
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Carla Miranda | + 2378 word(s) | 2378 | 2021-09-07 09:57:20 |
Video Upload Options
A variety of lactic acid bacteria (LAB) strains, which are either part of the autochthonous microbiota or introduced into animal products, have potential beneficial applications for the preservation of such products and/or for consumer health. Many foods obtained from fermented products of animal origin, such as meat, fish, and dairy, contain living microorganisms that are phylogenetically similar to probiotic LAB as part of the microbiota that directs their fermentation process and is responsible for their unique character. Fermented foods, such as cultured milk, yogurt, cheese, fermented sausage, and certain types of wine, are obtained through enzymatic reactions resulting from controlled microbial growth, in which the main microbial effectors comprise, primarily, LAB and their metabolites.
1. Introduction
The unique characteristics that LAB have demonstrated in different biotechnological processes have fueled decades of research by the scientific community. This ongoing research effort has shown the importance of their ability to produce antimicrobial compounds for the control of pathogens, health-promoting applications, the process of food preservation, and agroindustry [1][2][3]. Antimicrobial peptides, such as bacteriocins, produced by LAB have been used against pathogenic microorganisms involved in distinct infections and to control food fermentations [4]. Bacteriocins can be produced directly in the food through the starter, adjunct, and/or bioprotective cultures, or they can be incorporated as preservatives, with the aim to improve food safety and quality [2][4][5]. LAB also hold promise as probiotics, which can aid in the management of pathological conditions, and as therapy adjuvants during the oral intake of antibiotics by modulating the intestinal microbiota of the patients [6][7][8][9].
Several studies have described the positive effects of LAB on human and animal health, as well as in food industry and agriculture [2][10][11]. In foods, LAB contribute to preservation and innovation [9][10]. In humans, the daily consumption of fermented foods and food supplements containing LAB has shown a global increasing trend [9] due to their multiple perceived benefits for human health [10]. The reported beneficial effects of LAB ingestion on human health mainly include the prevention of gut chronic diseases and colon cancer, immunomodulation, promotion of skin health, alleviation of allergic conditions, lactose intolerance, gastroenteritis, diarrhea and peptic ulcer, inhibition of uropathogens, control of plasma cholesterol levels, and production of neurotransmitters (e.g., gamma-aminobutyric acid) [2][9][10][12].
In animals, LAB are also employed to obtain health benefits. The gut microbiota of animals can easily undergo dysregulation (dysbiosis) due to stress, medication, and changes in diet. As probiotics, LAB are used in cattle, other ruminants, swine, poultry, and in beekeeping to improve overall animal health and enhance growth, reproductive performance, and disease resistance [11].
Many studies have reported a diversity of LAB applications in the control of infectious diseases, both in animals and humans, due to their significant antimicrobial activity and probiotic properties. These antimicrobial effects have also been applied to control and inhibit foodborne microorganisms, promoting the safety and preservation of foods and feeds. However, few studies have investigated the impacts of antimicrobial-resistant LAB emergence in animal products on humans and on the environment [13][14]. The main aim of this review is, therefore, to summarize and highlight the application of LAB in livestock and animal products, as well as to discuss the implications of LAB presence in animal products on the health of their human consumers.
2. LAB in Livestock Production
At any age, perturbations of the native gastrointestinal tract microbiota can have severe consequences for the health, productivity, and development in production animals [15]. To improve animal health by combatting dysbiosis, LAB have been administered to production animals as probiotics, enhancing growth and reproductive performance. In animals, probiotics also show beneficial effects on the treatment or prevention of infectious diseases, can contribute to the reduction in antibiotic use, or be employed as antibiotic alternatives [11][16].
The use of LAB as probiotics in livestock production has been the subject of numerous studies, in which novel strains with different origins and properties have been screened for use in several animal species. Dowarah et al. [17] isolated thirty LAB from piglet feces, most of which showed sensitivity to a variety of antibiotics, such as penicillin, lincomycin, erythromycin, chloramphenicol, and clindamycin, but were resistant to vancomycin and ciprofloxacin. They showed in vitro antibacterial activity against Klebsiella oxytoca, Escherichia coli, Salmonella enterica, and Staphylococcus intermedius. In addition, Pediococcus acidilactici demonstrated potential probiotic properties in vitro, leading to higher nutrient digestibility, antioxidant activity, and improved hematological profile in weaned piglets [17]. A diet supplemented with P. acidilactici not only maintained balance in the gastrointestinal microbiota, but also enhanced the physicochemical properties of swine meat and carcass quality in comparison to Lactobacillus acidophilus supplementation [18][19].
