
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Malvi Savani | + 2909 word(s) | 2909 | 2021-12-01 09:12:43 | | | |
| 2 | Catherine Yang | Meta information modification | 2909 | 2022-01-19 02:05:10 | | |
Video Upload Options
Immunotherapy approaches for head and neck squamous cell carcinoma (HNSCC) are rapidly advancing. Human papillomavirus (HPV) has been identified as a causative agent in a subset of oropharyngeal cancers (OPC). HPV-positive OPC comprises a distinct clinical and pathologic disease entity and has a unique immunophenotype. Immunotherapy with anti-PD1 checkpoint inhibitors has exhibited improved outcomes for patients with advanced HNSCC, irrespective of HPV status. To date, the clinical management of HPV-positive HNSCC and HPV-negative HNSCC has been identical, despite differences in the tumor antigens, immune microenvironment, and immune signatures of these two biologically distinct tumor types. Numerous clinical trials are underway to further refine the application of immunotherapy and develop new immunotherapy approaches. The aim of this entry is to highlight the developing role of immunotherapy in HPV-positive HNSCC along with the clinical evidence and preclinical scientific rationale behind emerging therapeutic approaches, with emphasis on promising HPV-specific immune activators that exploit the universal presence of foreign, non-self tumor antigens.
1. Introduction
HPV-positive HNSCC represents a distinct disease entity with molecular, pathological, and clinical features that lead to an improved response to standard therapy and a favorable prognosis compared to HPV-negative HNSCC. Weinberger and colleagues initially identified an OPC molecular profile associated with favorable prognosis: specifically, the presence of HPV16 DNA and overexpression of p16. The latter implies functionally relevant HPV; conventional thinking is that p16 is upregulated as a consequence of retinoblastoma (Rb) degradation by the oncoprotein E7 [1]. Alternately, McLaughlin-Drubin et al. have shown that p16 upregulation is Rb-independent but indeed E7-dependent, as E7 drives the upregulation of KDM6B histone demethylase [2]. Ultimately, HPV status and tobacco history were identified as the two most important prognostic factors in OPC [3], and in 2017 the American Joint Commission on Cancer codified a new staging system for p16-positive OPC to improve hazard discrimination.
These poor outcomes have driven increased research into novel immunotherapeutic and targeted approaches in HNSCC. Immunotherapy has emerged as a promising therapeutic avenue in HPV-positive HNSCC as HPV-driven carcinogenesis is propelled by loss of immunologic control with chronic viral infection resulting in a unique, non-self, antigenic target [4]. In this review, we highlight the role of immunotherapy as an established standard of care, as well as explore emerging immunotherapies including novel checkpoint inhibitors, antibodies and fusion protein constructs, vaccines, and chimeric antigen receptor (CAR) T-cell therapy in HPV-positive HNSCC ( Figure 1 ).
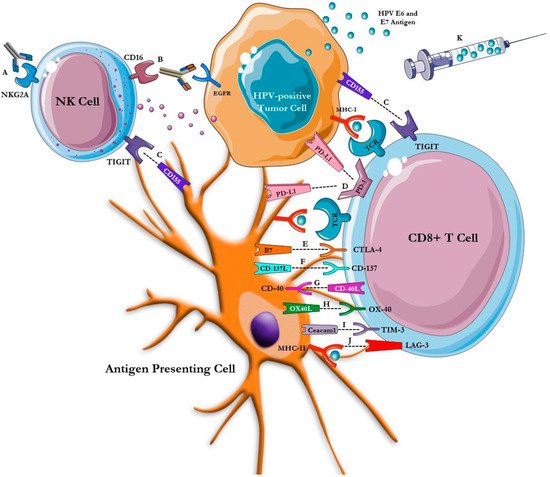
2. Primary Prevention of HPV-Positive HNSCC
HPV is a double-stranded DNA virus that infects the epidermal and mucosal surfaces of the oropharynx, genital, and anal regions and is an established causative agent in oropharynx, cervical, vulvar, anal, and penile cancers. HPV infection is common and occurs through mucosal surface contact with an infected sexual partner [5]. Three prophylactic HPV vaccinations are currently approved by the FDA including the quadrivalent HPV vaccine, Gardasil, a bivalent HPV vaccine, Cervarix, and a nine-valent vaccine, Gardasil 9, with the latter providing protection against HPV-6, 11, 16, 18, 31, 33, 45, 53, and 58 [5]. Each vaccine is recommended for a specific sex and age group. HPV vaccination for all individuals aged 9–26 years is recommended by the US Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices. Additionally, the FDA has approved HPV vaccination for all individuals aged 27–45 years who have not been adequately vaccinated previously [6]. The indications for these prophylactic HPV vaccinations were established based on impact on anogenital HPV infections. However, the effectiveness against oral HPV infection specifically has only been analyzed retrospectively but was shown to have 88–93% efficacy [7]. HPV vaccinations are only effective for primary prevention with the primary mechanism of action involving the induction of anti-L1 capsid antibodies, effectively blocking the initial step of viral entry. Although numerous epidemiological studies have shown an association between oral HPV DNA detection or anti-HPV16 E6 seropositivity and consequent risk of developing OPC, no validated screening test analogous to the Papanicolaou smear exists for HPV-positive HNSCC [8].
