
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Daniela B Vera | + 3087 word(s) | 3087 | 2021-12-29 04:57:53 | | | |
| 2 | Amina Yu | + 1 word(s) | 3088 | 2022-01-20 02:35:50 | | | | |
| 3 | Amina Yu | + 1 word(s) | 3088 | 2022-01-20 02:39:27 | | |
Video Upload Options
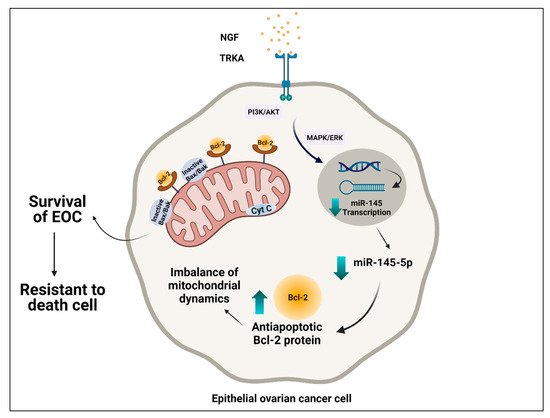
Ovarian cancer is the most lethal gynecological neoplasm, and epithelial ovarian cancer (EOC) accounts for 90% of ovarian malignancies. Nerve growth factor (NGF) and tropomyosin kinase A (TRKA), its high-affinity receptor, play a crucial role in pathogenesis through cell proliferation, angiogenesis, invasion, and migration. NGF/TRKA increase their expression during the progression of EOC by upregulation of oncogenic proteins as vascular endothelial growth factor (VEGF) and c-Myc. Otherwise, the expression of most oncoproteins is regulated by microRNAs (miRs). Our laboratory group reported that the tumoral effect of NGF/TRKA depends on the regulation of miR-145 levels in EOC. Currently, mitochondria have been proposed as new therapeutic targets to activate the apoptotic pathway in the cancer cell.
1. Introduction
Epithelial ovarian cancer (EOC), representing almost 90% of ovarian malignancies[1][2]. EOC comprises several subtypes, such as serous, endometrioid, mucinous, clear cell, and mixed. OC has been diagnosed in advanced stages due to a lack of specific symptoms and biomarkers that make it difficult for the early detection of the disease [3]. Five-year relative survival in patients with OC is below 45%, and the proportion of women who die from this disease has not improved substantially over time, unlike other common cancer types [4]. Standard treatment consists of cytoreductive surgery and, after, chemotherapy based on platinum and taxol compounds. Punctually, chemotherapy considers cycles of intravenous carboplatin plus paclitaxel [5][6]. In general, patients who receive therapy in early stages of OC tolerate the treatment well and go into remission, but cancer relapse is frequent[4][5] . Consequently, there is a need for new therapeutic approaches to prevent recurrence and avoid death by OC.
Among the different histological types of OC, the most common is epithelial ovarian cancer (EOC), representing almost 90% of ovarian malignancies [1][2]. EOC comprises several subtypes, such as serous, endometrioid, mucinous, clear cell, and mixed. High-grade serous ovarian cancer (HGSOC) is the most frequent histological subtype[2][6]. At the same time, cytoreductive surgery and chemotherapy are first-line therapies [8]. Eventually, the patients experience chemoresistance, which represents a problem and decreases the chances of eradicating cancer cells [7][8]. Unfortunately, current therapies are not as effective as expected because 70–80% of patients with OC suffer a relapse within the first 2 years [9]. Hence, it is essential to understand the physiopathology of this disease to develop new therapeutic strategies or enhance the existing ones.
Cancer cells amass metabolic alterations that allow them to access unusual nutrient sources to sustain accelerated proliferation. More recent studies revealed that mitochondria play a crucial role in carcinogenesis by modulating cell proliferation and resistance to apoptosis in cancer cells [9][10]. Evidence showed that mitochondrial activity still participates in tumor energy production[11][12] . Hence, cancer cells adapt their metabolism to acquire energy from the nutrient-poor environment to survive and proliferate. Accordingly, these adaptations in the tumor cells favor them to survive and proliferate, a phenomenon known as metabolic reprogramming [10][11][12] and considered one of the hallmarks in cancer cells [13].
