
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Liberty Chipise | + 4054 word(s) | 4054 | 2022-01-05 08:49:34 | | | |
| 2 | Conner Chen | Meta information modification | 4054 | 2022-01-19 03:17:07 | | |
Video Upload Options
Conventional beneficiation of the Platinum Group of Metals (PGMs) relies on the use of inorganic chemicals. With the depreciation of high grade deposits, these conventional processes are becoming less economically viable. Furthermore, the use of chemicals has serious negative impacts on the environment. To address the challenges of conventional PGM beneficiation, biobeneficiation has been proposed. Bio-beneficiation is the concentration of mineral species by employing microorganisms that interact with either the gangue or the valuable mineral species. Bio-beneficiation can also be described as the use of microorganisms to interact with minerals to subsequently induce processes such as magnetic separation, flotation, and flocculation.
1. Typical Beneficiation Process of PGMs
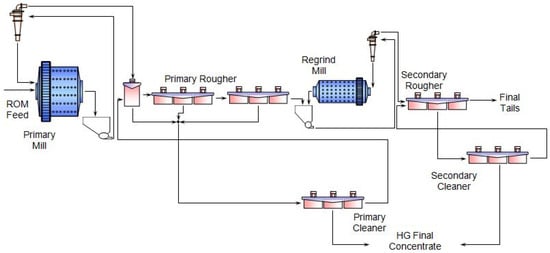
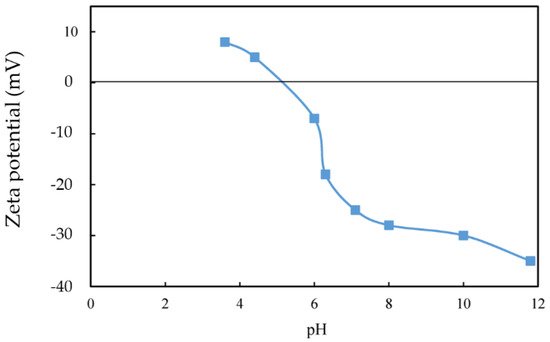
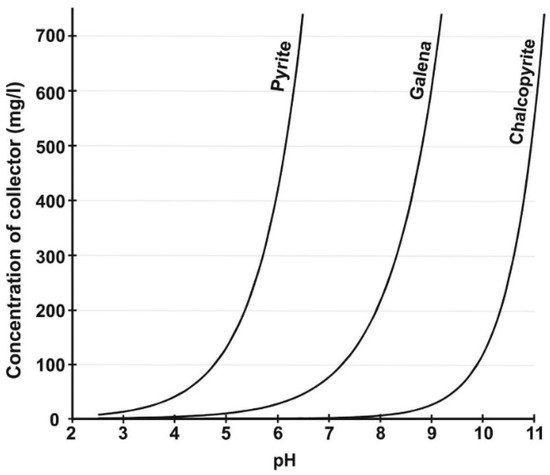
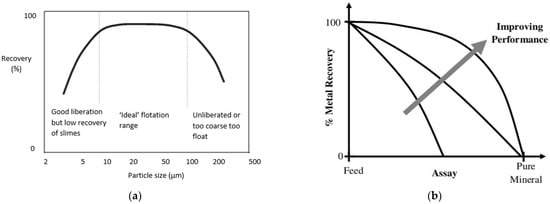
2. Biobeneficiation of Base Metal Sulphides Associated with PGMs
2.1. Bacillus polymyxa
2.2. Paenibacillus polymyxa
2.3. Mycobacterium phlei
2.4. Acidithiobacillus ferrooxidans
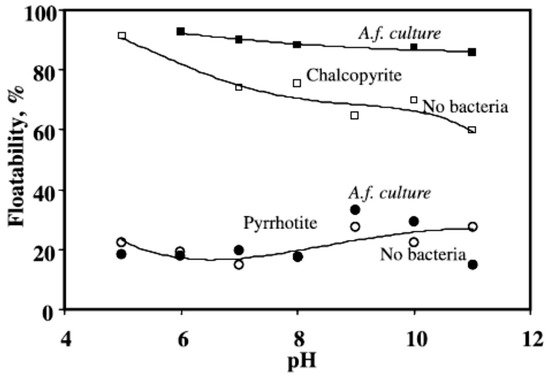
2.5. Acidithiobacillus thiooxidans
2.6. Leptospirillum ferrooxidans
2.7. Bacillus subtilis
2.8. Bacillus pumilus and Alicyclobacillus ferrooxidans
2.9. Halophilic Bacteria
2.10. Sulphate-Reducing Bacteria
2.11. Mixed Cultures
2.12. Biobeneficiation of Pentlandite
References
- Crabtree, E.H.; Vincent, J.D. The early days of froth flotation. In Froth Flotation—50th Anniversary; AIMME: New York, NY, USA, 1962; pp. 39–54.