3. Meta-Analysis of PD-1 Inhibition in HNSCC
In an effort to further understand the impact of HPV status on response to anti-PD1 immunotherapy, recent meta-analyses have been conducted. In a meta-analysis assessing results from 11 clinical trials including 1860 patients evaluating immunotherapy in R/M HNSCC, patients with HPV-positive HNSCC demonstrated an improved risk ratio (1.29, p = 0.24) and OS (11.5 vs. 6.3 months) in comparison to patients with HPV-negative disease [9]. The most common immunotherapy agents across the trials were anti-PD1 agents (pembrolizumab, nivolumab) and anti-PD-L1 inhibitors (durvalumab and atezolizumab). Of note, motolimod, a Toll-Like Receptor (TLR)-8 agonist, was assessed in two trials and monalizumab, a natural killer group 2 member A (NKG2A) inhibitor, was used in one. The data also found PD-L1 to be predictive in evaluating response to immunotherapy. The best outcomes to immunotherapy were observed in patients who had tumors expressing both HPV and PD-L1.
In another recent meta-analysis, data from 7 studies including 814 patients with R/M HNSCC were analyzed. This meta-analysis was restricted to patients treated with PD-1 or PD-L1 inhibitors as single agents. The objective response rate (ORR) of patients with HPV-positive HNSCC was significantly greater than that of their HPV-negative counterparts (OR = 1.77 ; 95% CI = 1.14–2.74; p = 0.01). The odds ratio (OR) was more impressive for the pooled anti-PD-L1 trials (OR = 2.66; 95% CI = 1.16–6.11; p = 0.02) in comparison to the pooled anti-PD-1 trials (OR = 1.51; 95% CI = 0.90–2.54; p = 0.12). Patients with HPV-positive HNSCC also were noted to have a lower risk of death in comparison to patients with HPV-negative HNSCC (HR = 0.77; 95% CI = 0.60–0.99; p = 0.04) [10].
Possible mechanisms underlying clinical benefit, including ORR and OS, in HPV-positive HNSCC treated with immunotherapy relates to the unique tumor microenvironment of HPV-positive tumors. Chiefly, the HPV-specific T-cells, type I-oriented CD4+ and CD8+ T-cells, dendritic cells, and dendritic-like macrophages, as well as the synthesis of E6 and E7 oncoproteins, induce the immune system to detect tumor cells [9]. Chakravarthy and colleagues have shown a difference in the tumor infiltrating lymphocyte levels between HPV-positive and HPV-negative OPC, reinforcing the hypothesis that a difference in immune response between the two groups may contribute to the observed survival benefit [11].