2. Role of Nerve Growth Factor and microRNAs in EOC
2.1. Role of Nerve Growth Factor in Epithelial Ovarian Cancer
2.2. Role of microRNAs in EOC
3. Mitochondria in Epithelial Ovarian Cancer: The Importance of Oxidative Phosphorylation, Chemoresistance, and miR-145 on NGF Regulation
3.1. Importance of Oxidative Phosphorylation in Epithelial Ovarian Cancer
3.2. Role of Mitochondria in Chemoresistance
3.3. Interplay between NGF/TRKA and miR-145 Levels: Possible Implications for Mitochondria
References
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef]
- Lheureux, S.; Braunstein, M.; Oza, A.M. Epithelial ovarian cancer: Evolution of management in the era of precision medicine. CA Cancer J. Clin. 2019, 69, 280–304. [Google Scholar] [CrossRef]
- Arend, R.; Martinez, A.; Szul, T.; Birrer, M.J. Biomarkers in ovarian cancer: To be or not to be. Cancer 2019, 125, 4563–4572. [Google Scholar] [CrossRef] [PubMed]
- Reid, B.M.; Permuth, J.B.; Seller, T.A. Epidemiology of ovarian cancer: A review. Cancer Biol. Med. 2017, 14, 9–32. [Google Scholar] [CrossRef] [PubMed]
- Kuroki, L.; Guntupalli, S.R. Treatment of epithelial ovarian cancer. BMJ 2020, 371, m3773. [Google Scholar] [CrossRef]
- Kurnit, K.C.; Fleming, G.F.; Lengyel, E. Updates and new options in advanced epithelial ovarian cancer treatment. Obstet. Gynecol. 2021, 137, 108–121. [Google Scholar] [CrossRef]
- Lee, J.; Minasian, L.; Kohn, E.C. New strategies in ovarian cancer treatment. Cancer 2019, 125, 4623–4629. [Google Scholar] [CrossRef]
- Lengyel, E. Ovarian cancer development and metastasis. Am. J. Pathol. 2010, 177, 1053–1064. [Google Scholar] [CrossRef]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef] [PubMed]
- Avolio, R.; Matassa, D.S.; Criscuolo, D.; Landriscina, M.; Esposito, F. Modulation of mitochondrial metabolic reprogramming and oxidative stress to overcome chemoresistance in cancer. Biomolecules 2020, 10, 135. [Google Scholar] [CrossRef]
- Pillai, S.R.; Damaghi, M.; Marunaka, Y.; Spugnini, E.P.; Fais, S.; Gillies, R.J. Causes, consequences, and therapy of tumors acidosis. Cancer Metastasis Rev. 2019, 38, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative stress. Ann. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, N.N.; Thompson, C.B. The emerging hallmarks of cancer metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef]
- Skaper, S.D. The biology of neurotrophins, signalling pathways, and functional peptide mimetics of neurotrophins and their receptors. Drug Targets 2008, 7, 46–62.
- Dissen, G.A.; Romero, C.; Hirshfield, A.N.; Ojeda, S.R. Nerve growth factor is required for early follicular development in the mammalian ovary. Endocrinology 2001, 142, 9.
- Kawamura, K.; Kawamura, N.; Mulders, S.M.; Sollewijn Gelpke, M.D.; Hsueh, A.J.W. Ovarian brain-derived neurotrophic factor (BDNF) promotes the development of oocytes into preimplantation embryos. Proc. Natl. Acad. Sci. USA 2005, 102, 9206–9211.
- Anderson, R.A. Brainwork in the ovary: Kisspeptin and BDNF signaling converge to ensure oocyte survival. Endocrinology 2014, 155, 2751–2753.
- Streiter, S.; Fisch, B.; Sabbah, B.; Ao, A.; Abir, R. The importance of neuronal growth factors in the ovary. Mol. Hum. Reprod. 2016, 22, 3–17.
- Vera, C.; Tapia, V.; Vega, M.; Romero, C. Role of nerve growth factor and its TRKA receptor in normal ovarian and epithelial ovarian cancer angiogenesis. J. Ovarian Res. 2014, 7, 8.
- Tapia, V.; Gabler, F.; Muñoz, M.; Yazigi, R.; Paredes, A.; Selman, A.; Vega, M.; Romero, C. Tyrosine kinase A receptor (TrkA): A potential marker in epithelial ovarian cancer. Gynecol. Oncol. 2011, 121, 13–23.
- Julio-Pieper, M.; Lara, H.E.; Bravo, J.A.; Romero, C. Effects of nerve growth factor (NGF) on blood vessels area and expression of the angiogenic factors VEGF and TGFbeta I in the rat ovary. Reprod. Biol. Endocrinol. 2006, 4, 57.
