Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Antonino Bruno | + 2014 word(s) | 2014 | 2021-12-21 08:05:44 | | | |
| 2 | Lindsay Dong | Meta information modification | 2014 | 2022-01-20 04:26:51 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Bruno, A. Neutrophil and Natural Killer Cell Interactions in Cancers. Encyclopedia. Available online: https://encyclopedia.pub/entry/18490 (accessed on 16 January 2026).
Bruno A. Neutrophil and Natural Killer Cell Interactions in Cancers. Encyclopedia. Available at: https://encyclopedia.pub/entry/18490. Accessed January 16, 2026.
Bruno, Antonino. "Neutrophil and Natural Killer Cell Interactions in Cancers" Encyclopedia, https://encyclopedia.pub/entry/18490 (accessed January 16, 2026).
Bruno, A. (2022, January 19). Neutrophil and Natural Killer Cell Interactions in Cancers. In Encyclopedia. https://encyclopedia.pub/entry/18490
Bruno, Antonino. "Neutrophil and Natural Killer Cell Interactions in Cancers." Encyclopedia. Web. 19 January, 2022.
Copy Citation
Neutrophils are the most abundant circulating leukocytes, accounting for 50–70% of blood cells. Natural killer (NK) cells are large granular lymphocytes from innate immunity, participating in virus-infected and malignant-transformed cells recognition and elimination.
neutrophils
natural killer cells
neutrophil-NK cell crosstalk
tumor
1. A Dangerous Liaison in the Immunosuppressive TME
Several studies have demonstrated that TANs can directly or indirectly (via crosstalk with other immune cells) contribute to the generation of an immunosuppressive TME. Studies of neutrophil-induced immunosuppression have mostly focused on their ability to inhibit T cell functions [1][2]. Here, we focused on neutrophil-NK cell interactions as a critical step in the immunosuppressive TME.
In a colorectal cancer murine model, generated by CT-26 cells intramuscularly injected into the flanks of BALB/c mice, neutrophils have been shown to suppress the NK cell infiltration, by downregulating CCR1 and to impair anti-tumor capabilities (Figure 1A) by cell-to-cell interactions, through the PD-L1/PD-1 axis [3] (Figure 1B).
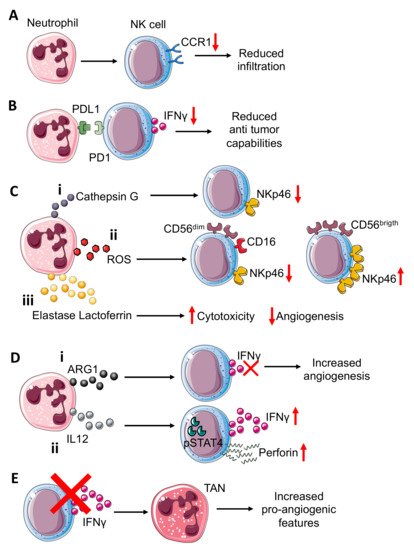
Figure 1. Neutrophil–NK cell crosstalk in tumor microenvironment. Neutrophils can (A) induce reduction in CCR1 expression on NK cells, impairing the NK cells’ infiltration capability. Interference with PDL1-PD1 (B) interactions in the TME, resulting in reduced NK cell capability to release IFNγ. Neutrophils can also modulate the expression of activating receptor NKp46 on NK cells through (C) the release of different molecules that include: (i) neutrophil-derived Cathepsin G (CG) reduces NKp46 on NK cells; similar; (ii) reactive oxygen species (ROS) can downmodulate NKp46 on cytotoxic CD56dimCD16+NK cells while they can upregulate this receptor on cytokine-producer CD56brigthCD16− NK cells; (iii) elastase and lactoferrin release exert a wide effect increasing cytotoxicity and reducing angiogenesis. NK-derived IFNγ, a key mediator in TME, can be inversely modulated by ARG1 and IL12 from neutrophils (D). (i) Indeed, neutrophil-derived ARG1 can abrogate IFNγ released from NK, improving NK pro-angiogenic features; (ii) while neutrophil-derived IL12 through STAT4 activation increases IFNγ and perforin production by NK cells. NK cell–neutrophil crosstalk (E) can be modulated by NK-derived IFNγ, which acts by decreasing pro-angiogenic features of tumor associated neutrophils (TANs). Indeed, NK cells or IFNγ depletion increase TANs’ pro-angiogenic features.