4. Novel Immune Therapies
| Treatment | Indication | Clinical Trial ID |
|---|---|---|
| Anti-PD-1/L1 Checkpoint Inhibitor Plus Standard of Care Combinations | ||
| Durvalumab (anti-PD-L1), cetuximab (anti-EGFR), and radiation therapy | Locally advanced HNSCC | NCT03051906 |
| Ipilimumab (anti-CTLA4), nivolumab (anti-PD1), and radiation therapy | HPV-positive advanced OPCs | NCT03799445 |
| Maintenance cemiplimab (anti-PD1) | Locally advanced HNSCC | NCT04831450 |
| Nivolumab plus ipilimumab compared to the standard of care (EXTREME Regimen) | First-line R/M HNSCC | NCT02741570 |
| Nivolumab/carboplatin/paclitaxel | HPV-positive OPC | NCT03342911 |
| Nivolumab plus paclitaxel | R/M HNSCC | NCT04282109 |
| Novel Immune Checkpoint Inhibitors | ||
| Atezolizumab (anti-PD-L1) plus tiragolumab (anti-TIGIT) | R/M PD-L1 positive HNSCC | NCT04665843 |
| Nivolumab plus relatlimab (anti-LAG-3) | R/M HNSCC progressed on prior immunotherapy | NCT04326257 |
| TSR-022 (anti-TIM-3) | Advanced solid tumors | NCT02817633 |
| MBG453 (anti-TIM-3) ± PDR001 (anti-PD1) | Advanced solid tumors | NCT02608268 |
| T Cell Receptor Costimulatory Agonists | ||
| Anti-OX40 Antibody (OX40 agonist) | Head and neck cancers | NCT02274155 |
| PF-04518600 (OX40 agonist) alone/or in combination with PF-05082566 (4-1BB agonist) | Advanced or metastatic carcinoma | NCT02315066 |
| PF-05082566 plus pembrolizumab (anti-PD1) | Advanced solid tumors | NCT02179918 |
| Avelumab (anti-PD-L1)in combination with utomilumab (4-1BB agonist) | locally advanced or metastatic solid tumors | NCT02554812 |
| Urelumab (4-1BB agonist) and cetuximab | Advanced/metastatic head and neck cancers | NCT02110082 |
| CP-870,893 (CD40 agonist) | Advanced solid tumors | NCT02225002 |
| Novel Antibodies and Fusion Proteins | ||
| Monalizumab (NK cell NKG2A inhibitor) plus cetuximab | R/M HNSCC | NCT04590963 |
| CUE-101 (HPV-16 E7 T cell activator) | HPV-positive R/M HNSCC | NCT03978689 |
| Eftilagimod alpha (soluble LAG3 protein) and pembrolizumab | R/M HNSCC | NCT03625323 |
| PDS0101 (liposomal multipeptide vaccine targeting HPV-16 E6 and E7) + NHS-IL12 (Interleukin-12) + M7824 (bifunctional fusion protein targeting TGF-β and PD-L1) | HPV-positive cancers | NCT04287868 |
| Vaccines | ||
| Utomilumab and ISA101b vaccine (synthetic long HPV16 E6/E7 peptides vaccine) | HPV-positive OPCs | NCT03258008 |
| ISA101b and pembrolizumab plus cisplatin | HPV-positive HNSCC | NCT04369937 |
| ADX 11-001 Vaccine (live attenuated Listeria monocytogenes bacterium) | HPV-positive OPC | NCT02002182 |
| PRGN-2009 Vaccine (novel gorilla adenovirus vaccine) alone or in combination with M7824 (anti-PDL1/TGF-beta trap) | HPV-positive cancers | NCT04432597 |
| DPX-E7 Vaccine (synthetic peptide-based vaccine of HPV16 E711-19) | HPV-positive OPC, cervical and anal cancers | NCT02865135 |
| BNT113 Vaccine (RNA-lipoplex (RNA-LIP)-based mRNA vaccine encoding HPV-16 E6 and E7) plus pembrolizumab | HPV-positive and PD-L1 expressing HNSCC | NCT04534205 |
| PDS0101 plus pembrolizumab | HPV-positive HNSCC | NCT04260126 |
| Adoptive T-cell Transfer | ||
| RPTR-168 (Autologous IL-12/multi-targeted primed T cells) | HPV-positive HNSCC, cervical and melanoma | NCT04762225 |
| E7 TCR T cells | HPV-positive cancers | NCT02858310 |
| T Cell Receptor Immunotherapy Targeting HPV-16 E6 | HPV-positive cancers | NCT02280811 |
4.1. Checkpoint Inhibitor Combinations with Standard Therapies
4.2. Novel Immune Checkpoint Inhibitors
4.3. Costimulatory T Cell Receptor Agonists
4.4. Novel Antibodies and Fusion Protein Constructs
4.5. Vaccines
4.6. Adoptive T Cell Transfer
References
- Weinberger, P.M.; Yu, Z.; Haffty, B.G.; Kowalski, D.; Harigopal, M.; Brandsma, J.; Sasaki, C.; Joe, J.; Camp, R.L.; Rimm, D.L.; et al. Molecular classification identifies a subset of human papillomavirus--associated oropharyngeal cancers with favorable prognosis. J. Clin. Oncol. 2006, 24, 736–747.
- McLaughlin-Drubin, M.E.; Crum, C.P.; Munger, K. Human papillomavirus E7 oncoprotein induces KDM6A and KDM6B histone demethylase expression and causes epigenetic reprogramming. Proc. Natl. Acad. Sci. USA 2011, 108, 2130–2135.
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tan, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl J. Med. 2010, 363, 24–35.
- Cramer, J.D.; Burtness, B.; Ferris, R.L. Immunotherapy for head and neck cancer: Recent advances and future directions. Oral Oncol. 2019, 99, 104460.
