Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | HIMA ZAFAR | + 3782 word(s) | 3782 | 2022-01-17 06:50:47 | | | |
| 2 | Rita Xu | Meta information modification | 3782 | 2022-01-18 03:17:14 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zafar, H. Comprehensive Review on Wearable Sweat-Glucose Sensors for CGM. Encyclopedia. Available online: https://encyclopedia.pub/entry/18367 (accessed on 07 February 2026).
Zafar H. Comprehensive Review on Wearable Sweat-Glucose Sensors for CGM. Encyclopedia. Available at: https://encyclopedia.pub/entry/18367. Accessed February 07, 2026.
Zafar, Hima. "Comprehensive Review on Wearable Sweat-Glucose Sensors for CGM" Encyclopedia, https://encyclopedia.pub/entry/18367 (accessed February 07, 2026).
Zafar, H. (2022, January 17). Comprehensive Review on Wearable Sweat-Glucose Sensors for CGM. In Encyclopedia. https://encyclopedia.pub/entry/18367
Zafar, Hima. "Comprehensive Review on Wearable Sweat-Glucose Sensors for CGM." Encyclopedia. Web. 17 January, 2022.
Copy Citation
The incidence of diabetes is increasing at an alarming rate, and regular glucose monitoring is critical in order to manage diabetes. Currently, glucose in the body is measured by an invasive method of blood sugar testing. Blood glucose (BG) monitoring devices measure the amount of sugar in a small sample of blood, usually drawn from pricking the fingertip, and placed on a disposable test strip. Therefore, there is a need for non-invasive continuous glucose monitoring, which is possible using a sweat sensor-based approach.
biosensor
diabetes
hypoglycemia
sweat based sensing
wearable electronics
1. Introduction
Wearable and digital technologies are bringing innovations to enable individuals with the ability to monitor their fitness and health conditions regularly and non-invasively. These technologies can measure a wide range of physiological parameters, including heart rate and physical activity, but it is lacking in the capability to quantify biochemical parameters that are necessary for the management of a wide range of pathological health conditions. For example, hypoglycemia in which blood glucose level decreases lower than the normal range is a risk for diabetic patients, particularly after they perform intense exercise [1].
Similarly, diabetes being a chronic illness is characterized by an unusual increase in the level of blood sugar, which eventually causes serious damage to the heart, blood vessels, eyes, and kidneys. According to the World Health Organization (WHO) [2], around 422 million people worldwide suffer from diabetes. Regular blood sugar monitoring is very crucial to manage type 1 or type 2 diabetes. It aids the subject to know how the numbers go up or down when eating different foods, taking medicine, or being physically active. The awareness and ability to monitor blood glucose (BG) has led to a significant improvement in the management of diabetes. Combined with therapy and the right protocols, it will gradually halt the rise in diabetes or hypoglycemia issues among people.
Figure 1 presents the vast field of glucose measurement techniques and distinguishes three different categories: invasive, minimally invasive, and non-invasive approaches. Minimally Invasive (MI) technologies are those that need to extract some form of fluid from the body (i.e., tears, saliva, sweat, and interstitial fluid (ISF)) to measure the glucose concentration through an enzymatic approach. In invasive monitoring, there is direct detection of glucose in the blood. In noninvasive or at least minimally invasive methods, biological fluids are obtained which contain glucose at concentrations that correlate with that in blood. Because of this correlation, these alternative biofluids have become novel analytes of interest for the painless monitoring of glucose in the body.
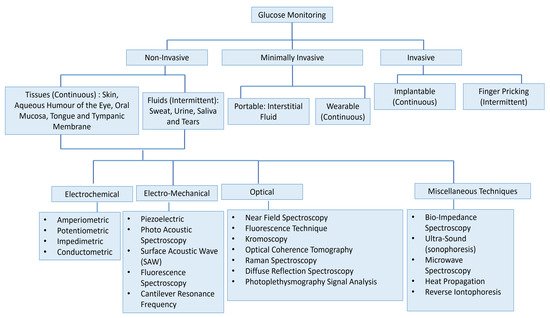
Figure 1. Overview of the numerous approaches for the measuring of glucose for intense insulin therapy. The figure concept adapted from [3].
Present BG tracking techniques, however, are invasive, unpleasant, and painful. Currently, a finger-prick test is a common method to obtain an insight of blood glucose level, which is enzyme-based and analyzed by in vitro methods using test strips and a glucometer [4][5]. The effectiveness of this method depends on strict compliance, which can be affected by time constraints or pain [6]. Additionally, it is not a continuous monitoring process and requires testing multiple times in a day in order to manage elevated glucose levels [7][8], especially after performing exercise, having meals, and dosing insulin. Furthermore, a non-continuous approach can miss periods of hyper- or hypoglycemia [6]. Nonetheless, there are implantable glucose monitoring systems that contribute in offering regular glucose monitoring, but these methods are not recommended for all diabetic patients due to their invasive nature, and some of these approaches have been reported to show inaccuracies up to 21% [9]. Thus, there is high consumer interest for a persistent glucose checking framework that can measure glucose levels without incessant calibration. In all the invasive techniques, the blood remains the most studied body fluid. However, in a non-invasive method, various considerations are needed to safeguard the accuracy and quality of measurements of glucose concentrations from these biofluids such as tears, ISF, saliva, and sweat. Among the major considerations, the glucose level in these biofluids is lower than that in blood. In the case of tears, the interference from impurities is relatively small but there are challenges associated with the energy supply for the glucose sensor on the contact lens to operate autonomously and transmit data wirelessly. In the case of saliva, the analyte is easily collected by spitting, but the large amount of impurities in saliva makes it difficult to isolate the inherent level of glucose from the fluid. In the case of ISF, a novel design for a continuous glucose monitoring system has been proposed, but it requires the subcutaneous injection of a needle, which can be uncomfortable to its prospective users. In comparison, the sweat-based glucose sensors are considered as one of the least intrusive solutions to estimate blood sugar level indirectly. However, certain challenges still exist regarding the use of sweat for sampling, the first being its clinical relevancy. Several well-known journals have already reviewed a number of wearable sweat glucose sensors, technologies, and devices, some of which are included hereafter. Toghill and Compton’s survey of electrochemical glucose sensing methods [10] over the previous decade gave an excellent overview of the many kinds of sensors that have been explored. Raman and infrared spectroscopy have received a lot of interest as non-invasive glucose detection techniques, and accordingly, spectroscopic techniques have increased in popularity [11][12][13]. Jayoung Kim et al. [14], in their review article, examined current developments and difficulties in the development of non-invasive epidermal electro-chemical glucose sensing devices focusing on skin interstitial fluid (ISF) and sweat bioanalytes. How to consistently collect a set amount of sweat before analyzing it was addressed in the review paper by Emma et al. [15]. The challenges of sweat sensing in conventional healthcare facilities have been discussed, outlining the fundamentals of human sweat, its properties and characteristics, sweat gland endocrine modeling, and ending with wearable sweat sensing devices being developed for research and commercialization.
