
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Juan Carlos Roa | + 3644 word(s) | 3644 | 2021-09-26 04:14:07 | | | |
| 2 | Conner Chen | Meta information modification | 3644 | 2022-01-20 03:38:04 | | |
Video Upload Options
Gallbladder cancer has a mortality of 85,000 deaths worldwide and an incidence rate of 0.9 cases per 100,000 individuals. The incidence of GBC varies among the different geographical areas of the world, showing a higher incidence among descendants of North and South American natives, and in several Asian countries. The highest GBC incidence rate is found in Chile between individuals who descend from the Mapuche people, with 12.3 cases per 100,000 in men and 27.3 cases per 100,000 in women.
Unfortunately, as symptomatology is unspecific and routine biochemical assays are not accurate, GBC is usually diagnosed late, sometimes as an accidental finding in patients with cholelithiasis. Due to this late diagnosis, GBC is generally found in an advanced stage, which causes that these patients have a poor prognosis and short life expectancy. For instance, the 5-year survival rate of GBC in advanced stages (T3 and T4 stages) is less than 5%, while if this cancer were detected in the initial stages (T1 stage) there would be an increase up to 75% in this 5-year survival rate.
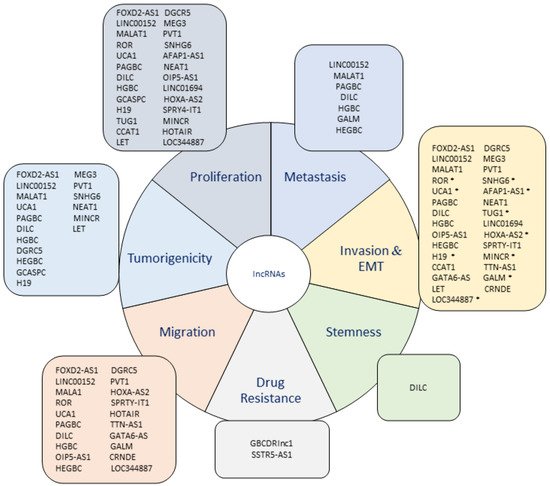
The research about lncRNAs and their participation in the acquisition of a malignant tumor phenotype has evidenced a dramatic increase because a large number of them have been demonstrated to actively participate in several mechanisms that contribute to the progression of cancer. Regarding this, the development of metastatic and tumorigenic characteristics is closely related to a more aggressive phenotype in cancer because these features provide cancer cells the capacity of expanding to other tissues and form new tumors, indicating a worse prognosis in cancer patients.
1. Upregulated lncRNAs in GBC
2. Downregulated lncRNAs in GBC
References
- Brannan, C.I.; Dees, E.C.; Ingram, R.S.; Tilghman, S.M. The product of the H19 gene may function as an RNA. Mol. Cell Biol. 1990, 10, 28–36.
- Isin, M.; Dalay, N. LncRNAs and neoplasia. Clin. Chim. Acta 2015, 444, 280–288.
- Ma, F.; Wang, S.H.; Cai, Q.; Zhang, M.D.; Yang, Y.; Ding, J. Overexpression of LncRNA AFAP1-AS1 predicts poor prognosis and promotes cells proliferation and invasion in gallbladder cancer. Biomed. Pharm. 2016, 84, 1249–1255.
- Liu, B.; Shen, E.D.; Liao, M.M.; Hu, Y.B.; Wu, K.; Yang, P.; Zhou, L.; Chen, W.D. Expression and mechanisms of long non-coding RNA genes MEG3 and ANRIL in gallbladder cancer. Tumor Biol. 2016, 37, 9875–9886.
- Liu, Z.; Chen, Q.; Hann, S.S. The functions and oncogenic roles of CCAT1 in human cancer. Biomed. Pharm. 2019, 115, 108943.
- Ma, M.Z.; Chu, B.F.; Zhang, Y.; Weng, M.Z.; Qin, Y.Y.; Gong, W.; Quan, Z.W. Long non-coding RNA CCAT1 promotes gallbladder cancer development via negative modulation of miRNA-218-5p. Cell Death Dis. 2015, 6, e1583.
- Ma, Y.C.; Yang, J.Y.; Yan, L.N. Relevant markers of cancer stem cells indicate a poor prognosis in hepatocellular carcinoma patients: A meta-analysis. Eur. J. Gastroenterol. Hepatol. 2013, 25, 1007–1016.
