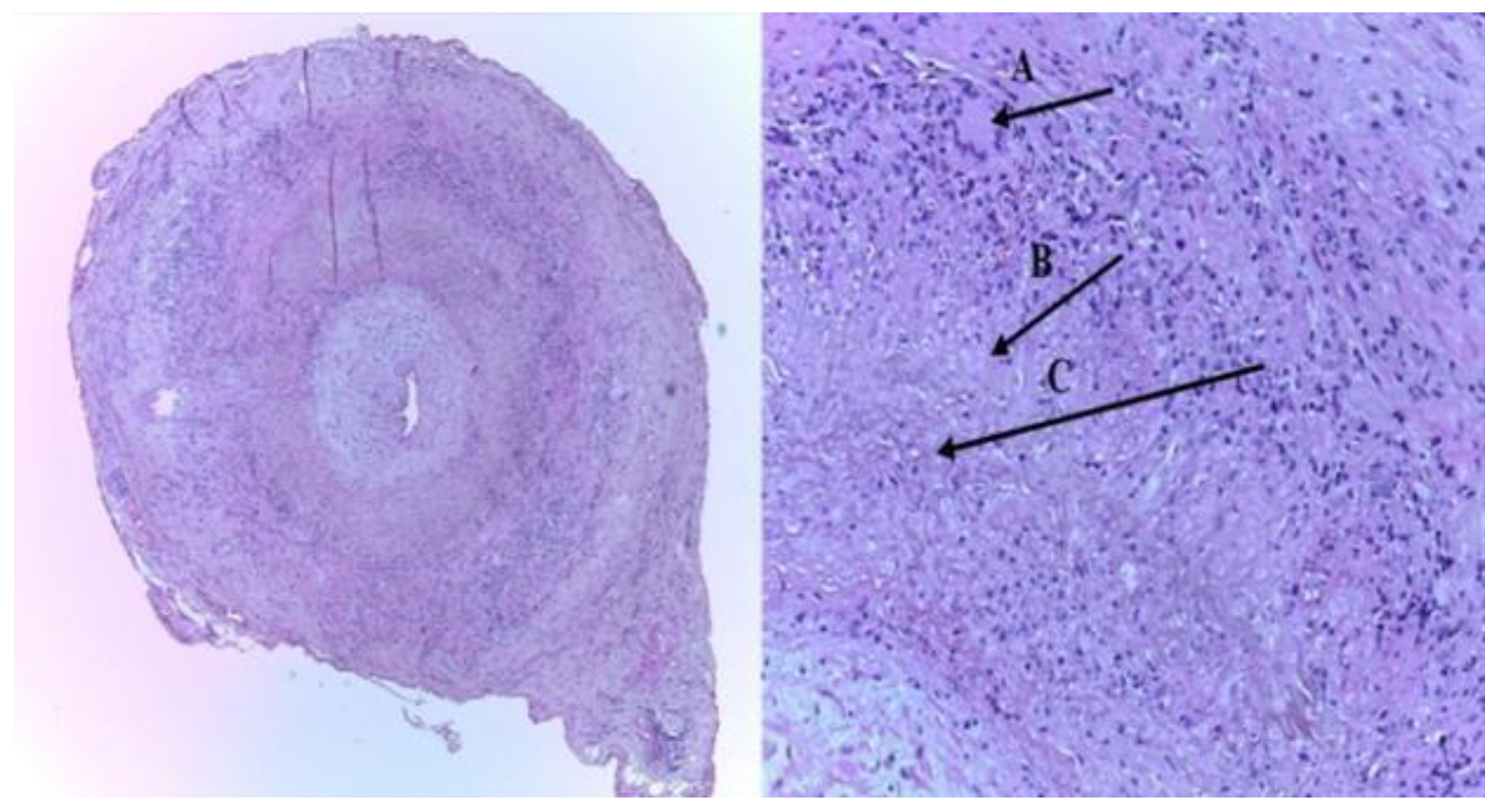
2. Ultrasonography (US) in Giant Cell Arteritis (GCA)
2.1. Background
-
Other medium-sized arteries (branches of the ECAs): the internal maxillary artery (claudication of the jaw on mastication), the renine artery (claudication of the tongue on mastication or on deglutition), the facial, and the occipital arteries,
-
Large size arteries: the common carotid arteries (CCAs), the ECAs, the internal carotid arteries (ICA’s), the vertebral, the subclavian, and the axillary arteries,
2.2. Ultrasonography (US) of the Temporal Arteries (TAs) and Other Medium Size Arteries