2. History of Wearable Biosensors
In past years, wearable biosensors have spurred new developments in many innovative technologies in several domains, from environmental to biomedical. Wearable biosensors have the potential to provide continuous, real-time physiological information via dynamic, noninvasive measurements of biochemical markers in biofluids, such as sweat, tears, saliva, and interstitial fluid. These sensors can measure analytes that are extremely crucial to rapidly monitor changing biological fluids in real time. It comprises of an element of a biological recognition layer in the sensors which may interact particularly with a target molecule and a transducer that can convert this interaction into a measurable signal. The presence of a biological element (to be analyzed) and its easy availability under normal conditions are the basis of any biosensor. Sweat-based sensors rely on sweat’s ready availability and easy collection process to facilitate providing the particular bioanalyte for further detection and amplification. In wearable biosensors, numerous known components such as receptors, nucleic acids, cells, and several types of enzymes are used. These are the foundation blocks for creating and characterizing biosensors that use optical, colorimetric, or acoustic sensing principles. The working of these biosensors is explained in Figure 2. Sample containing the target analytes is collected through various mechanisms, which, in case of sweat, may take the form of a microfluidic mechanism. A bio-recognition layer performs the detection of the target analyte in the presence of many. A transducer layer converts the detector response to a measurable response, and subsequently, the result is presented via the readout system, wirelessly elsewhere or locally.
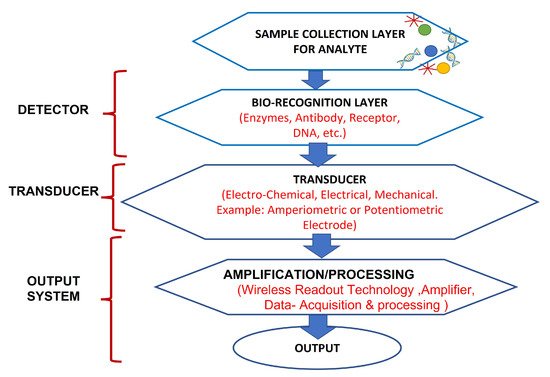
Figure 2. Working of biosensors: A close relationship between certain bio-recognition layers and electrical signal transaction. The figure concept adapted from [16].
In the beginning, blood glucose was targeted, and bio-recognition of glucose was facilitated through an enzyme-based electrode. In the 1960s, a glucose-oxidase-enzyme-based biosensor was established. Since then, several wearable electrochemical biosensors have been studied [17]. Specifically, in 1962, Clark and Lyons proposed the idea of enzyme-based electrodes, which relied on the following reaction:
(1)
It involves the GOx catalyzed oxidation of glucose by with the production of gluconic acid and . The generated is oxidized at the Pt (Noble Metal) electrode, and the electron flow induced by this oxidation reaction is proportional to the amount of glucose present in the blood sample. This is considered as first generation glucose sensor and has been widely used for the self-monitoring of BG levels. Later, GOx is immobilized on an amperometric oxygen electrode that measures the oxygen used by the biocatalytic process [16]. In 1963, 40 years after the discovery of insulin, Kadish [18] invented an insulin pump delivering insulin and glucagon (to prevent hypoglycemia). The first commercial subcutaneous insulin pump—the Auto Syringe—was introduced by Dean Kamen [19]. The need for blood glucose testing quickly grew.
In 1965, Ames developed the first blood glucose test strip [20], called Dextrostix, using glucose oxidase. This early strip was for physicians’ laboratories, not for home use. The variation of humidity and the low solubility in biological fluids (known as the “oxygen deficit”) could significantly influence the responses of this first generation glucose sensor performance. To overcome this limitation, the second generation glucose sensors were introduced in the mid-1970s. The concept of patients using BG data at home was contemplated by replacing the sensing layer of the sensor with a non-physiological redox mediator that can transport electrons from the GOx to the surface of the sensing electrode.