- Engelbrecht, J. Potential Changes in the Physical Beneficiation Processes That Can Improve the Recovery Grade or Costs for the Platinum Group Metals. In Proceedings of the Fifth International Platinum Conference: A Catalyst for Change, Sun City, South Africa, 18–20 September 2012.
- Junge, M.; Oberthür, T.; Kraemer, D.; Melcher, F.; Piña, R.; Derrey, I.T.; Manyeruke, T.; Strauss, H. Distribution of platinum-group elements in pristine and near-surface oxidized Platreef ore and the variation along strike, northern Bushveld Complex, South Africa. Miner. Depos. 2019, 54, 885–912.
- Filippov, L.O.; Filippova, I.V.; Severov, V.V. The use of collectors mixture in the reverse cationic flotation of magnetite ore: The role of Fe-bearing silicates. Miner. Eng. 2010, 23, 91–98.
- Wark, I.W.; Cox, A.B. Principles of flotation, III. Trans. AIME 1934, 112, 267–301.
- Chandraprabha, M.N.; Natarajan, K.A. Surface chemical and flotation behaviour of chalcopyrite and pyrite in the presence of Acidithiobacillus thiooxidans. Hydrometallurgy 2006, 83, 146–152.
- Deo, N.; Natarajan, K.A. Interaction of Bacillus polymyxa with some oxide minerals with reference to mineral beneficiation and environmental control. Miner. Eng. 1997, 10, 1339–1354.
- Patra, P.; Natarajan, K.A. Role of mineral specific bacterial proteins in selective flocculation and flotation. Int. J. Miner. Process. 2008, 88, 53–58.
- Sharma, P.K.; Rao, K.H. Role of a heterotrophic Paenibacillus polymyxa bacteria in the bioflotation of some sulfide minerals. Mining Metall. Explor. 1999, 16, 35–41.
- Hosseini, T.R.; Kolahdoozan, M.; Tabatabaei, Y.S.M.; Oliazadeh, M.; Noaparast, M.; Eslami, A.F.S.A.R.; Manafi, Z.; Alfantazi, A. Bioflotation of Sarcheshmeh copper ore using Thiobacillus ferrooxidans bacteria. Miner. Eng. 2005, 18, 371–374.
- Botero, A.E.C.; Torem, M.L.; de Mesquita, L.M.S. Fundamental studies of Rhodococcus opacus as a biocollector of calcite and magnesite. Miner. Eng. 2007, 20, 1026–1032.
- Vasanthakumar, B.; Ravishankar, H.; Subramanian, S. A novel property of DNA—As a bioflotation reagent in mineral processing. PLoS ONE 2012, 7, e39316.
- Behera, S.K.; Mulaba-Bafubiandi, A.F. Microbes assisted mineral flotation a future prospective for mineral processing industries: A review. Miner. Process. Extr. 2017, 38, 96–105.
- Wills, B.A.; Naier-Munn, T. Froth Flotation. In Mineral Processing Technology: An Introduction to the Practical Aspects of Ore Treatment and Mineral Recovery, 7th ed.; Elsevier Science and Technology Books: Amsterdam, The Netherlands, 2013; pp. 267–352.
- Sanwani, E.; Chaerun, S.; Mirahati, R.; Wahyuningsih, T. Bioflotation: Bacteria-mineral interaction for eco-friendly and sustainable mineral processing. Procedia Chem. 2016, 19, 666–672.
- Sheni, N.; Corin, K.; Wiese, J. Considering the effect of pulp chemistry during flotation on froth stability. Miner. Eng. 2018, 116, 15–23.
- Pearse, M.J. An overview of the use of chemical reagents in mineral processing. Miner. Eng. 2005, 18, 139–149.
- Klassen, V.I.; Mokrousov, V.A. An Introduction to the Theory of Flotation; Butterworth: Oxford, UK, 1963.
- Bulatovic, S.M. Handbook of Flotation Reagents: Chemistry, Theory and Practice: Volume 1: Flotation of Sulfide Ores; Elsevier: Amsterdam, The Netherlands, 2007.