- Campos, X.; Muñoz, Y.; Selman, A.; Yazigi, R.; Moyano, L.; Weinstein-Oppenheimer, C.; Lara, H.E.; Romero, C. Nerve growth factor and its high-affinity receptor TrkA participate in the control of vascular endothelial growth factor expression in epithelial ovarian cancer. Gynecol. Oncol. 2007, 104, 168–175.
- Garrido, M.P.; Torres, I.; Vega, M.; Romero, C. Angiogenesis in gynecological cancers: Role of neurotrophins. Front. Oncol. 2019, 9, 913.
- Garrido, M.P.; Hurtado, I.; Valenzuela-Valderrama, M.; Salvatierra, R.; Hernández, A.; Vega, M.; Selman, A.; Quest, A.F.G.; Romero, C. NGF-enhanced vasculogenic properties of epithelial ovarian cancer cells are reduced by inhibition of the COX-2/PGE2 signaling axis. Cancers 2019, 11, 1970.
- Reichard, A.; Asosingh, K. The role of mitochondria in angiogenesis. Mol. Biol. Rep. 2019, 46, 1393–1400.
- Lim, D.; Do, Y.; Kwon, B.S.; Chang, W.; Lee, M.-S.; Kim, J.; Cho, J.G. Angiogenesis and vasculogenic mimicry as therapeutic targets in ovarian cancer. BMB Rep. 2020, 53, 291–298.
- Urzúa, U.; Tapia, V.; Geraldo, M.; Selman, A.; Vega, M.; Romero, C. Nerve growth factor stimulates cellular proliferation of human epithelial ovarian cancer. Horm. Metab. Res. 2012, 44, 656–661.
- Xintaropoulou, C.; Ward, C.; Wise, A.; Queckborner, S.; Turnbull, A.; Michie, C.O.; Williams, A.R.W.; Rye, T.; Gourley, C.; Langdon, S.P. Expression of glycolytic enzymes in ovarian cancers and evaluation of the glycolytic pathway as a strategy for ovarian cancer treatment. BMC Cancer 2018, 18, 636.
- Martinou, I.; Desagher, S.; Eskes, R.; Antonsson, B.; André, E.; Fakan, S.; Martinou, J.-C. The release of cytochrome c from mitochondria during apoptosis of NGF-deprived sympathetic neurons is a reversible event. J. Cell Biol. 1999, 144, 883–889.
- Siu, M.K.; Wong, O.G.; Cheung, A.N. TrkB as a therapeutic target for ovarian cancer. Expert Opin. Ther. Targets 2009, 13, 1169–1178.
- Kang, M.; Chong, K.Y.; Hartwich, T.M.P.; Bi, F.; Witham, A.K.; Patrick, D.; Morrisson, M.J.; Cady, S.L.; Cerchia, A.P.; Kelk, D.; et al. Ovarian BDNF promotes survival, migration, and attachment of tumor precursors originated from P53 mutant fallopian tube epithelial cells. Oncogenesis 2020, 9, 55.
- Matsuda, S.; Fujita, T.; Kajiya, M.; Takeda, K.; Shiba, H.; Kawaguchi, H.; Kurihara, H. Brain-derived neurotrophic factor induces migration of endothelial cells through a TrkB-ERK-integrin AVβ3-FAK cascade. J. Cell. Physiol. 2012, 227, 2123–2129.
- Li, B.; Cai, S.; Zhao, Y.; He, Q.; Yu, X.; Cheng, L.; Zhang, Y.; Hu, X.; Ke, M.; Chen, S.; et al. Nerve growth factor modulates the tumor cells migration in ovarian cancer through the WNT/β-catenin pathway. Oncotarget 2016, 7, 81026–81048.
- Tsuyoshi, H.; Orisaka, M.; Fujita, Y.; Asare-Werehene, M.; Tsang, B.K.; Yoshida, Y. Prognostic impact of Dynamin related protein 1 (Drp1) in epithelial ovarian cancer. BMC Cancer 2020, 20, 467.
- Peng, Y.; Croce, C.M. The role of MicroRNAs in human cancer. Sig. Transduct. Target Ther. 2016, 1, 15004.
- Garrido, M.P.; Torres, I.; Avila, A.; Chnaiderman, J.; Valenzuela-Valderrama, M.; Aramburo, J.; Oróstica, L.; Durán-Jara, E.; Lobos-Gonzalez, L.; Romero, C. NGF/TRKA decrease MiR-145-5p levels in epithelial ovarian cancer cells. Int. J. Mol. Sci. 2020, 21, 7657.
- Deb, B.; Uddin, A.; Chakraborty, S. MiRNAs and ovarian cancer: An overview. J. Cell Physiol. 2018, 233, 3846–3854.