In 1971, in the United States, Anton Clemens [21] filed the first Patent for a BG monitor, also known as an Ames Reflectance Meter (AMF), for reliable point-of-care (POC) use in diabetic patients that may be performed at home. This second generation glucose meter (AMF) was used with the Dextrostix, requiring small volume of a blood (approximately 50–100 µL) for known and unknown concentrations of BG by automatically assessing the color changes of the strip. BG was previously calculated from a chart by interpreting the change in color, which was visible. The AMF followed the common Ames Eyetone, which was confined to clinical settings such as medical centers and hospital wards. When the possibility for external BG regulation was established by studies using intravenous glucose measurement and infusion of glucose and insulin [22], by Yellow Spring Instrument (YSI) Company, Yellow Springs, OH, USA purchased Clark’s electrochemical biosensor technology in 1975 and launched the first specialized BG analyzer (YSI Model 23 Analyzer).
Biosensors gained prominence in the late 1980s, when scientists attempted to develop revolutionary technologies that resulted in the development of a new class of glucose sensors known as enzyme-free or third and fourth generation glucose sensors [23] that enable wearable biosensors to monitor a person’s physiological biomarkers in real time. This decade observed the introduction of novel biosensor transduction concepts, including fiber-optic and mass sensitive (piezoelectric) devices, in response to the increasing focus on biotech. In the 1980s, commercial self-testing BG strips based on mediator-based enzyme electrodes were also launched. Efforts to adopt subcutaneous Continuous Glucose Monitoring (CGM) yielded a number of effective devices in the 1990s. Generally, the implanted amperometric biosensors monitor changes in glucose levels in the ISF dynamically and provide constant warnings when glucose levels drop. This non-invasive sensing approach displays the strong affiliation between the ISF and blood glucose levels. Despite the demonstrated benefits of CGM, its adoption was slow.
Due to the advent of nanotechnology in the late 1990s, a wide range of nanomaterial-based biosensors have been developed that make use of the appealing qualities of various nanomaterials, such as silicon and gold nanoparticles, for label-free and amplified biosensors, respectively, starting the fifth generation of the Advanced Wearable Biosensor Platform. A number of distinct DNA biosensors were developed in the late 1990s [24]. This led to significant advancement in biosensor technology over the last few years, paving the path for wearable biosensors. These subcutaneously implantable glucose sensors moved in the early 2000s to commercial wearable biosensors that track the real-time glucose level in the ISF, along with diabetes-relevant trends and patterns. A brief representative history of BG monitoring techniques is shown in Figure 3. As can be seen in the figure, the trend has always been towards noninvasive and continuous glucose monitoring. Today, sweat-based glucose sensing is the top contender there, though wearable optical methods are also very interesting, having similar benefits except for the technological challenges of some underlying mechanisms.
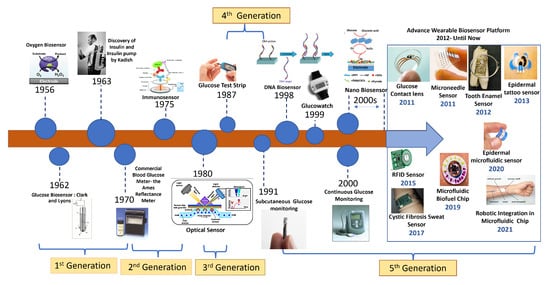
Figure 3. History of biosensor development for wearables from the beginning to the present.
3. Sweat as an Alternative Source of Glucose Monitoring
Sweat is considered as one of the crucial bio-liquids useful for non-invasive, continuous monitoring applications because of its particular nature. Sweat is the most freely available source of glucose, having the most sampling sites outside the body, consistent access, simplicity of assortment device placement, and availability of physiologically significant electrolytes and metabolites. In human subjects, sweat glucose (SG) has been successfully measured and reported in [25][26]. The association between SG and BG is also explicitly demonstrated [27][28]. Since sweat reaction occurs quickly and the sweat gland is highly vascularized, glucose levels inside the body can be estimated from sweat samples [29]. The concentration of glucose in human sweat is from 0.06–0.2 mM and corresponds to 3.3–17.3 mM in BG [29][30]. However, significant challenges remain in obtaining accurate sweat glucose data, such as changes in environmental parameters such as temperature, contamination from skin, sporadic sampling without iontophoretic incitement, low production rate, and the mixing of old samples with the new samples. Despite the good interaction, glucose level observation in sweat is extremely difficult in view of its low concentration (~100 times lower than BG), which therefore needs highly sensitive devices.
The growing demand of wearable sensors has helped researchers to develop an insight into various technical issues. A device named SwEatch was developed for sodium investigation in sweat and was manufactured utilizing 3D printing techniques by analysts in [31]. A similar device could be handily adjusted for glucose detecting in sweat by integrating a glucose sensor into the platform. Researchers in [32] manufactured sweat-sensing patches which can animate perspiration creation and detect analyte (sweat) concentrations wirelessly using a smartphone. Several other studies demonstrate sweat glucose monitoring systems using a patch type wearable platforms as illustrated in Table 1. Included in the table are some studies for comparison’s sake that are minimally invasive [33][34], making use of ISF as biofluid for glucose detection.