Neutrophils isolated from the CT-26 tumor-bearing mice, when co-cultured both with naïve and tumor-bearing NK cells, displayed a decreased production of IFNγ; treatment with PD-L1 neutralizing antibody was effective in limiting tumor-bearing neutrophil inhibitory effects on tumor-bearing NK cells, but did not exert the same inhibitory effect on naïve NK cells [3]. Additionally, administration of PD-1 neutralizing antibodies was able to antagonize the inhibitory effect of neutrophils on NK cells [3].
The NKp46 NCR is considered a key molecule for NK-related killing capacity; Valayer et al., showed that NKp46 decreasing on NK cells is related to activated neutrophil-derived serine proteases and in particular, cathepsin G (CG) is responsible for NKp46 extracellular cleavage [4] (Figure 1Ci), thus causing defective activation of NK cells in in vitro experiments using human-derived cells. The use of a specific CG inhibitor, α1ACT, abrogated the capability of neutrophil-derived conditioned media to decrease NKp46 on the NK cell surface [4].
Neutrophils can activate or suppress the NK cells cytotoxic functions and therefore can indirectly interfere with the angiogenic process. Romero et al., demonstrated that neutrophil-derived ROS can modulate NKp46 expression on NK targeted cells [5] (Figure 1Cii). Indeed, neutrophil-derived ROS downmodulated NKp46 in CD56dim NK cells [5] (Figure 1Cii), while the opposite effect was exerted in CD56bright NK cells, in which NKp46 was increased, probably due to high anti-oxidative intrinsic capability [5] (Figure 1Cii), and this modulation is revered by catalase [5]. Thus, neutrophil-derived ROS, by NKp46 modulation of NK cells, can enhance or reduce their cytotoxic effect against endothelial cells (ECs), as reported by Dondero and colleagues, that showed that NKp46 is involved in ECs killing in multiple myeloma (MM). Similar to ROS and CG, elastase and lactoferrin act on NK cells, increasing their cytotoxicity [6] (Figure 2Ciii). Arginase I (ARG1), released by TANs, also participate in supporting NK cell pro-angiogenic features, suppressing NK cell capability to produce anti-tumor factors such as IFNγ [7] (Figure 2Di).
IL12 is also produced by neutrophils and is crucial for optimal IFNγ and perforin production by both murine and human NK cells [8]. IL12 signaling, through STAT4 activation, induces IFNγ production in NK cells, as confirmed in a mouse model lacking Stat4, in which NK cells display lower IFNγ production and thus decreased cytolytic function [9] (Figure 1Dii). The same mechanism occurs in humans, as shown by using the human NKL cell line in which STAT4 activation by IL12 is directly related to perforin expression in in vitro experiments [10]. Moreover, IFNγ can exert its effect on TANs. As observed in C57BL/6 mice implanted with the MCA205 murine fibrosarcoma cell line, NK cell depletion and IFNγ deficiency allowed for an increased tumor growth compared to the control mice [11]. This effect is mediated by TANs, thus the absence of NK cells increased the pro-angiogenic features of TANs [11] (Figure 1E).
Neutrophil and NK cell interactions have been reported to be crucial in supporting the metastatic process. Spiegel et al., using a murine model (BALB/c mice) injected with 4T1 mammary carcinoma cells, showed that neutrophils can suppress intraluminal NK-mediated tumor cell elimination and enhance extravasation of disseminated carcinoma cells [12].
2. Contribution to Tumor Angiogenesis
Neutrophils represent one of the first infiltrating cell type within TME and can shape TME promoting tumor growth and metastasis formation by angiogenesis stimulation [13][14]. In a skin model of wound healing in CD18-deficient mice that lack neutrophils, it has been shown that neovascularization is compromised compared to wild-type animals [15], suggesting that neutrophil infiltration ameliorates/improves angiogenesis.