- Patra, P.; Natarajan, K.A. Microbially-induced flocculation and flotation for pyrite separation from oxide gangue minerals. Miner. Eng. 2003, 16, 965–973.
- Chandraprabha, M.N.; Natarajan, K.A. Microbially induced mineral beneficiation. Miner. Process. Extr. 2009, 31, 1–29.
- Patra, P.; Natarajan, K.A. Microbially enhanced removal of pyrite and chalcopyrite from oxide gangue minerals with reference to desulfurization of tailings. Mining Metall. Explor. 2004, 21, 169–178.
- Santhiya, D.; Subramanian, S.; Natarajan, K.A. Surface chemical studies on sphalerite and galena using extracellular polysaccharides isolated from Bacillus polymyxa. J. Colloid Interface Sci. 2002, 256, 237–248.
- Sharma, P.K.; Rao, K.H. Adhesion of Paenibacillus polymyxa on chalcopyrite and pyrite: Surface thermodynamics and extended DLVO theory. Colloids Surf. B Biointerfaces 2003, 29, 21–38.
- Misra, M.; Smith, R.W.; Dubel, J.; Chen, S. Selective flocculation of fine coal with hydrophobic Mycobacterium phlei. Mining Metall. Explor. 1993, 10, 20–23.
- Raichur, A.M.; Misra, M.; Smith, R.W. Differential adhesion of hydrophobic bacteria onto coal and associated minerals. Coal Prep. 1995, 16, 51–63.
- Raichur, A.M.; Misra, M.; Bukka, K.; Smith, R.W. Flocculation and flotation of coal by adhesion of hydrophobic Mycobacterium phlei. Colloids Surf. B Biointerfaces 1996, 8, 13–24.
- Chandraprabha, M.N.; Natarajan, K.A.; Modak, J.M. Selective separation of pyrite and chalcopyrite by biomodulation. Colloids Surf. B Biointerfaces 2004, 37, 93–100.
- Nagaoka, T.; Ohmura, N.; Saiki, H. A novel mineral flotation process using Thiobacillus ferrooxidans. Appl. Environ. 1999, 65, 3588–3593.
- Dwyer, R.; Bruckard, W.J.; Rea, S.; Holmes, R.J. Bioflotation and bioflocculation review: Microorganisms relevant for mineral beneficiation. Miner. Process. Extr. 2012, 121, 65–71.
- Kolahdoozan, M.; Tabatabaei Yazdi, S.M.; Yen, W.T.; Hosseini Tabatabaei, R.; Shahverdi, A.R.; Oliazadeh, M.; Noaparast, M.; Eslami, A.; Manafi, Z. Bioflotation of the low grade Sarcheshmeh copper sulfide. Trans. Indian Inst. Met. 2004, 57, 485–490.
- Atkins, A.S.; Bridgwood, E.W.; Davis, A.J.; Pooley, F.D. A study of the suppression of pyritic sulphur in coal froth flotation by Thiobacillus ferrooxidans. Coal Prep. 1987, 5, 1–13.
- Blake II, R.C.; Sasaki, K.; Ohmura, N. Does aporusticyanin mediate the adhesion of Thiobacillus ferrooxidans to pyrite? Hydrometallurgy 2001, 59, 357–372.
- Misra, M.; Chen, S. The effect of growth medium of Thiobacillus ferrooxidans on pyrite and galena flotation. Miner. Eng. 1996, 9, 157–168.
- Natrajan, K.A. Biotechnology for metal extraction, mineral beneficiation and environmental control. In Proceedings of the International Seminar on Mineral Processing Technology and Indo-Korean Workshop on Resource Recycling (MPT-2006), Chennai, India, 8–10 March 2006; Volume 1, pp. 68–81.
- Mehrabani, J.V.; Noaparast, M.; Mousavi, S.M.; Dehghan, R.; Rasooli, E.; Hajizadeh, H. Depression of pyrite in the flotation of high pyrite low-grade lead–zinc ore using Acidithiobacillus ferrooxidans. Miner. Eng. 2010, 23, 10–16.
- Vilinska, A.; Rao, K.H. Leptosririllum ferrooxidans-sulfide mineral interactions with reference to bioflotation and bioflocculation. Trans. Nonferrous Met. Soc. China 2008, 18, 1403–1409.
- Natarajan, K.A.; Das, A. Surface chemical studies on ‘Acidithiobacillus’ group of bacteria with reference to mineral flocculation. Int. J. Miner. Process 2003, 72, 189–198.