Table 1. Summarized studies on epidermal wearable glucose level monitoring systems.
| Wearability | Biofluid Type |
Sampling Method | Benefits | Next Steps | Reference |
|---|---|---|---|---|---|
| Eyeglasses sensor | Sweat | Exercise | Continuous monitoring of sweat glucose. Integration with wireless electronics. | Detail study with validation is required | [35] |
| Wearable patch with multimodal glucose sensor | Sweat | Exercise | Controlled sweat uptake. Improved accuracy of glucose sensing using multimodal sensing array and correction with sweat pH value. | Validation results required for continuous monitoring and replacement of commercial analyzer. | [36] |
| Graphene-based stretchable patch | Sweat | Exercise | Accurate monitoring by combination of pH, temperature, and humidity. Nanomaterials-based sensitive glucose sensor. | Increase sampling frequency and large-scale validation needed. | [37] |
| Wearable patch coupled with induced sweating | Sweat | Iontophoresis (stimulated) | Integration of iontophoresis sweat generation with glucose sensing. | Extension to on-body monitoring. | [38] |
| Multiplexed wearable, flexible array patch | Sweat | Exercise | Simultaneous multiplexed sweat sensing. Integration of customized wireless electronics. | Establish correlation to blood glucose, validation is also required. | [39] |
| Temporary tattoo | ISF | Reverse iontophoresis | Cost effective, easy to wear, and no skin irritation. | Single use, study stability, and reproducibility towards continuous use. | [33] |
| GlucoWatch | ISF | Reverse iontophoresis | FDA approved, provide continuous monitoring and electronics for measurement. | Minimize skin irritation, shorter warming up period, interference by sweat generation, and time lag compared to blood glucose. | [34] |
Sweat measurement is currently being used in applications such as the management of certain sicknesses, the prevention of alcohol dependence, monitoring of athletic training and recovery [39], etc. Observing glucose levels is important for managing fatigue levels in sports persons [40]. There are frequent users of advanced fitness wearables with built-in sweat sensors that aid in the detection of dehydration and water levels in the body. In developing nations, there is a rise in the adoption of wearable technology by people for patient care, early disease diagnosis, and point-of-care (POC) health monitoring. Consequently, sweat sensor companies are now selling hydration sensors to athletes and sports players to help them track their hydration needs [41]. Sports players and athletes are looking forward to a market with devices that sense and generate data for each body fluid to monitor various conditions. For example, cortisol sensors [42] are now used particularly for a few days to help measure human stress levels [43].
Although these research works achieved promising results in SG sensing accuracy, there are a few obstacles to overcome, such as real-time signal correction, long-term stability for continuous monitoring, and also the reproducibility between sensors and various patients and customized wireless electronics. The possible options for direct glucose monitoring include body fluids such as sweat, saliva [44], and tears [45]. The glucose density is 1 to 10 per cent of the blood density in these fluids. The long-term view is to develop sensors for integration in wearables such as clothing [46][47], bracelets [48][49][50], patches [51][52], and tattoos [39][41][53], which can sample a number of body indicators continuously. Some examples are given in Figure 4.
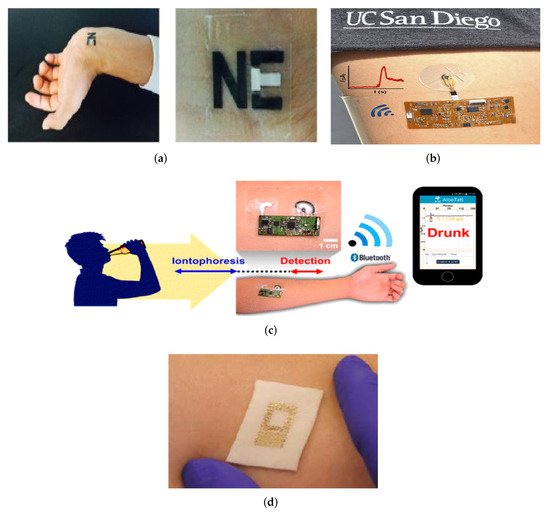
Figure 4. The direct application of sensors to skin, e.g., (a) Tattoo [54] (Reused with the permission of Copyright 2019, Elsevier), (b) Epidermal Microfluidic Electrochemical Detection System [55] (Reprinted with permission of Copyright© 2017, American Chemical Society), (c) Wearable Tattoo-Based Iontophoretic-Biosensing System for detecting Alcohol in Sweat [56] (Reused with permission of Copyright© 2016, American Chemical Society), (d) A passive wireless capacitive sensor [50] (Reused with the permission of Copyright 2014, John Wiley and Sons), addresses several difficulties in history. The technology is mechanically compatible with the skin, and all of the examples are important as a first step towards reducing perspiration between the skin and the sensors.
It is important to have a thorough understanding of sweat gland features and functionality before determining the most appropriate location for these sweat glucose monitoring sensors to be installed. Sweat glands are present all over the body, but are most numerous on the forehead, the armpits, the palms and the soles of the feet, as shown in Figure 5.
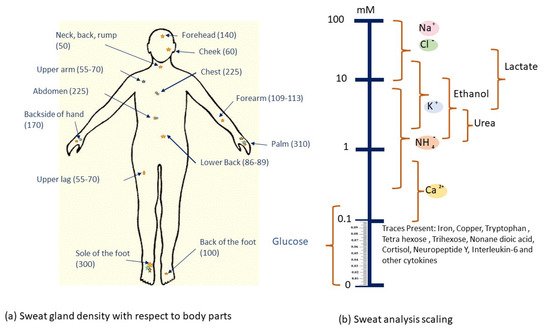
There are millions of eccrine glands [64] that are distributed across human skin and secrete liters of sweat per day. Eccrine sweat glands are present in several areas throughout the body with densities of more than 100 glands/cm2, allowing for a vast array of viable sampling sites. As a result, the argument for utilizing a wearable device to sample and detect sweat is clear. The main advantages and challenges of eccrine sweat are discussed further, and the issues will be quickly described as technological (perhaps directly solvable with enough effort) or fundamental (only indirect ways can be solved).
3.1. Benefits of Noninvasive Sweat Access to Bio Fluid
The easy availability of sweat on the skin allows for noninvasive and continuous access and sensing of a bioanalyte that contains health markers for a large number of conditions. One such technique is shown in Figure 6.