Angiogenesis stimulation can be mainly mediated by the release of soluble factors including the main pro-angiogenic master regulator VEGF [16][17]. Vessel-associated neutrophils are a key source of VEGF upon stimulation with CXCL1 [18] and G-CSF [19] are one of the main producers of MMP9, which contributes to angiogenic switch in Rip-tag pancreatic cancer in the in vivo mouse model, where neutrophil depletion at the early stage inhibits angiogenesis progression [20]. In addition, through gene expression analysis, Schruefer and colleagues identified novel pro-angiogenic factors produced by human neutrophils such as ephrin A2 and B2, thrombospondin, TGFβ receptor 2 and 3 (TGFβR2 and TGFβR3), tissue inhibitor of metalloproteinase 2, and restin [15]. Conversely, neutrophils can also produce anti-angiogenic [21] factors as they can release enzymatic activities that in vitro generate active angiostatin fragments that in turn inhibit basic fibroblast growth factor (bFGF/FGF2) and VEGF-induced EC proliferation [21], thus negatively interfering with the angiogenic process. On ECs, VEGF can increase ICAM-1 and VCAM-1 expression, which in turn, can respectively mediate neutrophil and NK cell recruitment by interaction with CD18 expressed by both neutrophils and NK and CD49d on the NK cell surface [17][22][23].
NK cells can play a dual role related to angiogenesis by acting as the inhibitor and promoter of this process. In response to IL12 [22], cytotoxic NK cells start producing IFNγ [23], which exerts an anti-angiogenic effect through IFNγ inducible protein-10 (IP-10) accumulation [24] (Figure 2A).
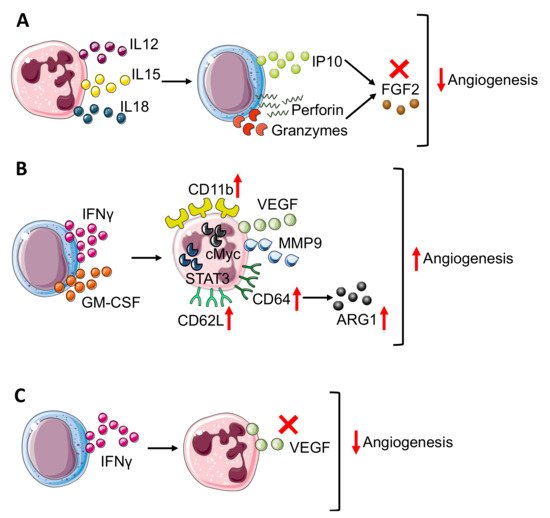
Figure 2. Neutrophil–NK cell crosstalk in angiogenesis modulation. The bidirectional crosstalk between neutrophils and NK cells can negatively modulate angiogenesis thus (A) neutrophil derived IL12, 15, and 18 stimulate IP10, perforin, and granzyme production in NK cells that block FGF2 effect. In TME, NK cells can improve angiogenesis (B) through the production of IFNγ and granulocyte-macrophages colony-stimulating factor (GM-CSF), which results in neutrophil expression of CD11b, CD62L, and CD64 surface antigens and release or VEGF and MMP9, in a STAT3 and c-Myc dependent manner. Moreover, in neutrophils, CD64 expression is linked to increasing production of ARG1. On the other hand, (C) NK-derived IFNγ prevents VEGF release from neutrophils, reducing angiogenesis stimulation. Finally, panels B and C show how IFNγ, produced by NK cells, act as a double edge sword by exerting both pro-angiogenic and anti-angiogenic activities.
Indeed, IL12 increased the production of cytolytic mediators as granzyme and perforin by NK cells and blocked the pro-angiogenic effect of FGF2 by IP-10 (CXCL10) (Figure 2A), causing tumor necrosis and vascular damage in experimental Burkitt lymphomas. Interestingly, IP-10/CXCL10, which sustains the NK cells’ anti-angiogenic activity, can also be produced by neutrophils upon IFNα stimulation [25], thus affecting both NK and neutrophil behavior.
The anti-angiogenic loop that involves IP-10 can be driven by both NK cells and neutrophils that together can contribute to angiogenesis inhibition. The anti-angiogenic effect of IFNγ can also be boosted by other neutrophil-derived cytokines as IL12, IL15, and IL18 [26][27] (Figure 2A) that, due to their pro-inflammatory function, activates NK cells and increases NK-derived IFNγ, thus sustaining angiogenesis inhibition [26][27] as also shown by an in vitro coculture experiment in which neutrophils enhanced NK production of IFNγ [28].
CXCL8 can work as a bridge-molecule between NK cells and neutrophils. CXCL8 can target neutrophils, increasing their recruitment, which in turn, sustains angiogenesis together with tumor progression in Ras oncogene driven tumors [29]. In addition, in vitro coculture of NK cells and neutrophils increased neutrophil CXCL8 production [28], corroborating the crosstalk between NK and neutrophils.