- Joseph, C.; Arshad, M.; Kurozomi, S.; Althobiti, M.; Miligy, I.M.; Al-Izzi, S.; Toss, M.S.; Goh, F.Q.; Johnston, S.J.; Martin, S.G.; et al. Overexpression of the cancer stem cell marker CD133 confers a poor prognosis in invasive breast cancer. Breast Cancer Res. Treat. 2019, 174, 387–399.
- Han, Y.; Xue, X.; Jiang, M.; Guo, X.; Li, P.; Liu, F.; Yuan, B.; Shen, Y.; Zhi, Q.; Zhao, H. LGR5, a relevant marker of cancer stem cells, indicates a poor prognosis in colorectal cancer patients: A meta-analysis. Clin. Res. Hepatol. Gastroenterol. 2015, 39, 267–273.
- Liu, B.; Zhang, Y.; Liao, M.; Deng, Z.; Gong, L.; Jiang, J.; Lynn, L.; Wu, K.; Miao, X. Clinicopathologic and prognostic significance of CD24 in gallbladder carcinoma. Pathol. Oncol. Res. 2011, 17, 45–50.
- Nguyen, L.V.; Vanner, R.; Dirks, P.; Eaves, C.J. Cancer stem cells: An evolving concept. Nat. Rev. Cancer 2012, 12, 133–143.
- Liang, C.; Yang, P.; Han, T.; Wang, R.Y.; Xing, X.L.; Si, A.F.; Ma, Q.Y.; Chen, Z.; Li, H.Y.; Zhang, B. Long non-coding RNA DILC promotes the progression of gallbladder carcinoma. Gene 2019, 694, 102–110.
- Xue, C.; Chen, C.; Gu, X.; Li, L. Progress and assessment of lncRNA DGCR5 in malignant phenotype and immune infiltration of human cancers. Am. J. Cancer Res. 2021, 11, 1–13.
- Wang, R.; Dong, H.X.; Zeng, J.; Pan, J.; Jin, X.Y. LncRNA DGCR5 contributes to CSC-like properties via modulating miR-330-5p/CD44 in NSCLC. J. Cell Physiol. 2018, 233, 7447–7456.
- Dong, H.X.; Wang, R.; Jin, X.Y.; Zeng, J.; Pan, J. LncRNA DGCR5 promotes lung adenocarcinoma (LUAD) progression via inhibiting hsa-mir-22-3p. J. Cell Physiol. 2018, 233, 4126–4136.
- Wang, X.L.; Shi, M.; Xiang, T.; Bu, Y.Z. Long noncoding RNA DGCR5 represses hepatocellular carcinoma progression by inactivating Wnt signaling pathway. J. Cell Biochem. 2019, 120, 275–282.
- Jiang, D.; Wang, C.; He, J. Long non-coding RNA DGCR5 incudes tumorigenesis of triple-negative breast cancer by affecting Wnt/β-catenin signaling pathway. J. BUON 2020, 25, 702–708.
- Liu, S.; Chu, B.; Cai, C.; Wu, X.; Yao, W.; Wu, Z.; Yang, Z.; Li, F.; Liu, Y.; Dong, P.; et al. DGCR5 Promotes Gallbladder Cancer by Sponging MiR-3619-5p via MEK/ERK1/2 and JNK/p38 MAPK Pathways. J. Cancer 2020, 11, 5466–5477.
- Gao, J.; Dai, C.; Yu, C.; Yin, X.B.; Liao, W.-y.; Huang, Y.; Zhou, F. Silencing of long non-coding RNA FOXD2-AS1 inhibits the progression of gallbladder cancer by mediating methylation of MLH1. Gene Ther. 2020, 28, 306–318.
- Cai, Q.; Wang, S.; Jin, L.; Weng, M.; Zhou, D.; Wang, J.; Tang, Z.; Quan, Z. Long non-coding RNA GBCDRlnc1 induces chemoresistance of gallbladder cancer cells by activating autophagy. Mol. Cancer 2019, 18, 82.
- Ma, M.Z.; Li, C.X.; Zhang, Y.; Weng, M.Z.; Zhang, M.D.; Qin, Y.Y.; Gong, W.; Quan, Z.W. Long non-coding RNA HOTAIR, a c-Myc activated driver of malignancy, negatively regulates miRNA-130a in gallbladder cancer. Mol. Cancer 2014, 13, 156.