In poultry production, the effects of dietary supplementations with probiotics are well documented [16][20][21]. However, further studies are still necessary to determine the mechanisms of action and assess the optimal dose for multi-strain probiotics. Besides improving the health status of these animals, the use of probiotics can provide an alternative to antibiotic growth promoters, banned by the European Union in 2006, thereby contributing to address a global public health concern—the antibiotic resistance crisis [16][21]. For example, P. acidilactici from rectal samples of poultry showed significant antibacterial activities against E. coli and S. enterica [22]. The applications of LAB that have been selected on the basis of their in vitro immunomodulatory properties to control Salmonella infections in broilers proved to be advantageous. Feng et al. [23] reported that LAB with higher in vitro immunomodulatory activities, such as L. plantarum, L. salivarius, and P. acidilactici, were more efficient in achieving a reduction in Salmonella counts in the intestinal tract and in minimizing its adhesion to and invasion of broiler livers and spleens. Moreover, broilers fed diets containing Lactobacillus showed lower cecal coliform counts, as well as lower cholesterol levels than the control group [20].
In general, when applied as probiotics, beneficial LAB have the following positive effects in animals: prevention against colonization by (antibiotic-resistant) pathogens, modulation of the intestinal microbiota, reduction in inflammatory reactions, improvement of carcass traits, modulation of the immune response, performance enhancement, increase in nutrient digestibility and absorption, and decrease in urea and ammonia excretion (Figure 1) [11][16][21][24]. Therefore, dietary probiotics not only have effects on animal health and performance, but they also have potential commercial applications with impacts on the quality of direct products and byproducts resulting from livestock production [18][19][25].
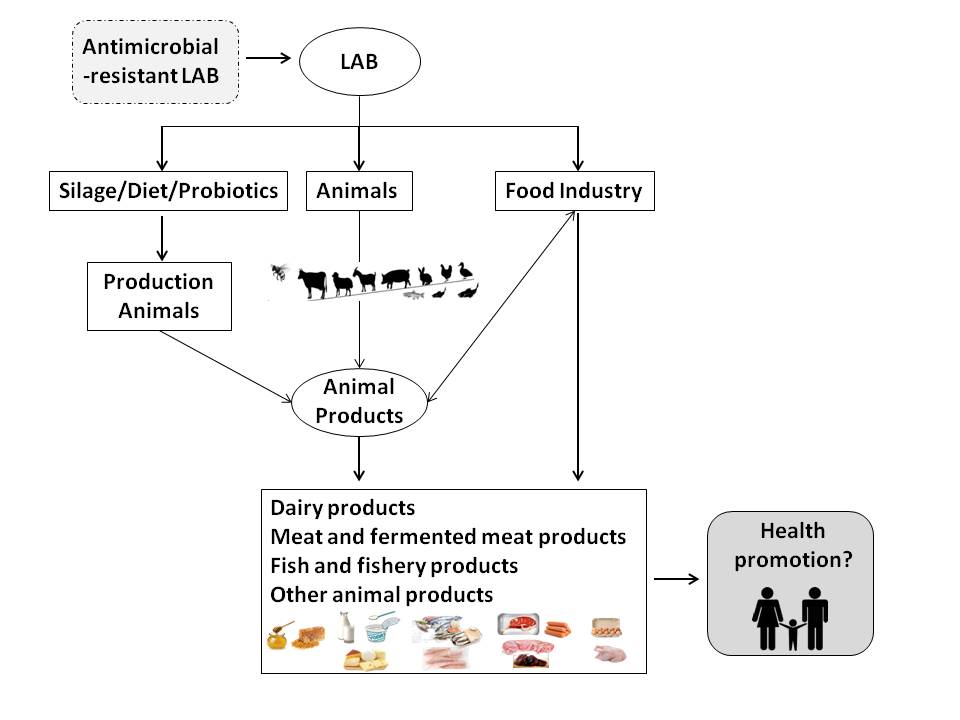
Figure 1. Application of lactic acid bacteria (LAB) in livestock production and animal products for human consumption and its potential implications for human health.