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297.
- Ha, M.; Kim, V.N. Regulation of MicroRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524.
- Matsuyama, H.; Suzuki, H.I. Systems and synthetic MicroRNA biology: From biogenesis to disease pathogenesis. Int. J. Mol. Sci. 2020, 21, 132.
- Vera, C.; Retamales-Ortega, R.; Garrido, M.; Vega, M.; Romero, C. Signaling pathways related to nerve growth factor and MiRNAs in epithelial ovarian cancer. In Ovarian Cancer—From Pathogenesis to Treatment; Devaja, O., Papadopoulos, A., Eds.; IntechOpen: London, UK, 2018.
- Zuberi, M.; Mir, R.; Das, J.; Ahmad, I.; Javid, J.; Yadav, P.; Masroor, M.; Ahmad, S.; Ray, P.C.; Saxena, A. Expression of serum MiR-200a, MiR-200b, and MiR-200c as candidate biomarkers in epithelial ovarian cancer and their association with clinicopathological features. Clin. Transl. Oncol. 2015, 17, 779–787.
- Wang, A.; Jin, C.; Li, H.; Qin, Q.; Li, L. LncRNA ADAMTS9-AS2 regulates ovarian cancer progression by targeting MiR-182-5p/FOXF2 signaling pathway. Int. J. Biol. Macromol. 2018, 120, 1705–1713.
- Báez-Vega, P.M.; Vargas, I.M.E.; Valiyeva, F.; Encarnación-Rosado, J.; Roman, A.; Flores, J.; Marcos-Martínez, M.J.; Vivas-Mejía, P.E. Targeting MiR-21-3p inhibits proliferation and invasion of ovarian cancer cells. Oncotarget 2016, 7, 36321–36337.
- Kleemann, M.; Schneider, H.; Unger, K.; Bereuther, J.; Fischer, S.; Sander, P.; Marion Schneider, E.; Fischer-Posovszky, P.; Riedel, C.U.; Handrick, R.; et al. Induction of apoptosis in ovarian cancer cells by MiR-493-3p directly targeting AKT2, STK38L, HMGA2, ETS1 and E2F5. Cell. Mol. Life Sci. 2019, 76, 539–559.
- Wu, H.; Xiao, Z.; Wang, K.; Liu, W.; Hao, Q. MiR-145 is downregulated in human ovarian cancer and modulates cell growth and invasion by targeting P70S6K1 and MUC1. Biochem. Biophys. Res. Commun. 2013, 441, 693–700.
- Yan, J.; Jiang, J.; Meng, X.-N.; Xiu, Y.-L.; Zong, Z.-H. MiR-23b targets cyclin G1 and suppresses ovarian cancer tumorigenesis and progression. J. Exp. Clin. Cancer Res. 2016, 35, 31.
- Zhu, X.; Li, Y.; Xie, C.; Yin, X.; Liu, Y.; Cao, Y.; Fang, Y.; Lin, X.; Xu, Y.; Xu, W.; et al. MiR-145 sensitizes ovarian cancer cells to paclitaxel by targeting Sp1 and Cdk6: MiR-145 sensitizes cancer cells to paclitaxel. Int. J. Cancer 2014, 135, 1286–1296.
- Xu, W.; Hua, Y.; Deng, F.; Wang, D.; Wu, Y.; Zhang, W.; Tang, J. MiR-145 in cancer therapy resistance and sensitivity: A comprehensive review. Cancer Sci. 2020, 111, 3122–3131.
- Sheng, Q.; Zhang, Y.; Wang, Z.; Ding, J.; Song, Y.; Zhao, W. Cisplatin-mediated down-regulation of MiR-145 contributes to up-regulation of PD-L1 via the C-Myc transcription factor in cisplatin-resistant ovarian carcinoma cells. Clin. Exp. Immunol. 2020, 200, 45–52.
- Chen, Q.; Hou, J.; Wu, Z.; Zhao, J.; Ma, D. MiR-145 Regulates the sensitivity of esophageal squamous cell carcinoma cells to 5-FU via targeting REV3L. Pathol. Res. Pract. 2019, 215, 152427.
- Pan, Y.; Ye, C.; Tian, Q.; Yan, S.; Zeng, X.; Xiao, C.; Wang, L.; Wang, H. MiR-145 suppresses the proliferation, invasion and migration of NSCLC cells by regulating the BAX/BCL-2 ratio and the caspase-3 cascade. Oncol. Lett. 2018, 15, 4337–4343.