2.2.1. Technical Requirements (According to Schmidt)
2.2.2. Machine Adjustments (According to Schmidt)
2.2.3. Sequence of the US Exam (According to Schmidt)
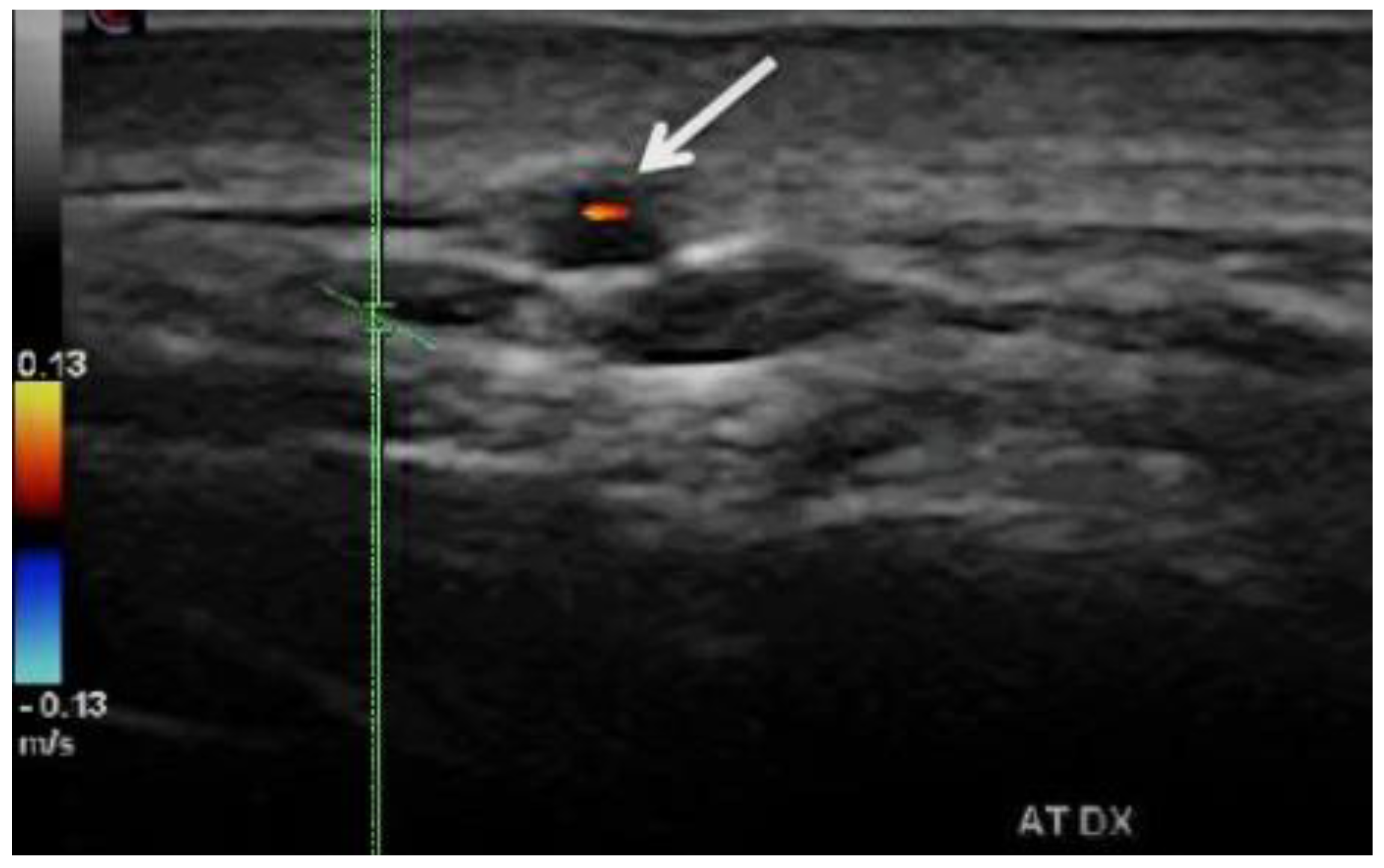
- b. Stenoses are characterized by aliasing and persistent diastolic flow by colour Doppler US. The peak systolic velocity (PSV) assessed within the stenosis area by pulsed-wave Doppler US is two or more times greater than the PSV recorded in the prestenotic segment of the vessel, with turbulence at the level of stenosis, associated with diminished velocities distal to the stenosis [10][11][12][13][14][15][16][17][26][27][28][29] (Figure 3) [19].