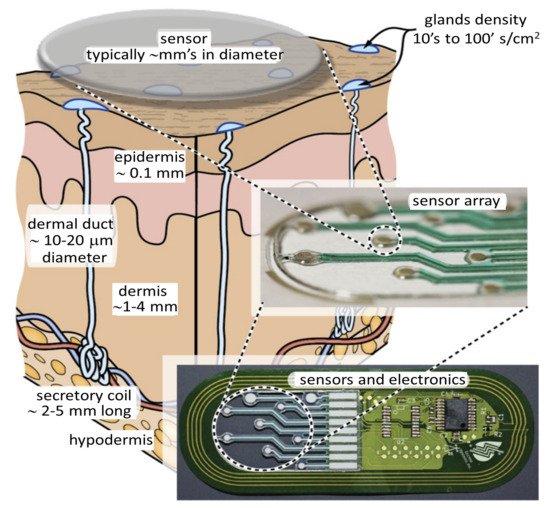
Unlike other unobtrusively accessible biofluids such as tears, urine, and saliva, sweat is more suitable for health monitoring. Tears can only be sampled once in a while, urine cannot be continuously accessed, and saliva cannot be used. Furthermore, saliva-based sensing may not be particularly accurate or very reliable as it is affected by the last meal the person had eaten [66]. Sweat-based sensing, however, suffers from often unknown correlation between the biomarkers present in sweat and those present in blood, considered the gold standard. While precise sweat-based detection is a challenging task, it is considered as an ideal candidate for continuous or semi-continuous monitoring over a prolonged period. This information can directly lead to detecting several pathologies. These sweat sensors can be placed in close proximity to sweat-generating sites, which allows for a fully wearable device with minimal sample degradation [67][68]. An analyte such as glucose is also present in the sweat, so using appropriate sweat collection method every several minutes, one can generate useful information about the level of glucose in the system.
3.2. Key Challenges in Using Sweat for Sensing
Despite the many benefits, sweat sensing requires huge efforts to address the following key challenges and in establishing a reliable correlation to gold standard BG measurements.
3.2.1. Exposure to Contaminants via the Skin
The skin may be contaminated by dead skin cells, sebum, analytes diffusing through the stratum carneum, and the condensate from trans-epidermal water loss, among other things. These issues must be taken into consideration while creating a sweat sensor, and special attention must be paid to signal analysis.
3.2.2. Quantity of Sweat Readily Available
For some patients, their sweat rate is in sub nano-liters per minute for each eccrine gland while they are in a resting condition. Small sampled quantities need to be rapidly moved between the collecting point and the sensing location via advanced microfluidics. In addition, the body’s temperature-regulating systems allows tiny skin droplets to evaporate quickly. Measures must be made in such a way that the microfluidic design is able to reduce evaporation, assure that sweat reaches the sensors, while avoiding the blockage of dried sweat components obstructing reliable and repeatable flow.
3.2.3. Deviations in Results Because of pH Differences
Changes in pH may affect sensor results, although this depends on the sensing technology used. Skin impurities, chronic conditions, or secreted components may all cause a pH shift. As a result, sensors need to be able to withstand large variations in operation without losing accuracy. Sweat sensors often need the addition of a pH sensor for calibration considerations.
3.2.4. Sweat Glands Periodic Activation
Sweat glands only generate sweat at certain times of the day or night. This must be considered while coming up with an appropriate collecting technique. Additionally, this has an impact on the resulting temporal resolution. This means that sampling within minutes may not be feasible since no sweat is produced when an eccrine gland is active for 30 s and then becomes in-active for 150 s [69].
3.2.5. Sampling Variability within and between People
There may be significant differences between individuals in terms of skin topography, sweat rate per gland, and the number of active sweat glands, as well as between measurement locations on a same person. Sweating output varies widely across individuals for a variety of reasons. In terms of inter-subject variability, physical development [70][71], hydration [72], diet [73], and adaptation to the new environment [74][75] are the most important factors. The time of day [76], the area of the body being sampled [73][77][78], and the technique of sweat induction [62] are major variables for inter-subject variability. Due to these factors, parameter estimation is complicated, and correlations between sweat and blood composition indicators may be disrupted. Because of this, procedures for sweat-based sensing may need more frequent measurements at various time intervals to track changes over the course of the measurement and statistical adjustments.
References
- Younk, L.M.; Mikeladze, M.; Tate, D.; Davis, S.N. Exercise-related hypoglycemia in diabetes mellitus. Expert Rev. Endocrinol. Metab. 2011, 6, 93–108.
- WHO. Diabetes. Available online: https://www.who.int/health-topics/diabetes#tab=tab_1 (accessed on 15 November 2021).
- Goodarzi, M.; Sharma, S.; Ramon, H.; Saeys, W. Multivariate calibration of NIR spectroscopic sensors for continuous glucose monitoring. TrAC Trends Anal. Chem. 2015, 67, 147–158.
- Bratlie, K.M.; York, R.L.; Invernale, M.A.; Langer, R.; Anderson, D.G. Materials for diabetes therapeutics. Adv. Healthc. Mater. 2012, 1, 267–284.
- Nathan, D.M.; DCCT/EDIC Research Group. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: Overview. Diabetes Care 2014, 37, 9–16.
- Bruen, D.; Delaney, C.; Florea, L.; Diamond, D. Glucose sensing for diabetes monitoring: Recent developments. Sensors 2017, 17, 1866.
- Badugu, R.; Lakowicz, J.R.; Geddes, C.D. Fluorescence sensors for monosaccharides based on the 6-methylquinolinium nucleus and boronic acid moiety: Potential application to ophthalmic diagnostics. Talanta 2005, 65, 762–768.