- Pecina-Treviño, E.T.; Ramos-Escobedo, G.T.; Gallegos-Acevedo, P.M.; López-Saucedo, F.J.; Orrantia-Borunda, E. Bioflotation of sulfide minerals with Acidithiobacillus ferrooxidans in relation to copper activation and surface oxidation. Can. J. Microbiol. 2012, 58, 1073–1083.
- Abramov, A.; Avdohin, V.M. Oxidation of Sulfide Minerals in Benefication Processes; Gordon and Breach Science Publishers: Amsterdam, The Netherlands, 1998.
- Leja, J. Collector Mechanism, I. Thiol collectors in sulfide minerals. In Surface Chemistry of Froth Flotation, 2nd ed.; Rao, S.R., Ed.; Springer: Boston, MA, USA, 2004; Volume 1, pp. 479–526.
- San Martín, F.; Kracht, W.; Vargas, T. Biodepression of pyrite using Acidithiobacillus ferrooxidans in seawater. Miner. Eng. 2018, 117, 127–131.
- San Martín, F.; Kracht, W.; Vargas, T.; Rudolph, M. Mechanisms of pyrite biodepression with Acidithiobacillus ferrooxidans in seawater flotation. Miner. Eng. 2020, 145, 106067.
- Attia, Y.A.; Elzeky, M.; Ismail, M. Enhanced separation of pyrite from oxidized coal by froth flotation using biosurface modification. Int. J. Miner. Process. 1993, 37, 61–71.
- Muzenda, E.; Afolabi, A.S.; Abdulkareem, A.S.; Ntuli, F. Effect of pH on the Recovery and grade of base metal sulphides (PGMs) by flotation. In Proceedings of the World Congress on Engineering and Computer Science, San Francisco, CA, USA, 19–21 October 2011; Volume 2, p. 2.
- La Vars, S.M.; Newton, K.; Quinton, J.S.; Cheng, P.Y.; Wei, D.H.; Chan, Y.L.; Harmer, S.L. Surface chemical characterisation of pyrite exposed to Acidithiobacillus ferrooxidans and associated extracellular polymeric substances. Minerals 2018, 8, 132.
- Jefferson, K.K. What drives bacteria to produce a biofilm? FEMS Microbiol. Lett. 2004, 236, 163–173.
- Das, A.; Modak, J.M.; Natarajan, K.A. Surface chemical studies of Thiobacillus ferrooxidans with reference to copper tolerance. Antonie Van Leeuwenhoek 1998, 73, 215–222.
- Bleeze, B.; Zhao, J.; Harmer, S.L. Selective attachment of Leptospirillum ferrooxidans for separation of chalcopyrite and pyrite through bio-flotation. Minerals 2018, 8, 86.
- Harneit, K.; Göksel, A.; Kock, D.; Klock, J.H.; Gehrke, T.; Sand, W. Adhesion to metal sulfide surfaces by cells of Acidithiobacillus ferrooxidans, Acidithiobacillus thiooxidans and Leptospirillum ferrooxidans. Hydrometallurgy 2006, 83, 245–254.
- He, Z.G.; Yang, Y.P.; Shan, Z.H.O.U.; Hu, Y.H.; Zhong, H. Effect of pyrite, elemental sulfur and ferrous ions on EPS production by metal sulfide bioleaching microbes. Trans. Nonferrous Met. Soc. China 2014, 24, 1171–1178.
- Díaz-López, C.V.; Pecina-Treviño, E.T.; Orrantia-Borunda, E. A study of bioflotation of chalcopyrite and pyrrhotite mixtures in presence of L. ferrooxidans. Can. Metall. Quart. 2012, 51, 118–125.
- Pecina, E.T.; Rodriguez, M.; Castillo, P.; Diaz, V.; Orrantia, E. Effect of Leptospirillum ferrooxidans on the flotation kinetics of sulphide ores. Miner. Eng. 2009, 22, 462–468.
- Sarvamangala, H.; Natarajan, K.A.; Girisha, S.T. Microbially-induced pyrite removal from galena using Bacillus subtilis. Int. J. Miner. Process 2013, 120, 15–21.
- Consuegra, G.L.; Kutschke, S.; Rudolph, M.; Pollmann, K. Halophilic bacteria as potential pyrite bio-depressants in Cu-Mo bioflotation. Miner. Eng. 2020, 145, 106062.
- Govender, Y.; Gericke, M. Extracellular polymeric substances (EPS) from bioleaching systems and its application in bioflotation. Miner. Eng. 2011, 24, 1122–1127.