-
Figure 3. Duplex ultrasound of the right temporal artery−longitudinal view. Indicates a “halo” sign and a stenosis revealed by a turbulent flow and a high PSV in the stenosis area (1 m/s), which is more than twice the PSV in the prestenotic segment of the artery [19].
- c. Acute occlusions, wherein the US image is similar to that of acute embolism in different other vessels, with lack of color Doppler signals (even with low pulse repetition frequency and high color gain) in a visible artery lumen filled with hypoechoic material (cloth) [10][11][12][13][14][15][16][17][26][27][28][29].
- d. Compression sign. The thickened vessel wall remains visible upon compression by the ultrasound examiner; the wall swelling is hypoechogenic (in acute temporal arteritis), contrasting with the mid-echogenic to hyperechogenic surrounding tissue [26].
2.3. Duplex and Color-Coded Duplex Sonography of the Large Cervical and Cervico-Brachial Vessels
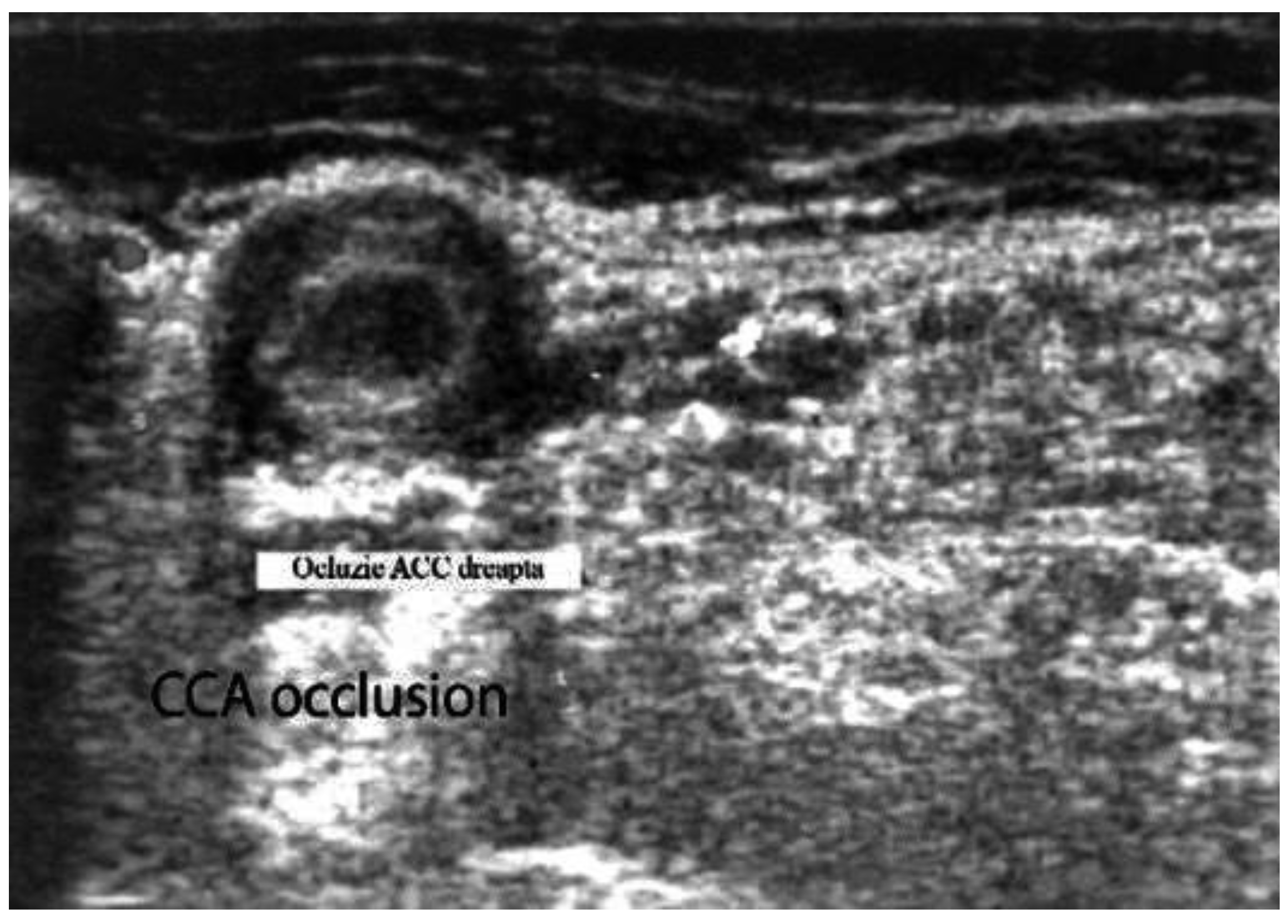 Figure 4. Large vessel GCA. Duplex ultrasound of the right CCA-transverse view. A dark “halo” sign-a hypoechoic circumferential wall thickening around the lumen (which represents arterial wall edema), and occlusion of the artery (the lumen of the vessel is obstructed) [18].
