Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Inken Flörkemeier | + 932 word(s) | 932 | 2021-12-22 03:01:31 | | | |
| 2 | Nora Tang | Meta information modification | 932 | 2022-01-19 05:14:04 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Flörkemeier, I. The Dual Topoisomerase Inhibitor P8-D6 in Breast Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/18406 (accessed on 07 February 2026).
Flörkemeier I. The Dual Topoisomerase Inhibitor P8-D6 in Breast Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/18406. Accessed February 07, 2026.
Flörkemeier, Inken. "The Dual Topoisomerase Inhibitor P8-D6 in Breast Cancer" Encyclopedia, https://encyclopedia.pub/entry/18406 (accessed February 07, 2026).
Flörkemeier, I. (2022, January 18). The Dual Topoisomerase Inhibitor P8-D6 in Breast Cancer. In Encyclopedia. https://encyclopedia.pub/entry/18406
Flörkemeier, Inken. "The Dual Topoisomerase Inhibitor P8-D6 in Breast Cancer." Encyclopedia. Web. 18 January, 2022.
Copy Citation
Breast cancer constitutes the leading cause of cancer deaths among females. However, numerous shortcomings, including low bioavailability, resistance and significant side effects, are responsible for insufficient treatment. The ultimate goal, therefore, is to improve the success rates and, thus, the range available treatment options for breast cancer. Consequently, the identification, development and evaluation of potential novel drugs such as P8-D6 with seminal antitumor capacities have a high clinical need. P8-D6 effectively induces apoptosis by acting as a dual topoisomerase I/II inhibitor.
breast cancer
drug development
dual topoisomerase inhibitor
apoptosis
2D
3D
1. Introduction
Breast cancer (BC) is the most lethal malignancy diagnosed in women worldwide [1], given that 13% of women will develop BC in their lifetime and 15% will die [2]. Even though BC incidence has increased in recent years, the mortality rate has decreased, mainly due to earlier diagnosis and better treatment options. The major cause of chemotherapy failure in BC treatment is chemoresistance. A variety of mechanisms to avoid the cytotoxic effects of drugs can be activated in cancer cells, e.g., decreased influx or increased efflux of drugs, activated survival pathways, or enhanced DNA repair mechanisms [3]. Thus, the development of new drugs is highly warranted.
2. The Dual Topoisomerase Inhibitor P8-D6 in Breast Cancer
The new anticancer drug P8-D6, an aza-analogous Benzo[c]phenanthridine, was synthesized with a new simple and optimized four-step approach [4]. Moreover, P8-D6 appeared as an extremely suitable anticancer drug candidate, due to its physicochemical properties and strong cytotoxic activities. Its cytotoxicity is induced by dual topoisomerase (topo) I/II inhibition. Thereby, P8-D6 functions as a topo poison and covalently stabilizes the DNA-topoisomerase complex of both topo enzymes I and II [4][5]. Human DNA topos are essential modulators of DNA topology as these enzymes regulate the untangling and unwinding of DNA strands during cellular processes such as DNA transcription, replication or recombination [6][7][8]. Due to the extension of the DNA strand breaks through the stabilization of the topo-DNA-intermediate by P8-D6, cell death is initiated by inducing apoptosis [9]. This leads to a reduction in tumor cell survival (Figure 1).
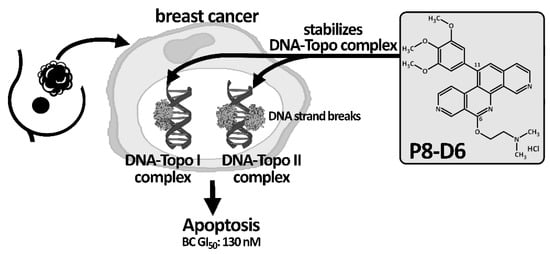
Figure 1. Schematic mechanism action of P8-D6. As a topo I/II poison, P8-D6 covalently stabilizes the enzyme–DNA complex and, thus, increases the amount of single- and double-strand DNA breaks and subsequently causes cell death. The effectiveness and the broad activity spectrum of P8-D6 has been verified in in vitro studies by various labs.