- Ghafouri-Fard, S.; Esmaeili, M.; Taheri, M. H19 lncRNA: Roles in tumorigenesis. Biomed. Pharm. 2020, 123, 109774.
- Wang, S.H.; Wu, X.C.; Zhang, M.D.; Weng, M.Z.; Zhou, D.; Quan, Z.W. Long noncoding RNA H19 contributes to gallbladder cancer cell proliferation by modulated miR-194-5p targeting AKT2. Tumor Biol. 2016, 37, 9721–9730.
- Wang, S.H.; Ma, F.; Tang, Z.H.; Wu, X.C.; Cai, Q.; Zhang, M.D.; Weng, M.Z.; Zhou, D.; Wang, J.D.; Quan, Z.W. Long non-coding RNA H19 regulates FOXM1 expression by competitively binding endogenous miR-342-3p in gallbladder cancer. J. Exp. Clin. Cancer Res. 2016, 35, 160.
- Wang, S.H.; Wu, X.C.; Zhang, M.D.; Weng, M.Z.; Zhou, D.; Quan, Z.W. Upregulation of H19 indicates a poor prognosis in gallbladder carcinoma and promotes epithelial-mesenchymal transition. Am. J. Cancer Res. 2016, 6, 15–26.
- Liu, L.; Yan, Y.; Zhang, G.; Chen, C.; Shen, W.; Xing, P. Knockdown of LINC01694 inhibits growth of gallbladder cancer cells via miR-340-5p/Sox4. Biosci. Rep. 2020, 40.
- Wu, X.C.; Wang, S.H.; Ou, H.H.; Zhu, B.; Zhu, Y.; Zhang, Q.; Yang, Y.; Li, H. The NmrA-like family domain containing 1 pseudogene Loc344887 is amplified in gallbladder cancer and promotes epithelial-mesenchymal transition. Chem. Biol. Drug Des. 2017, 90, 456–463.
- Seo, D.; Kim, D.; Kim, W. Long non-coding RNA linc00152 acting as a promising oncogene in cancer progression. Genom. Inf. 2019, 17, e36.
- Zhong, Y.; Wu, X.; Li, Q.; Ge, X.; Wang, F.; Wu, P.; Deng, X.; Miao, L. Long noncoding RNAs as potential biomarkers and therapeutic targets in gallbladder cancer: A systematic review and meta-analysis. Cancer Cell Int. 2019, 19, 169.
- Cai, Q.; Wang, Z.Q.; Wang, S.H.; Li, C.; Zhu, Z.G.; Quan, Z.W.; Zhang, W.J. Upregulation of long non-coding RNA LINC00152 by SP1 contributes to gallbladder cancer cell growth and tumor metastasis via PI3K/AKT pathway. Am. J. Transl. Res. 2016, 8, 4068–4081.
- Cai, Q.; Wang, Z.; Wang, S.; Weng, M.; Zhou, D.; Li, C.; Wang, J.; Chen, E.; Quan, Z. Long non-coding RNA LINC00152 promotes gallbladder cancer metastasis and epithelial-mesenchymal transition by regulating HIF-1α via miR-138. Open Biol. 2017, 7.
- Li, Z.X.; Zhu, Q.N.; Zhang, H.B.; Hu, Y.; Wang, G.; Zhu, Y.S. MALAT1: A potential biomarker in cancer. Cancer Manag. Res. 2018, 10, 6757–6768.
- Wu, X.S.; Wang, X.A.; Wu, W.G.; Hu, Y.P.; Li, M.L.; Ding, Q.; Weng, H.; Shu, Y.J.; Liu, T.Y.; Jiang, L.; et al. MALAT1 promotes the proliferation and metastasis of gallbladder cancer cells by activating the ERK/MAPK pathway. Cancer Biol. Ther. 2014, 15, 806–814.
- Sun, Y.; Ma, L. New Insights into Long Non-Coding RNA MALAT1 in Cancer and Metastasis. Cancers 2019, 11, 216.
- Huo, Y.; Li, Q.; Wang, X.; Jiao, X.; Zheng, J.; Li, Z.; Pan, X. MALAT1 predicts poor survival in osteosarcoma patients and promotes cell metastasis through associating with EZH2. Oncotarget 2017, 8, 46993–47006.
- Chen, Q.; Su, Y.; He, X.; Zhao, W.; Wu, C.; Zhang, W.; Si, X.; Dong, B.; Zhao, L.; Gao, Y.; et al. Plasma long non-coding RNA MALAT1 is associated with distant metastasis in patients with epithelial ovarian cancer. Oncol. Lett. 2016, 12, 1361–1366.