Figure 4. Large vessel GCA. Duplex ultrasound of the right CCA-transverse view. A dark “halo” sign-a hypoechoic circumferential wall thickening around the lumen (which represents arterial wall edema), and occlusion of the artery (the lumen of the vessel is obstructed) [18].
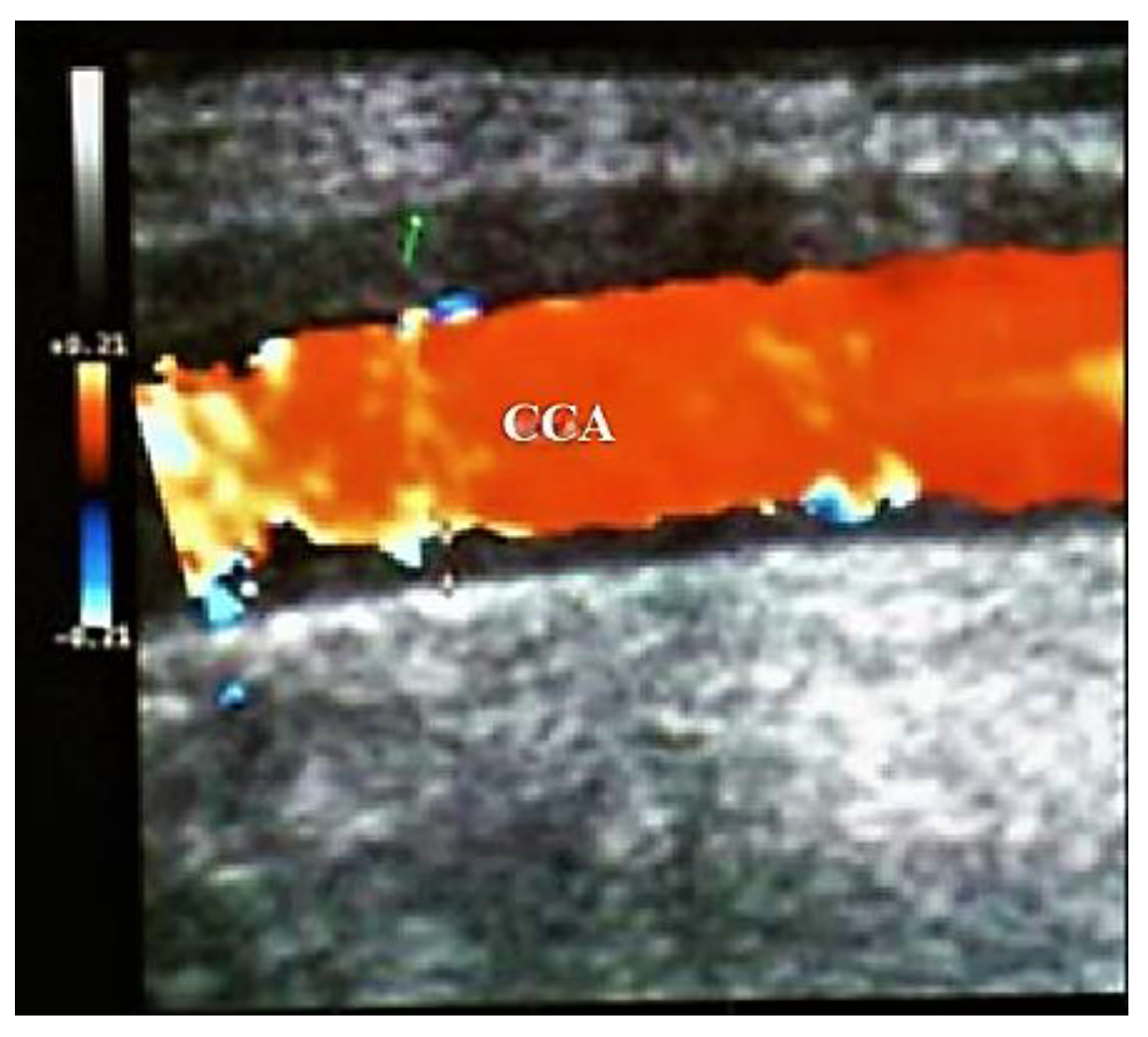
Figure 5. Large vessels GCA. Duplex ultrasound of the right CCA-longitudinal view. The artery presents a dark-hypoechoic circumferential wall thickening (which represents arterial wall edema) [18].
2.4. Color Doppler Imaging (CDI) of Orbital (Retro-Bulbar) Vessels
3. Conclusions
References
- Gonzalez-Gay, M. The diagnosis and management of patients with giant cell arteritis. J. Rheumatol. 2005, 32, 1186–1188.
- Salvarani, C.; Cantini, F.; Hunder, G.G. Polymyalgia rheumatica and giant-cell arteritis. Lancet 2008, 372, 234–245.
- Melson, M.R.; Weyand, C.M.; Newman, N.J.; Biousse, V. The diagnosis of giant cell arteritis. Rev. Neurol Dis. 2007, 4, 128–142.
- Hayreh, S.S.; Podhajsky, P.A.; Raman, R.; Zimmerman, B. Giant cell arteritis: Validity and reliability of various diagnostic criteria. Am. J. Ophthalmol. 1997, 123, 285–296.
- Levine, S.M.; Hellmann, D.B. Giant cell arteritis. Curr. Opin. Rheumatol. 2002, 14, 3–10.