Initially, evaluation of the effectiveness of P8-D6 in cancer was tested by the National Cancer Institute, Maryland [10]. The NCI-60 DTP Human Tumor Cell Line screening resulted in an average growth inhibition of 50 % (GI50) over all 60 human tumor cell lines of 49 nM [4]. For BC cells, the result showed a GI50 value of 0.13 µM compared to cisplatin with a GI50 of 23.55 µM, etoposide with a GI50 of 1.39 µM or epirubicin with a GI50 of 0.14 µM, respectively [10]. P8-D6 also proved to be highly active in comparative studies with current standard therapeutics [11]. Cell-based two-dimensional (2D)- monolayer cell culture assays are an effective and established approach for initial drug testing [12], but three-dimensional (3D) structure models provide a more complex network of cell–matrix and cell–cell interactions, simulating the function of biological complexity more closely to in vivo settings [13][14][15]. The induction of apoptosis in BC cells was significantly higher by P8-D6 treatment compared to standard therapeutics and negative control [11]. As an example, P8-D6 increased the rate of apoptosis in MCF-7 cells by 24-fold compared to cisplatin and by 6-fold compared to topotecan. This breast cancer study clearly determined the high antitumor property of P8-D6 in molecularly different BC cell lines and ex vivo primary patient cells in 2D monolayers and 3D culture [11].
The development of resistances during chemotherapy contributes to therapy failure. The inhibition of both topoisomerases (topo I/II) can prevent the development of resistance, since when only one of the two enzymes is inhibited, the other is upregulated [16][17]. In addition to the high effectiveness, this is an essential advantage of dual topo inhibition. P8-D6 acts as such a dual topo I/II inhibition.
Topo inhibitors play an important role in breast cancer treatment. Topo II inhibitors, such as doxorubicin, are recognized as highly active drugs for breast cancer treatment, despite their dose-dependent cardiac toxicity [17][18]. New liposomal formulations decrease the side effect profile with similar efficacy [19][20]. Topo I inhibitors are used less frequently in the treatment of metastatic breast cancer. The development of resistances during chemotherapy is the main reason for therapy failure. The inhibition of both topoisomerases (topo I/II) can prevent the development of resistance, since when only one of the two enzymes is inhibited, the other is upregulated [16][21]. In addition to the high effectiveness, this is an essential advantage of dual topo inhibition. P8-D6 acts as such a dual topo I/II inhibitor.
3. Conclusions
Breast cancer remains a challenging cancer type to treat.Continuing the development of novel drugs with higher efficacy, lower resistance potential and fewer side effects is still a clinical need in BC therapy. In order to create new therapeutic options, the targeted, cell-based preclinical testing of new active drugs is essential and forms the basis of drug development.
This present study outlines the outstanding apoptotic effect of the dual topo inhibitor P8-D6 in BC cell lines and in a translational approach in ex vivo BC primary patient cells, in both 2D monolayers and 3D culture, compared to standard therapeutics [11]. In order to prove the benefit of P8-D6 treatment for BC therapy in multiorgan systems and to verify potential toxic or side effects, further in vivo experiments would be beneficial.
References
- Hyuna Sung; Jacques Ferlay; Rebecca L. Siegel; Mathieu Laversanne; Isabelle Soerjomataram; Ahmedin Jemal; Freddie Bray; Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer Journal for Clinicians 2021, 71, 209-249, 10.3322/caac.21660.
- Rebecca L. Siegel; Kimberly D. Miller; Hannah E. Fuchs; Ahmedin Jemal; Cancer Statistics, 2021. CA: A Cancer Journal for Clinicians 2021, 71, 7-33, 10.3322/caac.21654.
- Xiwei Ji; Yuan Lu; Huifang Tian; Xiangrui Meng; Minji Wei; William C. Cho; Chemoresistance mechanisms of breast cancer and their countermeasures. Biomedicine & Pharmacotherapy 2019, 114, 108800, 10.1016/j.biopha.2019.108800.
- Christopher Meier; Tamara N. Steinhauer; Fabian Koczian; Birte Plitzko; Katharina Jarolim; Ulrich Girreser; Simone Braig; Dr. Doris Marko; Dr. Angelika M. Vollmar; Dr. Bernd Clement; et al. A Dual Topoisomerase Inhibitor of Intense Pro-Apoptotic and Antileukemic Nature for Cancer Treatment. ChemMedChem 2017, 12, 347-352, 10.1002/cmdc.201700026.
- Georg Aichinger; Falk-Bach Lichtenberger; Tamara N. Steinhauer; Inken Flörkemeier; Giorgia Del Favero; Bernd Clement; Doris Marko; The Aza-Analogous Benzo[c]phenanthridine P8-D6 Acts as a Dual Topoisomerase I and II Poison, thus Exhibiting Potent Genotoxic Properties. Molecules 2020, 25, 1524, 10.3390/molecules25071524.
- Yves Pommier; Yilun Sun; Shar-Yin N. Huang; John Nitiss; Roles of eukaryotic topoisomerases in transcription, replication and genomic stability. Nature Reviews Molecular Cell Biology 2016, 17, 703-721, 10.1038/nrm.2016.111.
