
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Anup Pandith | + 4034 word(s) | 4034 | 2022-01-11 03:30:19 | | | |
| 2 | Catherine Yang | -1718 word(s) | 2316 | 2022-01-19 07:18:05 | | |
Video Upload Options
The rising use of nonrenewable fossil fuels in recent decades has put human existence in grave danger. As a result, it is imperative to design environmentally friendly and cost-effective energy storage devices. Supercapacitors are a promising energy device because of their high power density, outstanding cycle stability, and quick charge/discharge process. However, supercapacitors' energy density is still lower than that of conventional batteries'. Supercapacitors' electrochemical performance is heavily influenced by the electrode materials, as is well-known to everyone.
1. Co3O4
The Cobalt oxide belongs to the spinel family and theoretical capacity of Co3O4 is found to be 3560 F/g [1] and moreover it is cheap and environment friendly compound along with excellent durability and stability. However, the capacitance is varied lot in many applications from theoretical capacitance value. The less conductivity, high volume expansion and contraction, slow kinetics and particle aggregation are the reasons behind this variation in capacitance [2][3].
2. Co3O4 Nanoparticles
3. Synthesis of Co3O4 Nanomaterials
Metal organic frameworks / MOFs type of materials have gained popularity in recent years in applications such as electro catalysis, adsorption of gas, degradation of pollutants, energy devices and so on. MOFs are also regarded to be an excellent template for the creation of Co3O4 nanoparticles because of their tunable porosity structure, variable pore size distribution, and large surface area [9][10]. The Zeolitic Imidazolate Frameworks 67 is acts as a precursor to synthesise Co3O4 NPs and Co3O4 material is converted by calcinations method to gain a good capacity of 190 F/g at 5 A/g. α-Co/Ni(OH)2 nanocages. The composite α-Co/Ni(OH)2@ Co3O4-70 prepared by Bao et al exhibits a large number reactive sites including good charge diffusion channels, because of this reason it shows excellent capacity of 1000 F/g at 1A/g current rate [1]. The addition of active carbon to α-Co/Ni(OH)2 increases the capacity retention of 72.3 % at current density of 10 A/g. In addition it delivers 0.075 kW/kg and 23.88 Wh/kg of power and energy density respectively [11]. In another way, Wei et al developed a process where thermal treatment converts ZIF-67 into ultrathin Co3O4 nanoparticles. A very good results in oxygen evolution reactions of 2D- Co3O4 ultrathin nanomaterials is because of its Tafel slope value of 74 mV/dec and potential of 230 mV. The 3D porous carbon developed by Li t al shows low specific capacitance of 423 F/g at ccurrent rate of 1 A/g. The low capacitance of material is due to the usage of 3D graphene / Co-metal organic framework (MOF) as a precursor, which slows down the transportation of electrons between electrolyte and active material [11].
4. MnO2
MnO2 has been thoroughly investigated as the high efficient TMO due to its abundent natural occurrence, lack of environmental pollution, and higher theoretical specific capacitance (1380 F/g) [12]. The MnO2 material is limited in supercapacitor applications; this is because of very less charge transfer rate [13].
5. Carbon@MnO2
The huge surface area and great electrical conductivity is the key factor behind the large use of carbonaceous materials like graphene, carbon nanofibres, carbon nanotubes and carbon nanowires. In order to achieve a good capacity of material, there should be very less path difference between the electrolyte and electrode surface, which can be seen in carbon materials [14]. The N-doped hollow HNC@MnO2 3D cores shell synthesised by Cai and co workers exhibits specific capacitance of 247 F/g at 0.5 A/g current rate [15]. Long and co workers prepared δ-MnO2 on carbon cloth and exhibited excellent power and energy density(Asymmetric device) of 1198 W/kg and 49 Wh/kg respectively. Lei et al developed MnO2 nanosheet@CNT framework through chemical vapour deposition method.
Commercial carbon compounds, on the other hand, are prohibitively expensive and difficult to prepare on a big scale due to their high cost and complicated preparation process. As a result, developing low-cost and renewable materials is critical in order to meet rising demand [16]. Biomass is a renewable resource with a large value of usage. Yang et al prepared MnO2/biomass-based porous carbon via hydrothermal approach by using banana peel as a carbon source, and which shows 139 F/g of specific capacitance at 300 mAh/g of current density and 70 F/g at current rate of 10 A/g [17][18].