- Weyand, C.M.; Tetzlaff, N.; Björnsson, J.; Brack, A.; Younge, B.; Goronzy, J.J. Disease patterns and tissue cytokine profiles in giant cell arteritis. Arthritis Rheum. 1997, 40, 19–26.
- Hunder, G.G.; Bloch, D.A.; Michel, B.A.; Stevens, M.B.; Arend, W.P.; Do, L.H.C.; Edworthy, S.M.; Fauci, A.S.; Leavitt, R.Y.; Lie, J.T.; et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990, 33, 1122–1128.
- Jennette, J.; Falk, R.J.; Bacon, P.A.; Basu, N.; Cid, M.C.; Ferrario, F.; Flores-Suarez, L.F.; Gross, W.L.; Guillevin, L.; Hagen, E.G.; et al. 2012 Revised international Chapell Hill consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013, 65, 1–11.
- Stanca, H.T.; Suvac, E.; Munteanu, M.; Jianu, D.C.; Motoc, A.G.M.; Roşca, G.C.; Boruga, O. Giant cell arteritis with arteritic anterior ischemic optic neuropathy. Rom. J. Morphol. Embryol. = Rev. Roum. Morphol. Embryol. 2017, 58, 281–285.
- Sturzenegger, M.H. Cervical artery vasculitides. In Manual of Neurosonology; Csiba, L., Baracchini, C., Eds.; Cambridge University Press: Cambridge, UK, 2016; Chapter 8; pp. 300–305.
- Schmidt, W.A. Ultrasound in the diagnosis and management of giant cell arteritis. Rheumatology 2018, 57, ii22–ii31.
- Schmidt, W.A. Takayasu and temporal arteritis. In Handbook on Neurovascular Ultrasound; Baumgartner, R.W., Ed.; Karger: Basel, Switzerland, 2006; Volume 21, pp. 96–104.