- Makaram, P.; Owens, D.; Aceros, J. Trends in nanomaterial-based non-invasive diabetes sensing technologies. Diagnostics 2014, 4, 27–46.
- Klonoff, D.C. Continuous glucose monitoring: Roadmap for 21st century diabetes therapy. Diabetes Care 2005, 28, 1231–1239.
- Toghill, K.E.; Compton, R.G. Electrochemical non-enzymatic glucose sensors: A perspective and an evaluation. Int. J. Electrochem. Sci. 2010, 5, 1246–1301.
- Yu, Z.; Jiang, N.; Kazarian, S.G.; Tasoglu, S.; Yetisen, A.K. Optical sensors for continuous glucose monitoring. Prog. Biomed. Eng. 2021, 3, 022004.
- Pandey, R.; Paidi, S.K.; Valdez, T.A.; Zhang, C.; Spegazzini, N.; Dasari, R.R.; Barman, I. Noninvasive monitoring of blood glucose with Raman spectroscopy. Accounts Chem. Res. 2017, 50, 264–272.
- Yadav, J.; Rani, A.; Singh, V.; Murari, B.M. Prospects and limitations of non-invasive blood glucose monitoring using near-infrared spectroscopy. Biomed. Signal Process. Control 2015, 18, 214–227.
- Kim, J.; Campbell, A.S.; Wang, J. Wearable non-invasive epidermal glucose sensors: A review. Talanta 2018, 177, 163–170.
- Moonen, E.J.; Haakma, J.R.; Peri, E.; Pelssers, E.; Mischi, M.; den Toonder, J.M. Wearable sweat sensing for prolonged, semicontinuous, and nonobtrusive health monitoring. View 2020, 1, 20200077.
- D’Orazio, P. Biosensors in clinical chemistry. Clin. Chim. Acta 2003, 334, 41–69.
- Clark, L.C., Jr.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45.
- Kadish, A.H. Automation Control of Blood Sugar. I. a Servomechanism for Glucose Monitoring and Control. Am. J. Med. Electron. 1964, 3, 82–86.
- Kesavadev, J.; Saboo, B.; Krishna, M.B.; Krishnan, G. Evolution of insulin delivery devices: From syringes, pens, and pumps to DIY artificial pancreas. Diabetes Ther. 2020, 11, 1251–1269.
- Hirsch, I.B. Introduction: History of glucose monitoring. In Role of Continuous Glucose Monitoring in Diabetes Treatment; American Diabetes Association: Arlington, VA, USA, 2018; Volume 1.
- Clemens, A.H. Blood Glucose Control Apparatus. U.S. Patent 4,151,845, 1 May 1979.
- Smith, E.; Kilpatrick, E. Intra-operative blood glucose measurements: The effect of haematocrit on glucose test strips. Anaesthesia 1994, 49, 129–132.
- Wang, J. Real-time electrochemical monitoring: Toward green analytical chemistry. Acc. Chem. Res. 2002, 35, 811–816.
- Robertson, D.L.; Joyce, G.F. Selection in vitro of an RNA enzyme that specifically cleaves single-stranded DNA. Nature 1990, 344, 467–468.
- Rothman, S. Physiology and Biochemistry of the Skin; University of Chicago Press: Chicago, IL, USA, 1954.
- Lobitz, W.; Osterberg, A. Chemistry of palmar sweat: III. Reducing substances (glucose). Arch. Dermatol. Syphilol. 1947, 56, 819–826.
- Olarte, O.; Chilo, J.; Pelegri-Sebastia, J.; Barbé, K.; Van Moer, W. Glucose detection in human sweat using an electronic nose. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine And Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 1462–1465.
- Wang, J. Electrochemical glucose biosensors. Chem. Rev. 2008, 108, 814–825.
- Moyer, J.; Wilson, D.; Finkelshtein, I.; Wong, B.; Potts, R. Correlation between sweat glucose and blood glucose in subjects with diabetes. Diabetes Technol. Ther. 2012, 14, 398–402.
- Bariya, M.; Nyein, H.; Javey, A. Wearable sweat sensors. Nat. Electron. 2018, 1, 160–171.
- Glennon, T.; O’Quigley, C.; McCaul, M.; Matzeu, G.; Beirne, S.; Wallace, G.; Stroiescu, F.; O’Mahoney, N.; White, P.; Diamond, D. ‘SWEATCH’: A wearable platform for harvesting and analysing sweat sodium content. Electroanalysis 2016, 28, 1283–1289.
- Heikenfeld, J. Let them see you sweat. IEEE Spectrum. 2014, 51, 46–63.
- Bandodkar, A.J.; Jia, W.; Yardımcı, C.; Wang, X.; Ramirez, J.; Wang, J. Tattoo-based noninvasive glucose monitoring: A proof-of-concept study. Anal. Chem. 2015, 87, 394–398.
- Tierney, M.J.; Tamada, J.A.; Potts, R.O.; Jovanovic, L.; Garg, S.; Cygnus Research Team. Clinical evaluation of the GlucoWatch® biographer: A continual, non-invasive glucose monitor for patients with diabetes. Biosens. Bioelectron. 2001, 16, 621–629.
- Sempionatto, J.R.; Nakagawa, T.; Pavinatto, A.; Mensah, S.T.; Imani, S.; Mercier, P.; Wang, J. Eyeglasses based wireless electrolyte and metabolite sensor platform. Lab Chip 2017, 17, 1834–1842.
- Lee, H.; Song, C.; Hong, Y.S.; Kim, M.S.; Cho, H.R.; Kang, T.; Shin, K.; Choi, S.H.; Hyeon, T.; Kim, D.H. Wearable/disposable sweat-based glucose monitoring device with multistage transdermal drug delivery module. Sci. Adv. 2017, 3, e1601314.