6. Nickel Oxide
Nickel oxide (NiO) has got a huge amount of value in latest years because of its unique properties in terms of heat, light, electricity, sound, catalysis and magnetism properties [19]. As a result of their environmental friendliness and huge availability, they are often employed in the areas of supercapacitors. Because it has two or more oxidation states, it promotes rapid redox reactions, which contributes in storage techniques that are in charge. At 0.5 V potential winow, Nickel oxide has exhibits theoretical capacitance of 2584 F /g [20]. Unfortunately, due to NiO2's low electrical conductivity of 0.01 to 0.32 Sm-1 [21],the experimental findings never achieve the theoretical capacitance because it can expand which leads to destroying the active materials and causing electrical contact damage [22]. As of now, the SC values for NiO-type electrodes including a nanostructure and SSA have been 50 to 1776 F/g [23]. The link between NiO and NiOOH is described using two primary hypotheses. The energy storage mechanism occurs between NiO and NiOOH in one model, whereas the other, NiO converted to Ni(OH)2 in the influence of an alkaline medium, resulting from Ni(OH)2 and NiOOH reactions like given below [24][25].
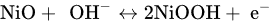
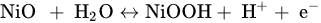
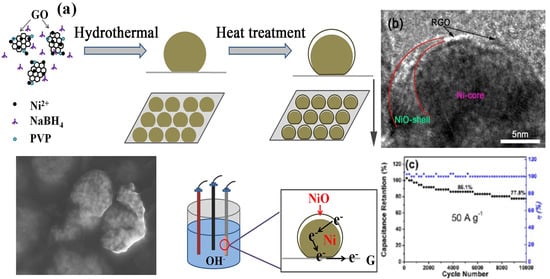
The existence of Gr produces the core shell semicoated NiO/Ni structure, as seen in Figure 1. Furthermore, it has an unusually high Csp (2048.3 F/g at 1.0 A/g), as well as exceptional cyclic stability (77.8%) and retention in capacity after 10000 cycles at a current rate of 50 A /g [26].
7. Copper Oxide
Copper oxides, like CuO and Cu2O-based SCs, have gained a lot of attention because of their abundance, low cost, nontoxicity, and ease of synthesis of diverse nanostructures [27]. Further, the capacity (loading storage) was diminished by very less electrical and cyclic abilities [28]. For example, Zhang et al reported the fabrication of flower-like CuO in a KOH electrolyte, yielding a specific capacitance of 133.6 F/g [29][30], whereas Li and co workers focused on developing CuO nanostructures immediately onto Cu foam surfaces, yielding a capacitance of 212 F/g in the same electrolyte [4][31]. To achieve a higher Csp of 569 F g1, Wang et al constructed CuO nanosheet arrays on Ni foam surface; yet, the synthesis approach is a pretty tricky approach with a very low yield [32][33][34].
8. Battery Type MOs (Metal Oxides)
Incorporating battery-type MO’s with MnO2-type of electrodes has been regarded to be an important technique to boost the energy density and capacity of supercapacitors [35][36], according to Nie's work. Synergistic effect, redox reactions and battery metal oxides are the main reasons to improve the capacitance of supercapacitors. In addition to that MnO2@NiO exhibits very high capacitance of 1277 F/g at 10 A/g with retention of 76% even after 10,000 cycles [37][38]. The Co3O4 on MnO2 shows extraordinary performance by exhibiting 616 F/g at 2 A/g current rates and moreover it was achieved 83% of capacity retention after 10,000 cycles. In other hand, AS electrode of Co3O4@ MnO2CC90 exhibits energy density and power density of 54 Wh/kg 1 kW/kg respectively [39].