Activated NK cells increased CD11b expression on neutrophils and promoted CD62L (L-selectin) shedding through IFNγ and granulocyte-macrophage colony stimulating factor (GM-CSF) release [30] (Figure 3B). In a transplantable tumor mouse model obtained with subcutaneous injection of melanoma and fibrosarcoma cells, Jablonska and colleagues have shown that CD11b+ neutrophils are responsible for angiogenesis stimulation since expressed high levels of genes encoding for VEGF and MMP9, together with transcription factor c-myc and STAT3, which are positive regulators of both VEGF and MMP9 [30] (Figure 2B). In addition to CD11b, NK modulated neutrophil expression of CD62L and CD64. In detail, NK-derived IFNγ and GM-CSF promoted CD64 expression [31] (Figure 2B).
In a work published by Romano et al., CD64 expression on neutrophils correlated with immune-suppression and tumor progression [32]. Studying MM patients compared with healthy donors and MGUS (monoclonal gammopathy of undetermined significance, early stage of myeloma) subjects, Romano and colleagues showed that CD64 expression on neutrophils increased from healthy to MGUS and to MM patients together with p-STAT3 [32]. The concomitant increase in CD64 expression on neutrophils during myeloma progression could be linked with angiogenesis promotion, considering that MM-neutrophils displayed a N2-like phenotype with pro-angiogenic features. In addition, CD64-expressing neutrophils showed increased ARG1 expression together with p-STAT3 during myeloma progression. As mentioned before, ARG1 [7] could play a positive role for angiogenesis and, in addition, STAT3 could increase pro-angiogenic VEGF and MMP9 production [30] (Figure 2B), therefore CD64-expressing neutrophils can contribute to angiogenic switch in MM.
In contrast, in a sarcoma transplantable model, the effect of NK-derived IFNγ regulated neutrophil function, preventing/impeding their pro-angiogenic activity [33]. Ogura and colleagues showed that IFNγ and NK cells negatively controlled neutrophil-derived VEGF-A [11][34] (Figure 2C). Indeed, the supernatant from IFNγ-KO mice switched on VEGF-A mRNA expression in neutrophils [11][34]. This modulation involved TME as VEGF-A protein expression is enhanced in NK-depleted mice. Finally, by the Matrigel plug angiogenesis assay with the MCA205 murine fibrosarcoma cell line, Ogura further confirmed the link between NK and neutrophils in angiogenesis control since vascularization is enhanced in the absence of NK cells (obtained with anti-asialo GM1 antibody) and this process is neutrophil-dependent since in neutrophil depleted mice (using anti-Ly6G antibody), angiogenesis is reduced as in the control [11][34] mice [11].
3. Conclusions
Immune cell plasticity can be envisaged as a relevant host-dependent hallmark of cancers, characterized by tumor-infiltrating and tumor-associated immune cell capability to acquire tumor-supporting phenotypes and functions. In this scenario, it is now clear that not only the immune cell phenotype/functional switch (pro-tumor/pro-angiogenic/pro-metastatic) but also the dangerous liaisons occurring between different immune cells in the tumor micro- and macro-environments, represent crucial events impacting cancer progression and the success of the therapeutic regimens. Based on these concepts, immunotherapy has emerged as the new next-generation approach in cancer therapy, with different success in some cancer types (melanoma, lung cancer) and with minor or null success in other cancer types. The still persistent window of “non-successful” immunotherapy further opens the need to deepen the investigation into the immune cell–TME interactions in cancer. This is a crucial point in perspective/future immunotherapeutic approaches, where modifying/re-educating the immune system should take into consideration not only the action on altered immune cells such as soloist elements, but also on the immune cells/TME interactions.
References
- Coffelt, S.B.; Wellenstein, M.D.; de Visser, K.E. Neutrophils in cancer: Neutral no more. Nat. Rev. Cancer 2016, 16, 431–446.
- Shaul, M.E.; Fridlender, Z.G. Tumour-associated neutrophils in patients with cancer. Nat. Rev. Clin. Oncol. 2019, 16, 601–620.
- Sun, R.; Xiong, Y.; Liu, H.; Gao, C.; Su, L.; Weng, J.; Yuan, X.; Zhang, D.; Feng, J. Tumor-associated neutrophils suppress antitumor immunity of NK cells through the PD-L1/PD-1 axis. Transl. Oncol. 2020, 13, 100825.