- Schmidt, W.A.; Kraft, H.E.; Vorpahl, K.; Völker, L.; Gromnica-Ihle, E.J. Color Duplex Ultrasonography in the Diagnosis of Temporal Arteritis. N. Engl. J. Med. 1997, 337, 1336–1342.
- Schmidt, W.A. Role of ultrasound in the understanding and management of vasculitis. Adv. Musculoskelet. Dis. 2013, 6, 39–47.
- Monti, S.; Floris, A.; Ponte, C.; Schmidt, W.A.; Diamantopoulos, A.P.; Pereira, C.; Piper, J.; Luqmani, R. The use of ultrasound to assess giant cell arteritis: Review of the current evidence and practical guide for the rheumatologist. Rheumatology 2018, 57, 227–235.
- Duftner, C.; Dejaco, C.; Moller-Dohn, U. Ultrasound definitions for vasculitis in cranial and large vessel giant cell arteritis: Results of a Delphi survey of the OMERACT ultrasound large vessel vasculitis group. Ann. Rheum. Dis. 2016, 75 (Suppl. S2), 626.
- Arida, A.; Kyprianou, M.; Kanakis, M.; Sfikakis, P.P. The diagnostic value of ultrasonography-derived edema of the temporal artery wall in giant cell arteritis: A second meta-analysis. BMC Musculoskelet. Disord. 2010, 11, 44.
- Jianu, D.C.; Jianu, S.N.; Petrica, L.; Serpe, M. Large Giant Cell Arteritis with Eye Involvement. In Advances in the Diagnosis and Treatment of Vasculitis-Luis; Amezcua-Guerra, M., Ed.; InTech: Rijeka, Croatia, 2011; Chapter 16; pp. 311–330.
- Jianu, D.C.; Jianu, S.N.; Munteanu, G.; Dan, T.F.; Gogu, A.E.; Petrica, L. Chapter–An Integrated Approach to the Role of Neurosonology in the Diagnosis of Giant Cell Arteritis . In Giant-Cell Arteritis-Imtiaz Chaudhry; IntechOpen: London, UK, 2021.
- Jianu, D.C.; Jianu, S.N. The role of Color Doppler Imaging in the study of optic neuropathies. In Color Doppler Imaging; Neuro-Ophthalmological Correlations; Jianu, D.C., Jianu, S.N., Eds.; Mirton: Timisoara, Romania, 2010; Chapter 8; pp. 154–174.
- Jianu, D.C.; Jianu, S.N. Giant Cell Arteritis and arteritic anterior ischemic optic neuropathies. In Updates in the Diagnosis and Treatment of Vasculitis; Sakkas, L., Katsiari, C., Eds.; InTech: Rijeka, Croatia, 2013; Chapter 5; pp. 111–130.
- Jianu, D.C.; Jianu, S.N.; Petrica, L.; Motoc, A.G.M.; Dan, T.F.; Lazureanu, D.C. Munteanu M-Clinical and color Doppler imaging features of one patient with occult giant cell arteritis presenting arteritic anterior ischemic optic neuropathy. Rom. J. Morphol. Embryol. 2016, 57, 579–583.
- Jianu, D.C.; Jianu, S.N.; Munteanu, M.; Petrica, L. Clinical and ultrasonographic features in anterior ischemic optic neuropathies-Vojnosanit. Pregl. 2018, 75, 773–779.
- Jianu, D.C.; Jianu, S.N. The role of Color Doppler Imaging in the study of central retinal artery obstruction. In Color Doppler Imaging; Neuro-Ophthalmological Correlations; Jianu, D.C., Jianu, S.N., Eds.; Mirton: Timisoara, Romania, 2010; Chapter 6; pp. 125–142.
- Jianu, D.C.; Jianu, S.N.; Munteanu, M.; Vlad, D.; Rosca, C.; Petrica, L. Color Doppler imaging features of two patients presenting central retinal artery occlusion with and without giant cell arteritis. Vojnosanit. Pregl. 2016, 73, 397–401.
- Coath, F.L.; Mukhtyar, C. Ultrasonography in the diagnosis and follow-up of giant cell arteritis. Rheumatology 2021, 60, 2528–2536.