9. ZnO
The specific capacity, stability and all other aspects of supercapacitors are controlled by choosing the good and capable material as an electrode. As a result, researchers have been investigating electrode materials that perform well electrochemically [40]. Because of their good theoretical capacitance, strong redox activity and affordable prices, TMOs have earned a lot of interest. ZnO has the features of environment friendly, wide availability, and constant capacitance [41]. Dhivya Angelin and co workers modify the ZnO by doping it with Zirconium, an appreciable capacitance of 518 F/g at 1 A/g was achieved in 9 Wt% Zr-Zno nanoparticle and capacity retention of 94% even after 5000 cycles is achieved. Zno nanomembranes exhibit different capacities in different electrolytes, like 846 F/g in 6 M KOH, 465 F/g in 1 M KCl,65 F/g in 6 M Na2SO4 each at 1 A/g of current densities [42].
10. ZnO Composites
Various types of ZnO composites are synthesised as supercapacitor electrodes, such as metal oxide-Zinc oxide,polymer-Zinc oxide,carbon-Zinc oxide to find out the most suitable material for electrochemical studies [43]. Graphene nanocapsules (GNCs) shows excellent capacitance of 194 F/g at 20 A/g current rate and moreover only 2.6% of capacity loss is found even after 15,000 cycles [44]. Chebrolu et al synthesised ZnO/NiO electrode and which exhibits extraordinary capacitance of 1248 F/g at 8 mA/cm2 than ZnO/PbO, ZnO/FeO, ZnO/CuO electrode materials. The reason behind this is, uniform surface area of nanosheets including good electrical conductivity. To avoid the distraction of ZnO framework, Di’s team synthesised ZnO with little quantity of Al2O3. The specific capacitance of 463 F/g with excellent stability of 96% is achieved. Later all studies proves that multicomponent compounds along with ZnO could increase the capacity and stability.The CoO3-CuO-ZnO @ GO nanocomposite prepared by Obodo et al and which delivered excellent Csp of 1950 F/g at 10 mV/s current density.
11. XCo2O4 (X-Cu, Ni, Mn)
The spinel structure exhibits good electrochemical activity and conductivity so these ternary transition materials used extensively for supercapacitor studies [45][46]. The MnCo2O4 electrode synthesised by shanmugavadivel et al by combustion method shows an excellent capacitance of 270 F/g. Moreover, electrodeposition method followed to prepare the same material shows increased Csp of 585 F/g at 0.2 mA/cm2 current rate [47].Later, by electrodeposition method NiCo2O4 is coated on nickel wire and which exhibits good capacitance of 315 C/g at 1 A/g with loss of 8.4% capacity after 50,000 cycles [48][49]. CuCo2O4 developed by Pawar et al by electrodeposition and annealing process [50]. An appreciable capacity of 1473 F/g even after 5,000 cycles is achieved at 1A/g current density [51]. The Csp of 1933 F/g at current rate of 1 A/g was achieved by Wang’s group by synthesising MnCo2O4 electrode material [52]. The NiCo2O4@MnO2 synthesised by Xu and co-workers exhibits a good specific capacitance of 1.23 F/cm-2 at 50 mA/cm-2 after 8000 cycles.
12. Transition Metal Molybdates
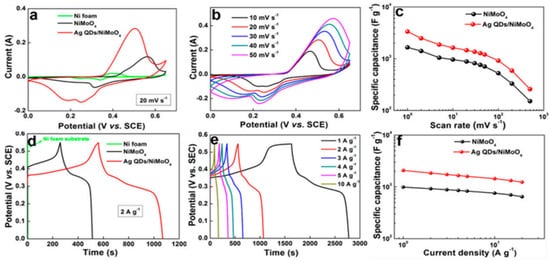
Due to a redox mechanism on materials surface, pseudocapacitive materials such as organic conductive materials and metal oxides show large specific capacitances than carbon materials [53]. Furthermore, due to large number of active sites, quick redox reactions ternary metal oxide could be the potential material to replace RuO2. The transition metal molybdates have got a lot of interest as main spot of mixed transition metal oxides because of their features such as abundant availability, higher specific, theoretical capacitance, and low prices [54][55]. The morphology and structure of supercapacitor electrode materials play a big role in their performance. As a result, it's critical to create electrode materials with distinct spatial structural features. The large specific surface area leads to the improved interfacial conductivity , increase in the number of active sites and porous structure, the Ag Quantum Dots/NiMoO4 nanopartilcles shows excellent specific capacitance of 3342 F /g at voltage of 1 mV/s and 2074 F/g at current density of 1 A/ g as shown in Figure-2 [56].