- Valayer, A.; Brea, D.; Lajoie, L.; Avezard, L.; Combes-Soia, L.; Labas, V.; Korkmaz, B.; Thibault, G.; Baranek, T.; Si-Tahar, M. Neutrophils can disarm NK cell response through cleavage of NKp46. J. Leukoc. Biol. 2017, 101, 253–259.
- Romero, A.I.; Thoren, F.B.; Brune, M.; Hellstrand, K. NKp46 and NKG2D receptor expression in NK cells with CD56dim and CD56bright phenotype: Regulation by histamine and reactive oxygen species. Br. J. Haematol. 2006, 132, 91–98.
- Costantini, C.; Cassatella, M.A. The defensive alliance between neutrophils and NK cells as a novel arm of innate immunity. J. Leukoc Biol. 2011, 89, 221–233.
- Oberlies, J.; Watzl, C.; Giese, T.; Luckner, C.; Kropf, P.; Muller, I.; Ho, A.D.; Munder, M. Regulation of NK cell function by human granulocyte arginase. J. Immunol. 2009, 182, 5259–5267.
- Gaggero, S.; Witt, K.; Carlsten, M.; Mitra, S. Cytokines Orchestrating the Natural Killer-Myeloid Cell Crosstalk in the Tumor Microenvironment: Implications for Natural Killer Cell-Based Cancer Immunotherapy. Front. Immunol. 2020, 11, 621225.
- Thierfelder, W.E.; van Deursen, J.M.; Yamamoto, K.; Tripp, R.A.; Sarawar, S.R.; Carson, R.T.; Sangster, M.Y.; Vignali, D.A.; Doherty, P.C.; Grosveld, G.C.; et al. Requirement for Stat4 in interleukin-12-mediated responses of natural killer and T cells. Nature 1996, 382, 171–174.
- Yamamoto, K.; Shibata, F.; Miyasaka, N.; Miura, O. The human perforin gene is a direct target of STAT4 activated by IL-12 in NK cells. Biochem. Biophys. Res. Commun. 2002, 297, 1245–1252.
- Liang, W.; Ferrara, N. The Complex Role of Neutrophils in Tumor Angiogenesis and Metastasis. Cancer Immunol. Res. 2016, 4, 83–91.
- Spiegel, A.; Brooks, M.W.; Houshyar, S.; Reinhardt, F.; Ardolino, M.; Fessler, E.; Chen, M.B.; Krall, J.A.; DeCock, J.; Zervantonakis, I.K.; et al. Neutrophils Suppress Intraluminal NK Cell-Mediated Tumor Cell Clearance and Enhance Extravasation of Disseminated Carcinoma Cells. Cancer Discov. 2016, 6, 630–649.
- Jaillon, S.; Ponzetta, A.; Di Mitri, D.; Santoni, A.; Bonecchi, R.; Mantovani, A. Neutrophil diversity and plasticity in tumour progression and therapy. Nat. Rev. Cancer 2020, 20, 485–503.
- Noonan, D.M.; De Lerma Barbaro, A.; Vannini, N.; Mortara, L.; Albini, A. Inflammation, inflammatory cells and angiogenesis: Decisions and indecisions. Cancer Metastasis Rev. 2008, 27, 31–40.
- Schruefer, R.; Sulyok, S.; Schymeinsky, J.; Peters, T.; Scharffetter-Kochanek, K.; Walzog, B. The proangiogenic capacity of polymorphonuclear neutrophils delineated by microarray technique and by measurement of neovascularization in wounded skin of CD18-deficient mice. J. Vasc. Res. 2006, 43, 1–11.
- Strieter, R.M. Masters of angiogenesis. Nat. Med. 2005, 11, 925–927.
- Adams, R.H.; Alitalo, K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat. Rev. Mol. Cell Biol. 2007, 8, 464–478.
- Scapini, P.; Morini, M.; Tecchio, C.; Minghelli, S.; Di Carlo, E.; Tanghetti, E.; Albini, A.; Lowell, C.; Berton, G.; Noonan, D.M.; et al. CXCL1/macrophage inflammatory protein-2-induced angiogenesis in vivo is mediated by neutrophil-derived vascular endothelial growth factor-A. J. Immunol. 2004, 172, 5034–5040.
- Ohki, Y.; Heissig, B.; Sato, Y.; Akiyama, H.; Zhu, Z.; Hicklin, D.J.; Shimada, K.; Ogawa, H.; Daida, H.; Hattori, K.; et al. Granulocyte colony-stimulating factor promotes neovascularization by releasing vascular endothelial growth factor from neutrophils. FASEB J. 2005, 19, 2005–2007.