- Lee, H.; Choi, T.K.; Lee, Y.B.; Cho, H.R.; Ghaffari, R.; Wang, L.; Choi, H.J.; Chung, T.D.; Lu, N.; Hyeon, T.; et al. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat. Nanotechnol. 2016, 11, 566–572.
- Emaminejad, S.; Gao, W.; Wu, E.; Davies, Z.A.; Nyein, H.Y.Y.; Challa, S.; Ryan, S.P.; Fahad, H.M.; Chen, K.; Shahpar, Z.; et al. Autonomous sweat extraction and analysis applied to cystic fibrosis and glucose monitoring using a fully integrated wearable platform. Proc. Natl. Acad. Sci. USA 2017, 114, 4625–4630.
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016, 529, 509–514.
- Seshadri, D.R.; Rowbottom, J.R.; Drummond, C.; Voos, J.E.; Craker, J. A review of wearable technology: Moving beyond the hype: From need through sensor implementation. In Proceedings of the 2016 8th Cairo International Biomedical Engineering Conference (CIBEC), Cairo, Egypt, 15–17 December 2016; IEEE: Piscataway, NJ, USA, 2016; pp. 52–55.
- Bandodkar, A.J.; Molinnus, D.; Mirza, O.; Guinovart, T.; Windmiller, J.R.; Valdés-Ramírez, G.; Andrade, F.J.; Schöning, M.J.; Wang, J. Epidermal tattoo potentiometric sodium sensors with wireless signal transduction for continuous non-invasive sweat monitoring. Biosens. Bioelectron. 2014, 54, 603–609.
- Tech Briefs. Wearable Device Measures Cortisol in Sweat. Available online: https://www.techbriefs.com/component/content/article/tb/pub/techbriefs/bio-medical/38343 (accessed on 15 November 2021).
- Parlak, O. Portable and wearable real-time stress monitoring: A critical review. Sens. Actuators Rep. 2021, 3, 100036.
- Jung, D.G.; Jung, D.; Kong, S.H. A lab-on-a-chip-based non-invasive optical sensor for measuring glucose in saliva. Sensors 2017, 17, 2607.
- Yao, H.; Shum, A.J.; Cowan, M.; Lähdesmäki, I.; Parviz, B.A. A contact lens with embedded sensor for monitoring tear glucose level. Biosens. Bioelectron. 2011, 26, 3290–3296.
- Wang, C.; Shim, E.; Chang, H.K.; Lee, N.; Kim, H.R.; Park, J. Sustainable and high-power wearable glucose biofuel cell using long-term and high-speed flow in sportswear fabrics. Biosens. Bioelectron. 2020, 169, 112652.
- Toi, P.T.; Trung, T.Q.; Dang, T.M.L.; Bae, C.W.; Lee, N.E. Highly electrocatalytic, durable, and stretchable nanohybrid fiber for on-body sweat glucose detection. ACS Appl. Mater. Interfaces 2019, 11, 10707–10717.
- Song, Y.; Min, J.; Yu, Y.; Wang, H.; Yang, Y.; Zhang, H.; Gao, W. Wireless battery-free wearable sweat sensor powered by human motion. Sci. Adv. 2020, 6, eaay9842.
- Song, Y.; Min, J.; Gao, W. Wearable and implantable electronics: Moving toward precision therapy. ACS Nano 2019, 13, 12280–12286.
- Huang, X.; Liu, Y.; Chen, K.; Shin, W.J.; Lu, C.J.; Kong, G.W.; Patnaik, D.; Lee, S.H.; Cortes, J.F.; Rogers, J.A. Stretchable, wireless sensors and functional substrates for epidermal characterization of sweat. Small 2014, 10, 3083–3090.
- Baker, L.B.; Ungaro, C.T.; Barnes, K.A.; Nuccio, R.P.; Reimel, A.J.; Stofan, J.R. Validity and reliability of a field technique for sweat Na+ and K+ analysis during exercise in a hot-humid environment. Physiol. Rep. 2014, 2, e12007.
- Huestis, M.A.; Oyler, J.M.; Cone, E.J.; Wstadik, A.T.; Schoendorfer, D.; Joseph, R.E., Jr. Sweat testing for cocaine, codeine and metabolites by gas chromatography–mass spectrometry. J. Chromatogr. Biomed. Sci. Appl. 1999, 733, 247–264.
- Chen, Y.; Lu, S.; Zhang, S.; Li, Y.; Qu, Z.; Chen, Y.; Lu, B.; Wang, X.; Feng, X. Skin-like biosensor system via electrochemical channels for noninvasive blood glucose monitoring. Sci. Adv. 2017, 3, e1701629.
- Kim, J.; de Araujo, W.R.; Samek, I.A.; Bandodkar, A.J.; Jia, W.; Brunetti, B.; Paixao, T.R.; Wang, J. Wearable temporary tattoo sensor for real-time trace metal monitoring in human sweat. Electrochem. Commun. 2015, 51, 41–45.
- Martín, A.; Kim, J.; Kurniawan, J.F.; Sempionatto, J.R.; Moreto, J.R.; Tang, G.; Campbell, A.S.; Shin, A.; Lee, M.Y.; Liu, X.; et al. Epidermal microfluidic electrochemical detection system: Enhanced sweat sampling and metabolite detection. ACS Sens. 2017, 2, 1860–1868.
- Kim, J.; Jeerapan, I.; Imani, S.; Cho, T.N.; Bandodkar, A.; Cinti, S.; Mercier, P.P.; Wang, J. Noninvasive alcohol monitoring using a wearable tattoo-based iontophoretic-biosensing system. ACS Sens. 2016, 1, 1011–1019.