13. Design of Transition Metal Oxides
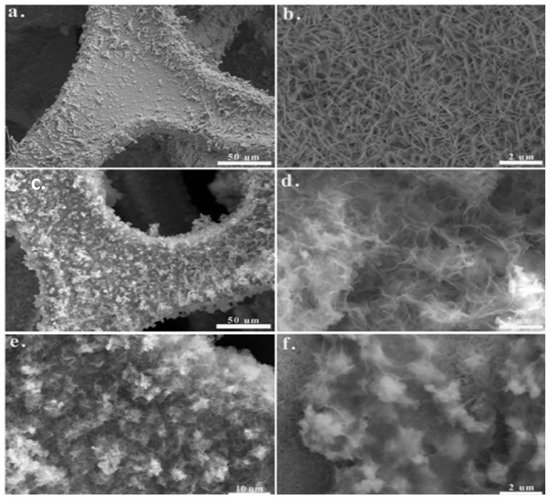
The construction of hetero type of composites has proven to be a promising technique to execute the electrochemical characteristics of TMOs, as per Yi's research [55]. Heterostructures include core–shell structures. The core–shell configuration can provide a lot of surface area with a lot of porosity. Likewise, core materials enhance electron transmission, while shell materials provide electrochemical redox active sites. Furthermore, each material's synergistic effect is used to boost the electrochemical behaviour of the electrode. 3D hierarchical core–shell (CoMoO4@CoS) was successively developed by Xuan and team, here they took rGO/Ni foam for the preparation. Figure-3 displays different magnifications of SEM images of CoS, CoMoO4 and CoMoO4@CoS materials [57].
References
- Cheng L, Zhang Q, Xu M, Zhai Q, Zhang C. Two-for-one strategy: Three-dimensional porous Fe-doped Co3O4 cathode and N-doped carbon anode derived from a single bimetallic metal-organic framework for enhanced hybrid supercapacitor. Journal of Colloid and Interface Science. 2021;583:299-309.
- Niu W, Xiao Z, Wang S, Zhai S, Qin L, Zhao Z, et al. Synthesis of nickel sulfide-supported on porous carbon from a natural seaweed-derived polysaccharide for high-performance supercapacitors. Journal of Alloys and Compounds. 2021;853:157123.
- Pettong T, Iamprasertkun P, Krittayavathananon A, Sukha P, Sirisinudomkit P, Seubsai A, et al. High-performance asymmetric supercapacitors of MnCo2O4 nanofibers and N-doped reduced graphene oxide aerogel. ACS applied materials & interfaces. 2016;8:34045-53.
- Xu P, Liu J, Liu T, Ye K, Cheng K, Yin J, et al. Preparation of binder-free CuO/Cu 2 O/Cu composites: A novel electrode material for supercapacitor applications. RSC advances. 2016;6:28270-8.
- Wang X, Yin S, Jiang J, Xiao H, Li X. A tightly packed Co3O4/C&S composite for high-performance electrochemical supercapacitors from a cobalt (III) cluster-based coordination precursor. Journal of Solid State Chemistry. 2020;288:121435.
- Lu J, Li J, Wan J, Han X, Ji P, Luo S, et al. A facile strategy of in-situ anchoring of Co3O4 on N doped carbon cloth for an ultrahigh electrochemical performance. Nano Research. 2021;14:2410-7.
- Chatterjee M, Sain S, Roy A, Das S, Pradhan SK. Enhanced electrochemical properties of Co3O4 with morphological hierarchy for energy storage application: A comparative study with different electrolytes. Journal of Physics and Chemistry of Solids. 2021;148:109733.
- Cao J, Li J, Zhou L, Xi Y, Cao X, Zhang Y, et al. Tunable agglomeration of Co3O4 nanowires as the growing core for in-situ formation of Co2NiO4 assembled with polyaniline-derived carbonaceous fibers as the high-performance asymmetric supercapacitors. Journal of Alloys and Compounds. 2021;853:157210.