- Nozawa, H.; Chiu, C.; Hanahan, D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc. Natl. Acad. Sci. USA 2006, 103, 12493–12498.
- Scapini, P.; Nesi, L.; Morini, M.; Tanghetti, E.; Belleri, M.; Noonan, D.; Presta, M.; Albini, A.; Cassatella, M.A. Generation of biologically active angiostatin kringle 1-3 by activated human neutrophils. J. Immunol. 2002, 168, 5798–5804.
- Sgadari, C.; Angiolillo, A.L.; Tosato, G. Inhibition of angiogenesis by interleukin-12 is mediated by the interferon-inducible protein 10. Blood 1996, 87, 3877–3882.
- Marcais, A.; Viel, S.; Grau, M.; Henry, T.; Marvel, J.; Walzer, T. Regulation of mouse NK cell development and function by cytokines. Front. Immunol. 2013, 4, 450.
- Yao, L.; Sgadari, C.; Furuke, K.; Bloom, E.T.; Teruya-Feldstein, J.; Tosato, G. Contribution of natural killer cells to inhibition of angiogenesis by interleukin-12. Blood 1999, 93, 1612–1621.
- Gasperini, S.; Marchi, M.; Calzetti, F.; Laudanna, C.; Vicentini, L.; Olsen, H.; Murphy, M.; Liao, F.; Farber, J.; Cassatella, M.A. Gene expression and production of the monokine induced by IFN-gamma (MIG), IFN-inducible T cell alpha chemoattractant (I-TAC), and IFN-gamma-inducible protein-10 (IP-10) chemokines by human neutrophils. J. Immunol. 1999, 162, 4928–4937.
- Shimasaki, N.; Jain, A.; Campana, D. NK cells for cancer immunotherapy. Nat. Rev. Drug Discov. 2020, 19, 200–218.
- Bassani, B.; Baci, D.; Gallazzi, M.; Poggi, A.; Bruno, A.; Mortara, L. Natural Killer Cells as Key Players of Tumor Progression and Angiogenesis: Old and Novel Tools to Divert Their Pro-Tumor Activities into Potent Anti-Tumor Effects. Cancers 2019, 11, 461.
- Jensen, K.N.; Omarsdottir, S.Y.; Reinhardsdottir, M.S.; Hardardottir, I.; Freysdottir, J. Docosahexaenoic Acid Modulates NK Cell Effects on Neutrophils and Their Crosstalk. Front. Immunol. 2020, 11, 570380.
- Karin, M. Inflammation and cancer: The long reach of Ras. Nat. Med. 2005, 11, 20–21.
- Bhatnagar, N.; Hong, H.S.; Krishnaswamy, J.K.; Haghikia, A.; Behrens, G.M.; Schmidt, R.E.; Jacobs, R. Cytokine-activated NK cells inhibit PMN apoptosis and preserve their functional capacity. Blood 2010, 116, 1308–1316.
- Costantini, C.; Micheletti, A.; Calzetti, F.; Perbellini, O.; Pizzolo, G.; Cassatella, M.A. Neutrophil activation and survival are modulated by interaction with NK cells. Int. Immunol. 2010, 22, 827–838.
- Romano, A.; Parrinello, N.L.; Simeon, V.; Puglisi, F.; La Cava, P.; Bellofiore, C.; Giallongo, C.; Camiolo, G.; D’Auria, F.; Grieco, V.; et al. High-density neutrophils in MGUS and multiple myeloma are dysfunctional and immune-suppressive due to increased STAT3 downstream signaling. Sci. Rep. 2020, 10, 1983.
- Molgora, M.; Supino, D.; Mavilio, D.; Santoni, A.; Moretta, L.; Mantovani, A.; Garlanda, C. The yin-yang of the interaction between myelomonocytic cells and NK cells. Scand. J. Immunol. 2018, 88, e12705.
- Ogura, K.; Sato-Matsushita, M.; Yamamoto, S.; Hori, T.; Sasahara, M.; Iwakura, Y.; Saiki, I.; Tahara, H.; Hayakawa, Y. NK Cells Control Tumor-Promoting Function of Neutrophils in Mice. Cancer Immunol. Res. 2018, 6, 348–357.
More
Information
Subjects:
Immunology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.3K
Revisions:
2 times
(View History)
Update Date:
29 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