- Thomson, M. A comparison between the number and distribution of functioning eccrine sweat glands in Europeans and Africans. J. Physiol. 1954, 123, 225.
- McSwiney, B. The Composition of Human Perspiration (Samuel Hyde Memorial Lecture): (Section of Physical Medicine). Proc. R. Soc. Med. 1934, 27, 839.
- Sonner, Z.; Wilder, E.; Heikenfeld, J.; Kasting, G.; Beyette, F.; Swaile, D.; Sherman, F.; Joyce, J.; Hagen, J.; Kelley-Loughnane, N.; et al. The microfluidics of the eccrine sweat gland, including biomarker partitioning, transport, and biosensing implications. Biomicrofluidics 2015, 9, 031301.
- Katchman, B.A.; Zhu, M.; Blain Christen, J.; Anderson, K.S. Eccrine sweat as a biofluid for profiling immune biomarkers. Proteom. -Clin. Appl. 2018, 12, 1800010.
- Calderón-Santiago, M.; Priego-Capote, F.; Turck, N.; Robin, X.; Jurado-Gámez, B.; Sanchez, J.C.; De Castro, M.D.L. Human sweat metabolomics for lung cancer screening. Anal. Bioanal. Chem. 2015, 407, 5381–5392.
- Verde, T.; Shephard, R.; Corey, P.; Moore, R. Sweat composition in exercise and in heat. J. Appl. Physiol. 1982, 53, 1540–1545.
- Aruoma, O.; Reilly, T.; MacLaren, D.; Halliwell, B. Iron, copper and zinc concentrations in human sweat and plasma; the effect of exercise. Clin. Chim. Acta 1988, 177, 81–87.
- Cui, C.Y.; Schlessinger, D. Eccrine sweat gland development and sweat secretion. Exp. Dermatol. 2015, 24, 644–650.
- Rose, D.P.; Ratterman, M.E.; Griffin, D.K.; Hou, L.; Kelley-Loughnane, N.; Naik, R.R.; Hagen, J.A.; Papautsky, I.; Heikenfeld, J.C. Adhesive RFID sensor patch for monitoring of sweat electrolytes. IEEE Trans. Biomed. Eng. 2014, 62, 1457–1465.
- Heikenfeld, J.; Jajack, A.; Feldman, B.; Granger, S.W.; Gaitonde, S.; Begtrup, G.; Katchman, B.A. Accessing analytes in biofluids for peripheral biochemical monitoring. Nat. Biotechnol. 2019, 37, 407–419.
- Rohit, A.; Stapleton, F.; Brown, S.H.; Mitchell, T.W.; Willcox, M.D. Comparison of tear lipid profile among basal, reflex, and flush tear samples. Optom. Vis. Sci. 2014, 91, 1391–1395.
- Morzel, M.; Truntzer, C.; Neyraud, E.; Brignot, H.; Ducoroy, P.; Lucchi, G.; Canlet, C.; Gaillard, S.; Nicod, F.; Nicklaus, S.; et al. Associations between food consumption patterns and saliva composition: Specificities of eating difficulties children. Physiol. Behav. 2017, 173, 116–123.
- Chen, X.; Gasecka, P.; Formanek, F.; Galey, J.B.; Rigneault, H. In vivo single human sweat gland activity monitoring using coherent anti-Stokes Raman scattering and two-photon excited autofluorescence microscopy. Br. J. Dermatol. 2016, 174, 803–812.
- Inoue, Y.; Shibasaki, M.; Ueda, H.; Ishizashi, H. Mechanisms underlying the age-related decrement in the human sweating response. Eur. J. Appl. Physiol. Occup. Physiol. 1999, 79, 121–126.
- Weschler, L.B. Sweat electrolyte concentrations obtained from within occlusive coverings are falsely high because sweat itself leaches skin electrolytes. J. Appl. Physiol. 2008, 105, 1376–1377.
- Wilke, K.; Martin, A.; Terstegen, L.; Biel, S. A short history of sweat gland biology. Int. J. Cosmet. Sci. 2007, 29, 169–179.
- Costa, F.; Galloway, D.H.; Margen, S. Regional and total body sweat composition of men fed controlled diets. Am. J. Clin. Nutr. 1969, 22, 52–58.
- Amano, T.; Gerrett, N.; Inoue, Y.; Nishiyasu, T.; Havenith, G.; Kondo, N. Determination of the maximum rate of eccrine sweat glands’ ion reabsorption using the galvanic skin conductance to local sweat rate relationship. Eur. J. Appl. Physiol. 2016, 116, 281–290.
- Chen, W.; Elizondo, R. Peripheral modification of thermoregulatory function during heat acclimation. J. Appl. Physiol. 1974, 37, 367–373.
- Waterhouse, J.; Aizawa, S.; Nevill, A.; Edwards, B.; Weinert, D.; Atkinson, G.; Reilly, T. Rectal temperature, distal sweat rate, and forearm blood flow following mild exercise at two phases of the circadian cycle. Chronobiol. Int. 2007, 24, 63–85.
- Baker, L.B. Physiology of sweat gland function: The roles of sweating and sweat composition in human health. Temperature 2019, 6, 211–259.
- La Count, T.D.; Jajack, A.; Heikenfeld, J.; Kasting, G.B. Modeling glucose transport from systemic circulation to sweat. J. Pharm. Sci. 2019, 108, 364–371.
More
Information
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.4K
Revisions:
2 times
(View History)
Update Date:
18 Jan 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