- Xiang C, Li M, Zhi M, Manivannan A, Wu N. A reduced graphene oxide/Co3O4 composite for supercapacitor electrode. Journal of Power Sources. 2013;226:65-70.
- Kumar R, Youssry SM, Soe HM, Abdel-Galeil MM, Kawamura G, Matsuda A. Honeycomb-like open-edged reduced-graphene-oxide-enclosed transition metal oxides (NiO/Co3O4) as improved electrode materials for high-performance supercapacitor. Journal of Energy Storage. 2020;30:101539.
- Gong Y, An J, Dai H, Chen R, Yu C, Chen Q, et al. Hierarchically tubular architectures composed of vertical carbon nanosheets embedded with oxygen-vacancy enriched hollow Co3O4 nanoparticles for improved energy storage. Electrochimica Acta. 2020;356:136843.
- Wang Q, Ma Y, Liang X, Zhang D, Miao M. Flexible supercapacitors based on carbon nanotube-MnO2 nanocomposite film electrode. Chemical Engineering Journal. 2019;371:145-53.
- Wu K, Ye Z, Ding Y, Zhu Z, Peng X, Li D, et al. Facile co-deposition of the carbon nanotube@ MnO2 heterostructure for high-performance flexible supercapacitors. Journal of Power Sources. 2020;477:229031.
- Zhao Y, Ran W, He J, Huang Y, Liu Z, Liu W, et al. High‐performance asymmetric supercapacitors based on multilayer MnO2/graphene oxide nanoflakes and hierarchical porous carbon with enhanced cycling stability. Small. 2015;11:1310-9.
- Wang L, Ouyang Y, Jiao X, Xia X, Lei W, Hao Q. Polyaniline-assisted growth of MnO2 ultrathin nanosheets on graphene and porous graphene for asymmetric supercapacitor with enhanced energy density. Chemical Engineering Journal. 2018;334:1-9.
- Bose N, Sundararajan V, Prasankumar T, Jose SP. α–MnO2 coated anion intercalated carbon nanowires: A high rate capability electrode material for supercapacitors. Materials Letters. 2020;278:128457.
- Sui Z, Chang Z, Xu X, Li Y, Zhu X, Zhao C, et al. Direct growth of MnO2 on highly porous nitrogen-doped carbon nanowires for asymmetric supercapacitors. Diamond and Related Materials. 2020;108:107988.
- Cakici M, Kakarla RR, Alonso-Marroquin F. Advanced electrochemical energy storage supercapacitors based on the flexible carbon fiber fabric-coated with uniform coral-like MnO2 structured electrodes. Chemical Engineering Journal. 2017;309:151-8.
- Kate RS, Khalate SA, Deokate RJ. Overview of nanostructured metal oxides and pure nickel oxide (NiO) electrodes for supercapacitors: A review. Journal of Alloys and Compounds. 2018;734:89-111.
- Sk MM, Yue CY, Ghosh K, Jena RK. Review on advances in porous nanostructured nickel oxides and their composite electrodes for high-performance supercapacitors. Journal of Power Sources. 2016;308:121-40.
- Xiao H, Yao S, Liu H, Qu F, Zhang X, Wu X. NiO nanosheet assembles for supercapacitor electrode materials. Progress in Natural Science: Materials International. 2016;26:271-5.
- Lu Q, Lattanzi MW, Chen Y, Kou X, Li W, Fan X, et al. Supercapacitor electrodes with high‐energy and power densities prepared from monolithic NiO/Ni nanocomposites. Angewandte Chemie International Edition. 2011;50:6847-50.
- Wang H, Casalongue HS, Liang Y, Dai H. Ni (OH) 2 nanoplates grown on graphene as advanced electrochemical pseudocapacitor materials. Journal of the American Chemical Society. 2010;132:7472-7.
- Liang K, Tang X, Hu W. High-performance three-dimensional nanoporous NiO film as a supercapacitor electrode. Journal of Materials Chemistry. 2012;22:11062-7.