3. LAB Applications in Animal Products
A variety of LAB strains, which are either part of the autochthonous microbiota or introduced into animal products, have potential beneficial applications for the preservation of such products and/or for consumer health (Figure 1). Many foods obtained from fermented products of animal origin, such as meat, fish, and dairy, contain living microorganisms that are phylogenetically similar to probiotic LAB as part of the microbiota that directs their fermentation process and is responsible for their unique character [26]. Fermented foods, such as cultured milk, yogurt, cheese, fermented sausage, and certain types of wine, are obtained through enzymatic reactions resulting from controlled microbial growth, in which the main microbial effectors comprise, primarily, LAB and their metabolites. During the fermentation process, a transformation of the substrate takes place; bioactive or bioavailable metabolic end-products are formed, enhancing the organoleptic (flavor and texture) and nutritional (e.g., vitamin and protein content and bioavailability) properties of the fermentate, and antimicrobial metabolites (such as bacteriocins) can accumulate, reducing the risk of contamination with pathogenic microorganisms and contributing to the preservation of the end-product. In spite of these potentially beneficial results of the fermentative processes, the number of studies that evaluate the health benefits of including fermented feeds in animal diets is still very limited [26][27].
Although LAB have demonstrated enormous environmental and health benefits in fermented food products, which could lead to an increase in the demand and consumption of such products, regulation has, in some instances, limited their application [5][28][29]. Some LAB (e.g., the enterococci) are still devoid of qualified presumption of safety (QPS) and generally regarded as safe statuses in the European Union and in the United States of America, respectively [30]. Although genetic determinants of virulence, such as genes encoding host adhesion factors, have been found in these bacteria, it remains to be assessed whether these are virulence traits or merely reflect bacterial adaptations to promote host colonization as part of its beneficial microbiota [31]. The advances in functional genomics will contribute to fill this gap by providing a better understanding of the LAB–food/feed–host interactions [32].
LAB are ubiquitous in dairy environment and are an important part of the microbiota present in raw milk, fermented milks (such as yogurt, kefir, and viili), and cheeses [10][33][34]. A recent review reported a high LAB diversity isolated from traditional fermented dairy products, such as cheese, yogurt, kajmak, and sour cream, manufactured with bovine, ovine, and caprine raw milk without the addition of a starter culture. From the 28 LAB species isolated, the most prevalent genera were Enterococcus, Lactobacillus (in its former taxonomic composition), Streptococcus, Lactococcus, Leuconostoc, Weissella, and Pediococcus [35].
LAB can either be used as meat starter cultures and/or as probiotics, interacting with native microorganisms in the product, or they can be part of the non-starter microbiota in fermented products. In both cases, their presence can have potential advantages for the end-products [36][37]. Fermentation allows the preservation of meat products and the production of a variety of fermented meats with different organoleptic characteristics as a result of the microbial and endogenous enzymatic reactions taking place within their primary component—the animal muscle. The use of starter cultures, including probiotic microorganisms with health-promoting potential, such as the ability to reduce cholesterol content, contributes to the promotion of product stability and safety, as well as consumer acceptance [37][38][39].
4. Presence of Antimicrobial-Resistant LAB in Animal Food Products
Most LAB used in animal feed or animal-derived foods occur naturally in the animals (e.g., in their gastrointestinal tract) or in the resulting products. Although LAB with probiotic potential are considered non-pathogenic, the risks associated with the possible genetic transfer of antimicrobial resistance or toxin production to pathogenic microorganisms cannot be disregarded [24][40]. Horizontal transfer of antibiotic resistance genes may occur through mobile elements (integrons, transposons, and plasmids). In some strains of the former Lactobacillus genus, the presence of antibiotic resistance genes has been reported [13]. Some LAB, beyond acquired antibiotic resistances, also show intrinsic antibiotic resistance. For example, the best characterized intrinsic resistance in LAB is that of some lactobacilli to vancomycin. The presence of intrinsic antibiotic resistance genes is undesirable but may not constitute a safety issue, since LAB are very rarely involved in infections, and these genes are not easily mobilized and transferred to other pathogens [37].