- Luan, C.; Li, Y.; Liu, Z.; Zhao, C. Long Noncoding RNA MALAT1 Promotes the Development of Colon Cancer by Regulating. OncoTargets Ther. 2020, 13, 3653–3665.
- Jen, J.; Tang, Y.A.; Lu, Y.H.; Lin, C.C.; Lai, W.W.; Wang, Y.C. Oct4 transcriptionally regulates the expression of long non-coding RNAs NEAT1 and MALAT1 to promote lung cancer progression. Mol. Cancer 2017, 16, 104.
- Schmidt, L.H.; Spieker, T.; Koschmieder, S.; Schäffers, S.; Humberg, J.; Jungen, D.; Bulk, E.; Hascher, A.; Wittmer, D.; Marra, A.; et al. The long noncoding MALAT-1 RNA indicates a poor prognosis in non-small cell lung cancer and induces migration and tumor growth. J. Thorac. Oncol. 2011, 6, 1984–1992.
- Jadaliha, M.; Zong, X.; Malakar, P.; Ray, T.; Singh, D.K.; Freier, S.M.; Jensen, T.; Prasanth, S.G.; Karni, R.; Ray, P.S.; et al. Functional and prognostic significance of long non-coding RNA MALAT1 as a metastasis driver in ER negative lymph node negative breast cancer. Oncotarget 2016, 7, 40418–40436.
- Huang, N.S.; Chi, Y.Y.; Xue, J.Y.; Liu, M.Y.; Huang, S.; Mo, M.; Zhou, S.L.; Wu, J. Long non-coding RNA metastasis associated in lung adenocarcinoma transcript 1 (MALAT1) interacts with estrogen receptor and predicted poor survival in breast cancer. Oncotarget 2016, 7, 37957–37965.
- Lin, N.; Yao, Z.; Xu, M.; Chen, J.; Lu, Y.; Yuan, L.; Zhou, S.; Zou, X.; Xu, R. Long noncoding RNA MALAT1 potentiates growth and inhibits senescence by antagonizing ABI3BP in gallbladder cancer cells. J. Exp. Clin. Cancer Res. 2019, 38, 244.
- Wang, S.H.; Zhang, W.J.; Wu, X.C.; Zhang, M.D.; Weng, M.Z.; Zhou, D.; Wang, J.D.; Quan, Z.W. Long non-coding RNA Malat1 promotes gallbladder cancer development by acting as a molecular sponge to regulate miR-206. Oncotarget 2016, 7, 37857–37867.
- Zhang, T.; Chen, L.; Xu, X.; Shen, C. Knockdown of Long Noncoding RNA. Cancer Biother. Radiopharm. 2020.
- Wang, S.H.; Zhang, W.J.; Wu, X.C.; Weng, M.Z.; Zhang, M.D.; Cai, Q.; Zhou, D.; Wang, J.D.; Quan, Z.W. The lncRNA MALAT1 functions as a competing endogenous RNA to regulate MCL-1 expression by sponging miR-363-3p in gallbladder cancer. J. Cell Mol. Med. 2016, 20, 2299–2308.
- Wang, S.H.; Yang, Y.; Wu, X.C.; Zhang, M.D.; Weng, M.Z.; Zhou, D.; Wang, J.D.; Quan, Z.W. Long non-coding RNA MINCR promotes gallbladder cancer progression through stimulating EZH2 expression. Cancer Lett. 2016, 380, 122–133.
- Yang, F.; Tang, Z.; Duan, A.; Yi, B.; Shen, N.; Bo, Z.; Yin, L.; Zhu, B.; Qiu, Y.; Li, J. Long Noncoding RNA. OncoTargets Ther. 2020, 13, 2357–2367.
- Wang, S.H.; Zhang, M.D.; Wu, X.C.; Weng, M.Z.; Zhou, D.; Quan, Z.W. Overexpression of LncRNA-ROR predicts a poor outcome in gallbladder cancer patients and promotes the tumor cells proliferation, migration, and invasion. Tumor Biol. 2016, 37, 12867–12875.
- Yang, L.; Cheng, X.; Ge, N.; Guo, W.; Feng, F.; Wan, F. Long non-coding RNA SPRY4-IT1 promotes gallbladder carcinoma progression. Oncotarget 2017, 8, 3104–3110.