- Xia X, Tu J, Mai Y, Chen R, Wang X, Gu C, et al. Graphene sheet/porous NiO hybrid film for supercapacitor applications. Chemistry–A European Journal. 2011;17:10898-905.
- Xu K, Zou R, Li W, Liu Q, Wang T, Yang J, et al. Carbon-coated mesoporous NiO nanoparticles as an electrode material for high performance electrochemical capacitors. New Journal of Chemistry. 2013;37:4031-6.
- Xu W, Dai S, Liu G, Xi Y, Hu C, Wang X. CuO nanoflowers growing on carbon fiber fabric for flexible high-performance supercapacitors. Electrochimica Acta. 2016;203:1-8.
- Shahsank M, Naik HB, Sumedha H, Nagaraju G. Implementing an in-situ carbon formation of MoO3 nanoparticles for high performance lithium-ion battery. Ceramics International. 2021;47:10261-7.
- Zhou L, He Y, Jia C, Pavlinek V, Saha P, Cheng Q. Construction of hierarchical CuO/Cu2O@ NiCo2S4 nanowire arrays on copper foam for high performance supercapacitor electrodes. Nanomaterials. 2017;7:273.
- Chuai M, Chen X, Zhang K, Zhang J, Zhang M. CuO–SnO 2 reverse cubic heterojunctions as high-performance supercapacitor electrodes. Journal of Materials Chemistry A. 2019;7:1160-7.
- Yang Y, Pei L, Xu X, Xu J, Shen J, Ye M. In-situ growth of self-assembled 3D Cu2O@ Cu foam with enhanced electrochemical properties. Electrochimica Acta. 2016;221:56-61.
- Saravanakumar B, Radhakrishnan C, Ramasamy M, Kaliaperumal R, Britten AJ, Mkandawire M. Copper oxide/mesoporous carbon nanocomposite synthesis, morphology and electrochemical properties for gel polymer-based asymmetric supercapacitors. Journal of Electroanalytical Chemistry. 2019;852:113504.
- Zhang H, Feng J, Zhang M. Preparation of flower-like CuO by a simple chemical precipitation method and their application as electrode materials for capacitor. Materials Research Bulletin. 2008;43:3221-6.
- Li Y, Chang S, Liu X, Huang J, Yin J, Wang G, et al. Nanostructured CuO directly grown on copper foam and their supercapacitance performance. Electrochimica Acta. 2012;85:393-8.
- Aghazadeh M, Ganjali MR. Samarium-doped Fe 3 O 4 nanoparticles with improved magnetic and supercapacitive performance: a novel preparation strategy and characterization. Journal of Materials Science. 2018;53:295-308.
- Serrapede M, Rafique A, Fontana M, Zine A, Rivolo P, Bianco S, et al. Fiber-shaped asymmetric supercapacitor exploiting rGO/Fe2O3 aerogel and electrodeposited MnOx nanosheets on carbon fibers. Carbon. 2019;144:91-100.
- Qu Q, Yang S, Feng X. 2d sandwich‐like sheets of iron oxide grown on graphene as high energy anode material for supercapacitors. Advanced materials. 2011;23:5574-80.
- Eskusson J, Rauwel P, Nerut J, Jänes A. A hybrid capacitor based on Fe3O4-graphene nanocomposite/few-layer graphene in different aqueous electrolytes. J Electrochem Soc. 2016;163:A2768.
- Borenstein A, Hanna O, Attias R, Luski S, Brousse T, Aurbach D. Carbon-based composite materials for supercapacitor electrodes: a review. Journal of Materials Chemistry A. 2017;5:12653-72.
- Chen H-C, Lyu Y-R, Fang A, Lee G-J, Karuppasamy L, Wu JJ, et al. The design of ZnO nanorod arrays coated with MnOx for high electrochemical stability of a pseudocapacitor electrode. Nanomaterials. 2020;10:475.
- Mohamed IM, Yasin AS, Liu C. Synthesis, surface characterization and electrochemical performance of ZnO@ activated carbon as a supercapacitor electrode material in acidic and alkaline electrolytes. Ceramics International. 2020;46:3912-20.