Several studies on autochthonous food bacteria have highlighted the potential role of LAB as reservoirs of antibiotic resistance genes, from where resistance determinants could be transferred to the human microbiome via food chain [13][41][42][43]. For instance, in the production of dry-fermented sausages, antibiotic-resistant LAB may be introduced through contamination of the raw materials and added ingredients, fecal contamination, improper handling, environmental contamination, or cross-contamination, together with the lack of processing steps that allow the elimination of these microorganisms. The close contact among bacteria in these foods could facilitate the horizontal transfer of the antibiotic resistance genes they carry to pathogenic bacteria or to other LAB during processing [36][44]. The horizontal transfer of resistance genes between LAB ingested with foods and from LAB to other bacteria may also take place in the consumers’ gut [45].
Genetic determinants of tetracycline, vancomycin, and erythromycin resistance have been described in Enterococcus spp., Lac. lactis, and several lactobacilli from fermented meat and milk products [13][42]. The potential role of enterococci as reservoirs of antibiotic resistance genes in dairy products [44] and their particular propensity to trade these genetic determinants with other bacteria, including pathogens, is well known [46]. A recent study detected widespread resistance to several antibiotics in LAB from fermented dairy products (yogurt and a fermented dairy drink), of which several were lactobacilli (L. delbrueckii subsp. bulgaricus, L. plantarum, L. paracasei, and L. acidophilus) and Strep. salivarius subsp. thermophilus. In this study, most of the strains belonging to the former Lactobacillus genus were resistant to streptomycin (84%) and gentamycin (84%); less frequently, these strains showed resistance to erythromycin, sulfamethoxazole, and tetracycline. Most Strep. salivarius subsp. thermophilus also harbored resistance genes to the aminoglycosides streptomycin (92%) and gentamycin (87%), as well as to ciprofloxacin (79%), chloramphenicol (72%), and erythromycin (8%) [47]. In another study, L. plantarum isolated from raw-milk cheese showed erythromycin resistance [48]. The research on antibiotic resistance in probiotic microorganisms obtained from yogurts, yogurt-type fermented milk, and pharmaceutical products detected resistance to aztreonam, cycloserin, kanamycin, nalidixic acid, polymyxin B, and spectinomycin in all LAB strains belonging to the Streptococcus and former Lactobacillus genera, as well as in bifidobacteria [49]. Additionally, it has been demonstrated that L. delbrueckii subsp. bulgaricus and L. plantarum can successfully transfer the tetM and tetS tetracycline resistance genes to L. monocytogenes [47].
In meat products, all strains of Lac. lactis and L. plantarum demonstrated in vitro resistance to erythromycin, and some were also resistant to vancomycin, tetracycline, and chloramphenicol, as previously described [50]. Antibiotic resistance to vancomycin was also detected in LAB strains isolated from traditional salami [37]. Gevers et al. [44] analyzed LAB resistance to tetracycline along the process of fermented dry sausage, demonstrating the presence of resistant lactococci, lactobacilli, streptococci, and enterococci in the raw meat, but only resistant lactobacilli were persisted after fermentation. Both the tetM and tetS tetracycline resistance genes were found in the raw meat isolates, but tetM, found exclusively in the lactobacilli, was the only gene detected after sausage fermentation. This study confirms that raw meat already contains a subpopulation of resistant bacteria and that the resulting fermented meat can act as a vehicle for tetracycline-resistant LAB. However, Jha et al. [21] reported that probiotic use can reduce subtherapeutic antibiotic use in the poultry production and mitigate public health concerns with antibiotic resistance transfer via animal products. Additionally, there is not enough evidence that antibiotic resistance genes are transferred in poultry production by probiotic supplementation [21]. Therefore, at present, the benefits of antibiotic supplementation in food producing animals seem to outweigh the potential risks associated with the dissemination of antibiotic resistance genes (Figure 1). However, evaluating the resistome of LAB intended for food purposes is necessary to mitigate the dissemination of such genetic determinants to human communities, mediated by foods of animal origin.