- Cheng, L.; Wang, Y.; Du, J. Human Papillomavirus Vaccines: An Updated Review. Vaccines 2020, 8, 391.
- Meites, E.; Szilagyi, P.G.; Chesson, H.W.; Unger, E.R.; Romero, J.R.; Markowitz, L.E. Human Papillomavirus Vaccination for Adults: Updated Recommendations of the Advisory Committee on Immunization Practices. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 698–702.
- Herrero, R.; Quint, W.; Hildesheim, A.; Gonzalez, P.; Struijk, L.; Katki, H.A.; Porras, C.; Schiffman, M.; Rodriguez, A.C.; Solomon, D.; et al. Reduced prevalence of oral human papillomavirus (HPV) 4 years after bivalent HPV vaccination in a randomized clinical trial in Costa Rica. PLoS ONE 2013, 8, e68329.
- Taberna, M.; Mena, M.; Pavon, M.A.; Alemany, L.; Gillison, M.L.; Mesia, R. Human papillomavirus-related oropharyngeal cancer. Ann. Oncol. 2017, 28, 2386–2398.
- Galvis, M.M.; Borges, G.A.; Oliveira, T.B.; Toledo, I.P.; Castilho, R.M.; Guerra, E.N.S.; Kowalski, L.P.; Squarize, C.H. Immunotherapy improves efficacy and safety of patients with HPV positive and negative head and neck cancer: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2020, 150, 102966.
- Xu, Y.; Zhu, G.; Maroun, C.A.; Wu, I.X.Y.; Huang, D.; Seiwert, T.Y.; Liu, Y.; Mandal, R.; Zhang, X. Programmed Death-1/Programmed Death-Ligand 1-Axis Blockade in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma Stratified by Human Papillomavirus Status: A Systematic Review and Meta-Analysis. Front. Immunol. 2021, 12, 645170.
- Chakravarthy, A.; Henderson, S.; Thirdborough, S.M.; Ottensmeier, C.H.; Su, X.; Lechner, M.; Feber, A.; Thomas, G.J.; Fenton, T.R. Human Papillomavirus Drives Tumor Development Throughout the Head and Neck: Improved Prognosis Is Associated With an Immune Response Largely Restricted to the Oropharynx. J. Clin. Oncol. 2016, 34, 4132–4141.
- Suresh, T. Burtness Barbara Immunotherapy in Head and Neck Squamos Cell Cancer. Am. J. Hematol. Oncol. 2017, 13, 20–27.
- Zhang, P.; Ma, Y.; Lv, C.; Huang, M.; Li, M.; Dong, B.; Liu, X.; An, G.; Zhang, W.; Zhang, J.; et al. Upregulation of programmed cell death ligand 1 promotes resistance response in non-small-cell lung cancer patients treated with neo-adjuvant chemotherapy. Cancer Sci. 2016, 107, 1563–1571.
- Sacco, A.G.; Chen, R.; Worden, F.P.; Wong, D.J.L.; Adkins, D.; Swiecicki, P.; Chai-Ho, W.; Oppelt, P.; Ghosh, D.; Bykowski, J.; et al. Pembrolizumab plus cetuximab in patients with recurrent or metastatic head and neck squamous cell carcinoma: An open-label, multi-arm, non-randomised, multicentre, phase 2 trial. Lancet Oncol. 2021, 22, 883–892.
- Gameiro, S.F.; Ghasemi, F.; Barrett, J.W.; Koropatnick, J.; Nichols, A.C.; Mymryk, J.S.; Maleki Vareki, S. Treatment-naive HPV+ head and neck cancers display a T-cell-inflamed phenotype distinct from their HPV- counterparts that has implications for immunotherapy. Oncoimmunology 2018, 7, e1498439.
- Ferris, R.L.; Haddad, R.; Even, C.; Tahara, M.; Dvorkin, M.; Ciuleanu, T.E.; Clement, P.M.; Mesia, R.; Kutukova, S.; Zholudeva, L.; et al. Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open-label phase III study. Ann. Oncol. 2020, 31, 942–950.
- Bristol Myers Squibb. Bristol Myers Squibb Provides Update on CheckMate-651 Trial Evaluating Opdivo (Nivolumab) plus Yervoy (Ipilimumab) Versus EXTREME Regimen as First-Line Treatment for Squamous Cell Carcinoma of the Head and Neck. Press Release, Bristol Myers Squibb Corporate News. 16 July 2021. Available online: https://news.bms.com/news/corporate-financial/2021/Bristol-Myers-Squibb-Provides-Update-on-CheckMate--651-Trial-Evaluating-Opdivo-nivolumab-Plus-Yervoy-ipilimumab-Versus-EXTREME-Regimen-as-First-Line-Treatment-for-Squamous-Cell-Carcinoma-of-the-Head-and-Neck/default.aspx (accessed on 16 July 2021).