- Sun L, Zhang Y, Si H, Shi Y, Sun C, Zhang Y. Porous Mo–C coverage on ZnO rods for enhanced supercapacitive performance. Dalton Transactions. 2020;49:5134-42.
- He D, Wan J, Liu G, Suo H, Zhao C. Design and construction of hierarchical α-Co (OH) 2-coated ultra-thin ZnO flower nanostructures on nickel foam for high performance supercapacitors. Journal of Alloys and Compounds. 2020;838:155556.
- Ding S, Li X, Jiang X, Hu Q, Yan Y, Zheng Q, et al. Core-shell nanostructured ZnO@ CoS arrays as advanced electrode materials for high-performance supercapacitors. Electrochimica Acta. 2020;354:136711.
- Wu D, Han H, Hong X, Tao S, Xu S, Qian B, et al. A novel self-branching MnCo2O4/nanographene hybrid composites on macroporous electrically conductive network as bifunctional electrodes for boosting miniature supercapacitors and sodium ion batteries. Journal of Alloys and Compounds. 2020;846:155720.
- Sumedha H, Alsaiari MA, Jalalah MS, Shashank M, Alharthi FA, Ahmad N, et al. Rapid Microwave Synthesis of β-SnWO4 Nanoparticles: An Efficient Anode Material for Lithium Ion Batteries. Crystals. 2021;11:334.
- Saren P, De Adhikari A, Khan S, Nayak GC. Self-assembled GNS wrapped flower-like MnCo2O4 nanostructures for supercapacitor application. Journal of Solid State Chemistry. 2019;271:282-91.
- Ma L, Shen X, Zhou H, Ji Z, Chen K, Zhu G. High performance supercapacitor electrode materials based on porous NiCo2O4 hexagonal nanoplates/reduced graphene oxide composites. Chemical Engineering Journal. 2015;262:980-8.
- Lei Y, Li J, Wang Y, Gu L, Chang Y, Yuan H, et al. Rapid microwave-assisted green synthesis of 3D hierarchical flower-shaped NiCo2O4 microsphere for high-performance supercapacitor. ACS applied materials & interfaces. 2014;6:1773-80.
- Li Z, Yao Y, Zheng Y, Gao T, Liu Z, Zhou G. Fabrication of core-shell Fe3O4@ C@ MnO2 microspheres and their application in supercapacitors. J Electrochem Soc. 2018;165:E58.
- Liu Y, Zhang X, Matras-Postolek K, Yang P. Ni2P nanosheets modified N-doped hollow carbon spheres towards enhanced supercapacitor performance. Journal of Alloys and Compounds. 2021;854:157111.
- Samuel E, Aldalbahi A, El-Newehy M, El-Hamshary H, Yoon SS. Nickel ferrite beehive-like nanosheets for binder-free and high-energy-storage supercapacitor electrodes. Journal of Alloys and Compounds. 2021;852:156929.
- Sumedha H, Shashank M, Alharthi FA, Santosh MS, Praveen B, Nagaraju G. Synthesis of novel pseudo-capacitive perovskite nanostructured flowerlike KTaO3 for lithium ion storage. International Journal of Hydrogen Energy. 2021;46:28214-20.
- Chen H, Du X, Sun J, Wu R, Wang Y, Xu C. Template-free synthesis of novel Co3O4 micro-bundles assembled with flakes for high-performance hybrid supercapacitors. Ceramics International. 2021;47:716-24.
- Dai M, Zhao D, Wu X. Research progress on transition metal oxide based electrode materials for asymmetric hybrid capacitors. Chinese Chemical Letters. 2020;31:2177-88.
- Zhang X, Li Z, Yu Z, Wei L, Guo X. Mesoporous NiMoO4 microspheres decorated by Ag quantum dots as cathode material for asymmetric supercapacitors: enhanced interfacial conductivity and capacitive storage. Applied Surface Science. 2020;505:144513.
- Xuan H, Li H, Yang J, Liang X, Xie Z, Han P, et al. Rational design of hierarchical core-shell structured CoMoO4@ CoS composites on reduced graphene oxide for supercapacitors with enhanced electrochemical performance. International Journal of Hydrogen Energy. 2020;45:6024-35.