- Liu, X.F.; Wang, K.; Du, H.C. LncRNA SNHG6 regulating Hedgehog signaling pathway and affecting the biological function of gallbladder carcinoma cells through targeting miR-26b-5p. Eur. Rev. Med. Pharm. Sci. 2020, 24, 7598–7611.
- Xue, Z.; Yang, B.; Xu, Q.; Zhu, X.; Qin, G. Long non-coding RNA SSTR5-AS1 facilitates gemcitabine resistance via stabilizing NONO in gallbladder carcinoma. Biochem. Biophys. Res. Commun. 2020, 522, 952–959.
- Ma, F.; Wang, S.H.; Cai, Q.; Jin, L.Y.; Zhou, D.; Ding, J.; Quan, Z.W. Long non-coding RNA TUG1 promotes cell proliferation and metastasis by negatively regulating miR-300 in gallbladder carcinoma. Biomed. Pharm. 2017, 88, 863–869.
- Cai, Q.; Jin, L.; Wang, S.; Zhou, D.; Wang, J.; Tang, Z.; Quan, Z. Long non-coding RNA UCA1 promotes gallbladder cancer progression by epigenetically repressing p21 and E-cadherin expression. Oncotarget 2017, 8, 47957–47968.
- Lin, Z.; Li, Y.; Shao, R.; Hu, Y.; Gao, H. LncRNA TTN-AS1 acts as a tumor promoter in gallbladder carcinoma by regulating miR-107/HMGA1 axis. World J. Surg. Oncol. 2021, 19, 163.
- Zhang, L.; Geng, Z.; Meng, X.; Meng, F.; Wang, L. Screening for key lncRNAs in the progression of gallbladder cancer using bioinformatics analyses. Mol. Med. Rep. 2018, 17, 6449–6455.
- Shen, S.; Liu, H.; Wang, Y.; Wang, J.; Ni, X.; Ai, Z.; Pan, H.; Shao, Y. Long non-coding RNA CRNDE promotes gallbladder carcinoma carcinogenesis and as a scaffold of DMBT1 and C-IAP1 complexes to activating PI3K-AKT pathway. Oncotarget 2016, 7, 72833–72844.
- Ma, M.Z.; Kong, X.; Weng, M.Z.; Zhang, M.D.; Qin, Y.Y.; Gong, W.; Zhang, W.J.; Quan, Z.W. Long non-coding RNA-LET is a positive prognostic factor and exhibits tumor-suppressive activity in gallbladder cancer. Mol. Carcinog. 2015, 54, 1397–1406.
- Li, K.; Tang, J.; Hou, Y. LncRNA GATA6-AS inhibits cancer cell migration and invasion in gallbladder cancer by downregulating miR-421. OncoTargets Ther. 2019, 12, 8047–8053.
- Bao, D.; Yuan, R.X.; Zhang, Y. Effects of lncRNA MEG3 on proliferation and apoptosis of gallbladder cancer cells through regulating NF-κB signaling pathway. Eur. Rev. Med. Pharm. Sci. 2020, 24, 6632–6638.
- Jin, L.; Cai, Q.; Wang, S.; Mondal, T.; Wang, J.; Quan, Z. Long noncoding RNA MEG3 regulates LATS2 by promoting the ubiquitination of EZH2 and inhibits proliferation and invasion in gallbladder cancer. Cell Death Dis. 2018, 9, 1017.
- Ma, M.Z.; Zhang, Y.; Weng, M.Z.; Wang, S.H.; Hu, Y.; Hou, Z.Y.; Qin, Y.Y.; Gong, W.; Zhang, Y.J.; Kong, X.; et al. Long Noncoding RNA GCASPC, a Target of miR-17-3p, Negatively Regulates Pyruvate Carboxylase-Dependent Cell Proliferation in Gallbladder Cancer. Cancer Res. 2016, 76, 5361–5371.
- Tian, J.; Hu, X.; Gao, W.; Zhang, J.; Chen, M.; Zhang, X.; Ma, J.; Yuan, H. Identification of the long non-coding RNA LET as a novel tumor suppressor in gastric cancer. Mol. Med. Rep. 2017, 15, 2229–2234.
- Gu, L.; Zhang, J.; Shi, M.; Zhan, Q.; Shen, B.; Peng, C. lncRNA MEG3 had anti-cancer effects to suppress pancreatic cancer activity. Biomed. Pharm. 2017, 89, 1269–1276.