- J. E. Deweese; N. Osheroff; The DNA cleavage reaction of topoisomerase II: wolf in sheep's clothing. Nucleic Acids Research 2008, 37, 738-748, 10.1093/nar/gkn937.
- Yves Pommier; Juana M. Barcelo; V. Ashutosh Rao; Olivier Sordet; Andrew G. Jobson; Laurent Thibaut; Ze‐Hong Miao; Jennifer A. Seiler; Hongliang Zhang; Christophe Marchand; et al.Keli AgamaJohn L. NitissChristophe Redon Repair of Topoisomerase I‐Mediated DNA Damage. Progress in Nucleic Acid Research and Molecular Biology 2006, 81, 179-229, 10.1016/s0079-6603(06)81005-6.
- Yves Pommier; Elisabetta Leo; Hongliang Zhang; Christophe Marchand; DNA Topoisomerases and Their Poisoning by Anticancer and Antibacterial Drugs. Chemistry & Biology 2010, 17, 421-433, 10.1016/j.chembiol.2010.04.012.
- National Cancer Institute (NCI). DTP homepage: Cancer Screen 09/2014: NCI-60 DTP Human Tumor Cell Line Screen. The 392 National Cancer Institute (NCI) is gratefully acknowledged for its excellent screening service. . National Cancer Institute. Retrieved 2022-1-10
- Inken Flörkemeier; Tamara N. Steinhauer; Nina Hedemann; Jörg Paul Weimer; Christoph Rogmans; Marion T. van Mackelenbergh; Nicolai Maass; Bernd Clement; Dirk O. Bauerschlag; High Antitumor Activity of the Dual Topoisomerase Inhibitor P8-D6 in Breast Cancer. Cancers 2021, 14, 2, 10.3390/cancers14010002.
- Rasheena Edmondson; Jessica Jenkins Broglie; Audrey F. Adcock; Liju Yang; Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors. ASSAY and Drug Development Technologies 2014, 12, 207-218, 10.1089/adt.2014.573.
- Simon Lagies; Manuel Schlimpert; Simon Neumann; Astrid Wäldin; Bernd Kammerer; Christoph Borner; Lukas Peintner; Cells grown in three-dimensional spheroids mirror in vivo metabolic response of epithelial cells. Communications Biology 2020, 3, 1-10, 10.1038/s42003-020-0973-6.
- Lauren C. Kimlin; Giovanna Casagrande; Victoria M. Virador; In vitro three-dimensional (3D) models in cancer research: An update. Molecular Carcinogenesis 2011, 52, 167-182, 10.1002/mc.21844.
- Goodman, T.T.; Olive, P.L.; Pun, S.H.; Increased nanoparticle penetration in collagenase-treated multicellular spheroids. Int J 401 Nanomedicine 2007, 2, 265–274.
- Aronson, J.K.. Side Effects of Drugs Annual 27: Cytostatic drugs; Lipp, H.- P; Hartmann, J T; Stanley, A, Eds.; Elsevier Science: Amsterdam, 2004; pp. 477–479.
- Lankelma, J.; Dekker, H.; Luque, F.R.; Luykx, S.; Hoekman, K.; van der Valk, P.; van Diest, P.J.; Pinedo, H.M.; Doxorubicin 423 gradients in human breast cancer. Clin. Cancer Res 1999, 5, 1703–1707.
- C L Shapiro; P H Hardenbergh; R Gelman; D Blanks; P Hauptman; A Recht; D F Hayes; J Harris; I C Henderson; Cardiac effects of adjuvant doxorubicin and radiation therapy in breast cancer patients.. Journal of Clinical Oncology 1998, 16, 3493-3501, 10.1200/jco.1998.16.11.3493.
- L Ansari; F Shiehzadeh; Z Taherzadeh; S Nikoofal-Sahlabadi; A A Momtazi-Borojeni; A Sahebkar; S Eslami; The most prevalent side effects of pegylated liposomal doxorubicin monotherapy in women with metastatic breast cancer: a systematic review of clinical trials. Cancer Gene Therapy 2017, 24, 189-193, 10.1038/cgt.2017.9.
- Yesinia L Franco; Tanaya R Vaidya; Sihem Ait-Oudhia; Anticancer and cardio-protective effects of liposomal doxorubicin in the treatment of breast cancer. Breast Cancer: Targets and Therapy 2018, ume 10, 131-141, 10.2147/bctt.s170239.
- J. Robert; Laurent Rivory; Pharmacology of irinotecan. Drugs of Today 1998, 34, 777, 10.1358/dot.1998.34.9.485276.
More
Information
Subjects:
Oncology; Medicine, Research & Experimental
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
809
Revisions:
2 times
(View History)
Update Date:
19 Jan 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