References
- Saeed A. Hayek; Salam A. Ibrahim; Current Limitations and Challenges with Lactic Acid Bacteria: A Review. Food and Nutrition Sciences 2013, 04, 73-87, 10.4236/fns.2013.411a010.
- Mduduzi Paul Mokoena; Lactic Acid Bacteria and Their Bacteriocins: Classification, Biosynthesis and Applications against Uropathogens: A Mini-Review. Molecules 2017, 22, 1255, 10.3390/molecules22081255.
- Rakhmanova A; Khan ZA; Shah K.; A mini review fermentation and preservation: Role of lactic acid bacteria. MOJ Food Process Technol. 2018, 6, 414-417, 10.15406/mojfpt.2018.06.00197.
- Mattia Pia Arena; Vittorio Capozzi; Pasquale Russo; Djamel Dridier; Giuseppe Spano; Daniela Fiocco; Immunobiosis and probiosis: antimicrobial activity of lactic acid bacteria with a focus on their antiviral and antifungal properties. European Journal of Applied Microbiology and Biotechnology 2018, 102, 9949-9958, 10.1007/s00253-018-9403-9.
- Khalid, K.; An overview of lactic acid bacteria. Int. J. Biosci 2011, 1, 13.
- Maria Jose Saez-Lara; Carolina Gomez-Llorente; Julio Plaza-Diaz; Angel Gil; The Role of Probiotic Lactic Acid Bacteria and Bifidobacteria in the Prevention and Treatment of Inflammatory Bowel Disease and Other Related Diseases: A Systematic Review of Randomized Human Clinical Trials. BioMed Research International 2015, 2015, 1-15, 10.1155/2015/505878.
- Francesca De Filippis; Edoardo Pasolli; Danilo Ercolini; The food-gut axis: lactic acid bacteria and their link to food, the gut microbiome and human health. FEMS Microbiology Reviews 2020, 44, 454-489, 10.1093/femsre/fuaa015.
- Leah T Stiemsma; Reine E Nakamura; Jennifer G Nguyen; Karin B Michels; Does Consumption of Fermented Foods Modify the Human Gut Microbiota?. The Journal of Nutrition 2020, 150, 1680-1692, 10.1093/jn/nxaa077.
- Theodoros Varzakas; Microbiology of Fermented Foods and Beverages. Foods 2020, 9, 1660, 10.3390/foods9111660.
- Muhammad Irfan Masood; Muhammad Imran Qadir; Jafir Hussain Shirazi; Ikram Ullah Khan; Beneficial effects of lactic acid bacteria on human beings. Critical Reviews in Microbiology 2010, 37, 91-98, 10.3109/1040841x.2010.536522.
- Ornella Yolanda Ramos; Marina Basualdo; Carina Libonatti; María Fernanda Vega; Current status and application of lactic acid bacteria in animal production systems with a focus on bacteria from honey bee colonies. Journal of Applied Microbiology 2019, 128, 1248-1260, 10.1111/jam.14469.
- Yanhua Cui; Kai Miao; Siripitakyotin Niyaphorn; Xiaojun Qu; Production of Gamma-Aminobutyric Acid from Lactic Acid Bacteria: A Systematic Review. International Journal of Molecular Sciences 2020, 21, 995, 10.3390/ijms21030995.
- Shalini Mathur; Rameshwar Singh; Antibiotic resistance in food lactic acid bacteria—a review. International Journal of Food Microbiology 2005, 105, 281-295, 10.1016/j.ijfoodmicro.2005.03.008.
- John A. Hudson; Lynn J. Frewer; Glyn Jones; Paul A. Brereton; Mark J. Whittingham; Gavin Stewart; The agri-food chain and antimicrobial resistance: A review. Trends in Food Science & Technology 2017, 69, 131-147, 10.1016/j.tifs.2017.09.007.
- Carl J. Yeoman; Bryan A. White; Gastrointestinal Tract Microbiota and Probiotics in Production Animals. Annual Review of Animal Biosciences 2014, 2, 469-486, 10.1146/annurev-animal-022513-114149.