- Lucido, C.T.; Vermeer, P.D.; Wieking, B.G.; Vermeer, D.W.; Lee, J.H. CD137 enhancement of HPV positive head and neck squamous cell carcinoma tumor clearance. Vaccines 2014, 2, 841–853.
- Cohen, E.E.W.; Pishvaian, M.J.; Shepard, D.R.; Wang, D.; Weiss, J.; Johnson, M.L.; Chung, C.H.; Chen, Y.; Huang, B.; Davis, C.B.; et al. A phase Ib study of utomilumab (PF-05082566) in combination with mogamulizumab in patients with advanced solid tumors. J. Immunother. Cancer 2019, 7, 342.
- Andre, P.; Denis, C.; Soulas, C.; Bourbon-Caillet, C.; Lopez, J.; Arnoux, T.; Blery, M.; Bonnafous, C.; Gauthier, L.; Morel, A.; et al. Anti-NKG2A mAb Is a CheckpoInt. Inhibitor that Promotes Anti-tumor Immunity by Unleashing Both T and NK Cells. Cell 2018, 175, 1731–1743.e13.
- Borel, C.; Jung, A.C.; Burgy, M. Immunotherapy Breakthroughs in the Treatment of Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma. Cancers 2020, 12, 2691.
- Cohen, R.B.; Posner, G.L.M.R.; Bauman, J.R.; Salas, S.; Even, C.; Saada-Bouzid, E.; Seiwert, T.; Colevas, D.; Calmels, F.; Zerbib, R.; et al. Monalizumab in combination with cetuximab in patients (pts) with recurrent or metastatic (R/M) head and neck cancer (SCCHN) previously treated or not with PD-(L)1 inhibitors (IO): 1-year survival data. Ann. Oncol. 2019, 30, v460.
- Cohen, R.B.; Bauman, J.R.; Salas, S.; Colevas, A.D.; Even, C.; Cupissol, D.; Posner, M.R.; Lefebvre, G.; Saada-Bouzid, E.; Bernadach, M.; et al. Combination of monalizumab and cetuximab in recurrent or metastatic head and neck cancer patients previously treated with platinum-based chemotherapy and PD-(L)1 inhibitors. J. Clin. Oncol. 2020, 38 (Suppl. 15), 6516.
- Peng, S.; Ferrall, L.; Gaillard, S.; Wang, C.; Chi, W.Y.; Huang, C.H.; Roden, R.B.S.; Wu, T.C.; Chang, Y.N.; Hung, C.F. Development of DNA Vaccine Targeting E6 and E7 Proteins of Human Papillomavirus 16 (HPV16) and HPV18 for Immunotherapy in Combination with Recombinant Vaccinia Boost and PD-1 Antibody. mBio 2021, 12, e03224-20.
- Aggarwal, C.; Cohen, R.B.; Morrow, M.P.; Kraynyak, K.A.; Sylvester, A.J.; Knoblock, D.M.; Bauml, J.M.; Weinstein, G.S.; Lin, A.; Boyer, J.; et al. Immunotherapy Targeting HPV16/18 Generates Potent Immune Responses in HPV-Associated Head and Neck Cancer. Clin. Cancer Res. 2019, 25, 110–124.
- Aggarwal, C.; Saba, N.F.; Algazi, A.P.; Sukari, A.; Seiwer, T.; Haigentz, M.; Porosnicu, M.; Bonomi, M.; Boyer, J.; Durham, N.; et al. Gasco Hernandez, Safety and efficacy of MEDI0457 plus durvalumab in patients (pts) with human papillomavirus-associated recurrent/metastatic head and neck squamous cell carcinoma (HPV+ R/M HNSCC). Ann. Oncol. 2020, 31, S661–S662.
- Fesnak, A.D.; June, C.H.; Levine, B.L. Engineered T cells: The promise and challenges of cancer immunotherapy. Nat. Rev. Cancer 2016, 16, 566–581.
- Jin, B.Y.; Campbell, T.E.; Draper, L.M.; Stevanovic, S.; Weissbrich, B.; Yu, Z.; Restifo, N.P.; Rosenberg, S.A.; Trimble, C.L.; Hinrichs, C.S. Engineered T cells targeting E7 mediate regression of human papillomavirus cancers in a murine model. JCI Insight 2018, 3, e99488.