- Serodio, J.F.; Trindade, M.; Favas, C.; Alvez, J.D. Chapter—Extra-Cranial Involvement in Giant Cell Arteritis . In Giant-Cell Arteritis-Imtiaz Chaudhry; IntechOpen: London, UK, 2021.
- Schmidt, W.A.; Krause, A.; Schicke, B.; Kuchenbecker, J.; Gromnica-Ihle, E. Do temporal artery duplex ultrasound findings correlate with ophthalmic complications in giant cell arteritis? Rheumatology 2009, 48, 383–385.
- Karassa, F.B.; Matsagas, M.I.; Schmidt, W.A.; Ioannidis, J.P. Meta-analysis: Test performance of ultrasonography fogiant-cell arteritis. Ann. Intern. Med. 2005, 142, 359–369.
- Diamantopoulos, A.P.; Haugeberg, G.; Lindland, A.; Myklebust, G. The fast-track ultrasound clinic for early diagnosis of giant cell arteritis significantly reduces permanent visual impairment: Towards a more effective strategy to improve clinical outcome in giant cell arteritis? Rheumatology 2016, 55, 66–70.
- Hayreh, S.S.; Podhajsky, P.A.; Zimmerman, B. Occult giant cell arteritis: Ocular manifestations. Am. J. Ophthalmol. 1998, 125, 521–526.
- Hayreh, S.S.; Zimmerman, B.; Kardon, R.H. Visual improvement with corticosteroid therapy in giant cell arteritis. Report of a large study and review of literature. Acta Ophthalmol. Scand. 2002, 80, 355–367.
- Daneshmeyer, H.; Savino, P.; Gamble, G. Poor Prognosis of Visual Outcome after Visual Loss from Giant Cell Arteritis. Ophthalmology 2005, 112, 1098–1103.
- González-Gay, M.A.; García-Porrúa, C.; Llorca, J.; Hajeer, A.H.; Brañas, F.; Dababneh, A.; González-Louzao, C.; Rodriguez-Gil, E.; Rodríguez-Ledo, P.; Ollier, W.E.R. Visual Manifestations of Giant Cell Arteritis: Trends and Clinical Spectrum in 161 Patients. Medicine 2000, 79, 283–292.
- Singh, A.G.; Kermani, T.A.; Crowson, C.S.; Weyand, C.M.; Matteson, E.L.; Warrington, K.J. Visual Manifestations in Giant Cell Arteritis: Trend over 5 Decades in a Population-based Cohort. J. Rheumatol. 2015, 42, 309–315.
- Arnold, A.C.; Wang, M.Y. Ischemic optic neuropathy. In Ophtalmology, 5th ed.; Ianoff, M., Duker, J.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 9.8; pp. 892–897.
- Biousse, V.; Newman, N.J. Ischemic Optic Neuropathies. N. Engl. J. Med. 2015, 372, 2428–2436.
- Hayreh, S.S. Ischemic optic neuropathies-where are we now? Graefes Arch. Clin. Exp. Oplnhalmol. 2013, 251, 1873–1884.
- Hayreh, S.S. Ischaemic optic neuropathy. Indian J. Ophthalmol. 2000, 48, 171–194.
- Collignon-Robe, N.J.; Feke, G.T.; Rizzo, J.F. Optic nerve head circulation in nonarteritic anterior ischemic optic neuropathy and optic neuritis. Ophthalmology 2004, 111, 1663–1672.
- Duker, J.S.; Duker, J.S. Retinal arterial obstruction. In Ophtalmology, 5th ed.; Yanoff, M., Duker, J.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 6.9; pp. 520–527.
- Ahuja, R.M.; Chaturvedi, S.; Elliot, D.; Joshi, N.; Puklin, J.E.; Abrams, G.W. Mechanism of retinal arterial occlusive disease in African, American and Caucasian patients. Stroke 1999, 30, 1506–1509.
- Connolly, B.P.; Krishnan, A.; Shah, G.K.; Whelan, J.; Brown, G.C.; Eagle Jr, R.C.; Shakin, E.P. Characteristics of patients presenting with central retinal artery occlusion with and without giant cell arteritis. Can. J. Ophthalmol. 2000, 35, 379–384.