- Seyed Amin Kazemi; Hamed Ahmadi; Mohammad A. Karimi Torshizi; Evaluating two multistrain probiotics on growth performance, intestinal morphology, lipid oxidation and ileal microflora in chickens.. Journal of Animal Physiology and Animal Nutrition 2019, 103, 1399-1407, 10.1111/jpn.13124.
- Runjun Dowarah; Ashok Kumar Verma; Neeta Agarwal; Putan Singh; Bhoj Raj Singh; Selection and characterization of probiotic lactic acid bacteria and its impact on growth, nutrient digestibility, health and antioxidant status in weaned piglets. PLoS ONE 2018, 13, e0192978, 10.1371/journal.pone.0192978.
- Runjun Dowarah; Ashok Kumar Verma; Neeta Agarwal; Putan Singh; Efficacy of species-specific probiotic Pediococcus acidilactici FT28 on blood biochemical profile, carcass traits and physicochemical properties of meat in fattening pigs. Research in Veterinary Science 2018, 117, 60-64, 10.1016/j.rvsc.2017.11.011.
- Mamata Joysowal; B. N. Saikia; Runjun Dowarah; Shantanu Tamuly; D. Kalita; K. B. Dev Choudhury; Effect of probiotic Pediococcus acidilactici FT28 on growth performance, nutrient digestibility, health status, meat quality, and intestinal morphology in growing pigs. Veterinary World 2018, 11, 1669-1676, 10.14202/vetworld.2018.1669-1676.
- L. Z. Jin; Y. W. Ho; N Abdullah; S Jalaludin; Growth performance, intestinal microbial populations, and serum cholesterol of broilers fed diets containing Lactobacillus cultures. Poultry Science 1998, 77, 1259-1265, 10.1093/ps/77.9.1259.
- Rajesh Jha; Razib Das; Sophia Oak; Pravin Mishra; Probiotics (Direct-Fed Microbials) in Poultry Nutrition and Their Effects on Nutrient Utilization, Growth and Laying Performance, and Gut Health: A Systematic Review. Animals 2020, 10, 1863, 10.3390/ani10101863.
- N. Noohi; G. Ebrahimipour; M. Rohani; M. Talebi; M. R. Pourshafie; Evaluation of potential probiotic characteristics and antibacterial effects of strains of Pediococcus species isolated from broiler chickens. British Poultry Science 2016, 57, 317-323, 10.1080/00071668.2016.1169247.
- Junchang Feng; Lihong Wang; Luoxiong Zhou; Xin Yang; Xin Zhao; Using In Vitro Immunomodulatory Properties of Lactic Acid Bacteria for Selection of Probiotics against Salmonella Infection in Broiler Chicks. PLOS ONE 2016, 11, e0147630, 10.1371/journal.pone.0147630.
- FAO. Probiotics in animal nutrition – Production, impact and regulation, by Yadav S. Bajagai, Athol V. Klieve, Peter J. Dart and Wayne L. Bryden. Editor Harinder P.S. Makkar. FAO Animal Production and Health Paper No. 179. Rome, Italy, 2016.
- D. Mikulski; Jan Jankowski; J. Naczmanski; M. Mikulska; V. Demey; Effects of dietary probiotic (Pediococcus acidilactici) supplementation on performance, nutrient digestibility, egg traits, egg yolk cholesterol, and fatty acid profile in laying hens. Poultry Science 2012, 91, 2691-2700, 10.3382/ps.2012-02370.
- Eirini Dimidi; Selina Cox; Megan Rossi; Kevin Whelan; Fermented Foods: Definitions and Characteristics, Impact on the Gut Microbiota and Effects on Gastrointestinal Health and Disease. Nutrients 2019, 11, 1806, 10.3390/nu11081806.
- Maria L Marco; Dustin Heeney; Sylvie Binda; Christopher J Cifelli; Paul Cotter; Benoit Foligne; Michael Gänzle; Remco Kort; Gonca Pasin; Anne Pihlanto; et al.Eddy J SmidRobert Hutkins Health benefits of fermented foods: microbiota and beyond. Current Opinion in Biotechnology 2017, 44, 94-102, 10.1016/j.copbio.2016.11.010.