- Lim, H.Y.; Ho, Q.S.; Low, J.; Choolani, M.; Wong, K.P. Respiratory competent mitochondria in human ovarian and peritoneal cancer. Mitochondrion 2011, 11, 437–443.
- Zampieri, L.X.; Grasso, D.; Bouzin, C.; Brusa, D.; Rossignol, R.; Sonveaux, P. Mitochondria participate in chemoresistance to cisplatin in human ovarian cancer cells. Mol. Cancer Res. 2020, 18, 1379–1391.
- Signorile, A.; De Rasmo, D.; Cormio, A.; Musicco, C.; Rossi, R.; Fortarezza, F.; Palese, L.; Loizzi, V.; Resta, L.; Scillitani, G.; et al. Human ovarian cancer tissue exhibits increase of mitochondrial biogenesis and cristae remodeling. Cancers 2019, 11, 1350.
- Bindra, S.; McGill, M.A.; Triplett, M.K.; Tyagi, A.; Thaker, P.H.; Dahmoush, L.; Goodheart, M.J.; Ogden, R.T.; Owusu-Ansah, E.; Karan, K.; et al. Ovarian tumor mitochondria exhibit abnormal phenotypes and blunted associations with biobehavioral factors. Sci. Rep. 2021, 11, 11595.
- Emmings, E.; Mullany, S.; Chang, Z.; Landen, C.N.; Linder, S.; Bazzaro, M. Targeting mitochondria for treatment of chemoresistant ovarian cancer. Int. J. Mol. Sci. 2019, 20, 229.
- Grieco, J.P.; Allen, M.E.; Perry, J.B.; Wang, Y.; Song, Y.; Rohani, A.; Compton, S.L.E.; Smyth, J.W.; Swami, N.S.; Brown, D.A.; et al. Progression-mediated changes in mitochondrial morphology promotes adaptation to hypoxic peritoneal conditions in serous ovarian cancer. Front. Oncol. 2021, 10, 600113.
- Kleih, M.; Böpple, K.; Dong, M.; Gaißler, A.; Heine, S.; Olayioye, M.A.; Aulitzky, W.E.; Essmann, F. Direct impact of cisplatin on mitochondria induces ROS production that dictates cell fate of ovarian cancer cells. Cell Death Dis. 2019, 10, 851.
- Wang, D.; You, D.; Li, L. Galectin-3 regulates chemotherapy sensitivity in epithelial ovarian carcinoma via regulating mitochondrial function. J. Toxicol. Sci. 2019, 44, 47–56.
- Dar, S.; Chhina, J.; Mert, I.; Chitale, D.; Buekers, T.; Kaur, H.; Giri, S.; Munkarah, A.; Rattan, R. Bioenergetic adaptations in chemoresistant ovarian cancer cells. Sci. Rep. 2017, 7, 8760.
- Harper, A.K.; Fletcher, N.M.; Fan, R.; Morris, R.T.; Saed, G.M. Heat shock protein 60 (HSP60) serves as a potential target for the sensitization of chemoresistant ovarian cancer cells. Reprod. Sci. 2020, 27, 1030–1036.
- Zhao, S.; Zhang, Y.; Pei, M.; Wu, L.; Li, J. MiR-145 inhibits mitochondrial function of ovarian cancer by targeting ARL5B. J. Ovarian Res. 2021, 14, 8.
- Vaux, D.L. Apoptogenic factors released from mitochondria. Biochim. Biophys. Acta 2011, 1813, 546–550.
- Jin, Z.; Gu, J.; Xin, X.; Li, Y.; Wang, H. Expression of hexokinase 2 in epithelial ovarian tumors and its clinical significance in serous ovarian cancer. Eur. J. Gynaecol. Oncol. 2014, 35, 519–524.
- Gross, A.; Katz, S.G. Non-apoptotic functions of BCL-2 family proteins. Cell Death Differ. 2017, 24, 1348–1358.
- Yu, Y.; Xu, L.; Qi, L.; Wang, C.; Xu, N.; Liu, S.; Li, S.; Tian, H.; Liu, W.; Xu, Y.; et al. ABT737 induces mitochondrial pathway apoptosis and mitophagy by regulating DRP1-dependent mitochondrial fission in human ovarian cancer cells. Biomed. Pharmacother. 2017, 96, 22–29.
- Han, Y.; Kim, B.; Cho, U.; Park, I.S.; Kim, S.I.; Dhanasekaran, D.N.; Tsang, B.K.; Song, Y.S. Mitochondrial fission causes cisplatin resistance under hypoxic conditions via ROS in ovarian cancer cells. Oncogene 2019, 38, 7089–7105.