- Hill, D.; Sugrue, I.; Arendt, E.; Hill, C.; Stanton, C.; Ross, R.P.; Recent advances in microbial fermentation for dairy and health. . F1000Res 2017, 6, 751, 10.12688/f1000research.
- Leroy, F.; De Vuyst, L.; Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends in Food Sci Technol 2004, 15, 67-78.
- Maria Dapkevicius; Bruna Sgardioli; Sandra Câmara; Patrícia Poeta; Francisco Malcata; Current Trends of Enterococci in Dairy Products: A Comprehensive Review of Their Multiple Roles. Foods 2021, 10, 821, 10.3390/foods10040821.
- Ken Graham; Helena Stack; Rosemary Rea; Safety, beneficial and technological properties of enterococci for use in functional food applications – a review. Critical Reviews in Food Science and Nutrition 2020, 60, 3836-3861, 10.1080/10408398.2019.1709800.
- Francois P Douillard; Willem M De Vos; Functional genomics of lactic acid bacteria: from food to health. Microbial Cell Factories 2014, 13, S8-S8, 10.1186/1475-2859-13-s1-s8.
- K. Makarova; A. Slesarev; Yuri Wolf; A. Sorokin; Boris Mirkin; E. Koonin; A. Pavlov; N. Pavlova; V. Karamychev; N. Polouchine; et al.V. ShakhovaIgor GrigorievY. LouD. RohksarS. LucasK. HuangDavid GoodsteinT. HawkinsV. PlengvidhyaD. WelkerJ. HughesYong Jun GohA. BensonK. BaldwinJ.- H. LeeI. Diaz-MunizB. DostiVladimir SmeianovW. WechterR. BaraboteG. LorcaEric AltermannRodolphe BarrangouB. GanesanY. XieH. RawsthorneD. TamirC. ParkerF. BreidtJeff BroadbentR. HutkinsD. O'SullivanJ. SteeleGulhan UnluM. SaierT. KlaenhammerP. RichardsonSergei KozyavkinB. WeimerD. Mills Comparative genomics of the lactic acid bacteria. Proceedings of the National Academy of Sciences 2006, 103, 15611-15616, 10.1073/pnas.0607117103.
- Fanny George; Catherine Daniel; Muriel Thomas; Elisabeth Singer; Axel Guilbaud; Frédéric J. Tessier; Anne-Marie Revol-Junelles; Frédéric Borges; Benoît Foligné; Occurrence and Dynamism of Lactic Acid Bacteria in Distinct Ecological Niches: A Multifaceted Functional Health Perspective. Frontiers in Microbiology 2018, 9, 2899, 10.3389/fmicb.2018.02899.
- Amarela Terzić-Vidojević; Katarina Veljović; Maja Tolinački; Milica Živković; Jovanka Lukić; Jelena Lozo; Đorđe Fira; Branko Jovčić; Ivana Strahinić; Jelena Begović; et al.Nikola PopovićMarija MiljkovićMilan KojicLjubiša TopisirovićNataša Golić Diversity of non-starter lactic acid bacteria in autochthonous dairy products from Western Balkan Countries - Technological and probiotic properties. Food Research International 2020, 136, 109494, 10.1016/j.foodres.2020.109494.
- Maria João Fraqueza; Antibiotic resistance of lactic acid bacteria isolated from dry-fermented sausages. International Journal of Food Microbiology 2015, 212, 76-88, 10.1016/j.ijfoodmicro.2015.04.035.
- Svetoslav Dimitrov Todorov; Saso Stojanovski; Ilia Iliev; Penka Moncheva; Luis Augusto Nero; Iskra Vitanova Ivanova; Technology and safety assessment for lactic acid bacteria isolated from traditional Bulgarian fermented meat product “lukanka”. Brazilian Journal of Microbiology 2017, 48, 576-586, 10.1016/j.bjm.2017.02.005.
- Frédéric Leroy; Jurgen Verluyten; Luc De Vuyst; Functional meat starter cultures for improved sausage fermentation. International Journal of Food Microbiology 2006, 106, 270-285, 10.1016/j.ijfoodmicro.2005.06.027.
- Rosa Capita; Sandra Llorente-Marigómez; Miguel Prieto; Carlos Alonso-Calleja; Microbiological Profiles, pH, and Titratable Acidity of Chorizo and Salchichón (Two Spanish Dry Fermented Sausages) Manufactured with Ostrich, Deer, or Pork Meat. Journal of Food Protection 2006, 69, 1183-1189, 10.4315/0362-028x-69.5.1183.
- Marion Bernardeau; Jean Paul Vernoux; Ségolène Henri-Dubernet; Micheline Guéguen; Safety assessment of dairy microorganisms: The Lactobacillus genus. International Journal of Food Microbiology 2008, 126, 278-285, 10.1016/j.ijfoodmicro.2007.08.015.
- Surya Chandra Rao Thumu; Prakash M. Halami; Presence of erythromycin and tetracycline resistance genes in lactic acid bacteria from fermented foods of Indian origin. Antonie van Leeuwenhoek 2012, 102, 541-551, 10.1007/s10482-012-9749-4.
- Yiming Li; Lili Li; Sofie Kromann; Miaorui Chen; Lei Shi; Hecheng Meng; Antibiotic Resistance of Lactobacillus spp. and Streptococcus thermophilus Isolated from Chinese Fermented Milk Products. Foodborne Pathogens and Disease 2019, 16, 221-228, 10.1089/fpd.2018.2516.
- S. P. A. Câmara; A. Dapkevicius; C. C. G. Silva; F. X. Malcata; Maria L. N. Enes Dapkevicius; Artisanal Pico cheese as reservoir of Enterococcus species possessing virulence and antibiotic resistance properties: implications for food safety. Food Biotechnology 2020, 34, 25-41, 10.1080/08905436.2019.1710844.
- Dirk Gevers; Liesbeth Masco; Leen Baert; Geert Huys; Johan Debevere; Jean Swings; Prevalence and Diversity of Tetracycline Resistant Lactic Acid Bacteria and their tet Genes Along the Process Line of Fermented Dry Sausages. Systematic and Applied Microbiology 2003, 26, 277-283, 10.1078/072320203322346137.
- Carol A. van Reenen; Leon M. T. Dicks; Horizontal gene transfer amongst probiotic lactic acid bacteria and other intestinal microbiota: what are the possibilities? A review. Archives of Microbiology 2010, 193, 157-168, 10.1007/s00203-010-0668-3.
- Guido Werner; Teresa M. Coque; Charles M.A.P. Franz; Elisabeth Grohmann; Kristin Hegstad; Lars Bogø Jensen; Willem van Schaik; Keith Weaver; Antibiotic resistant enterococci—Tales of a drug resistance gene trafficker. International Journal of Medical Microbiology 2013, 303, 360-379, 10.1016/j.ijmm.2013.03.001.
- Chao Yang; Tao Yu; Characterization and transfer of antimicrobial resistance in lactic acid bacteria from fermented dairy products in China. The Journal of Infection in Developing Countries 2019, 13, 137-148, 10.3855/jidc.10765.
- Louise Feld; Eliza Bielak; Karin Hammer; Andrea Wilcks; Characterization of a small erythromycin resistance plasmid pLFE1 from the food-isolate Lactobacillus plantarum M345. Plasmid 2009, 61, 159-170, 10.1016/j.plasmid.2009.01.002.
- Maria Rosaria D'Aimmo; Monica Modesto; Bruno Biavati; Antibiotic resistance of lactic acid bacteria and Bifidobacterium spp. isolated from dairy and pharmaceutical products. International Journal of Food Microbiology 2007, 115, 35-42, 10.1016/j.ijfoodmicro.2006.10.003.
- Izildinha Moreno; Elza Teresinha Grael Marasca; Patrícia Blumer Zacarchenco Rodrigues De Sá; Josiane De Souza Moitinho; Míriam Marquezini; Márcia Regina Cucatti Alves; Renata Bromberg; Evaluation of Probiotic Potential of Bacteriocinogenic Lactic Acid Bacteria Strains Isolated from Meat Products. Probiotics and Antimicrobial Proteins 2018, 10, 762-774, 10.1007/s12602-018-9388-9.





