Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Virgile GUENEAU | + 1855 word(s) | 1855 | 2021-12-30 04:56:14 | | | |
| 2 | Amina Yu | Meta information modification | 1855 | 2022-01-18 01:48:37 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gueneau, V. Environmental Biofilms in Livestock Buildings. Encyclopedia. Available online: https://encyclopedia.pub/entry/18343 (accessed on 07 February 2026).
Gueneau V. Environmental Biofilms in Livestock Buildings. Encyclopedia. Available at: https://encyclopedia.pub/entry/18343. Accessed February 07, 2026.
Gueneau, Virgile. "Environmental Biofilms in Livestock Buildings" Encyclopedia, https://encyclopedia.pub/entry/18343 (accessed February 07, 2026).
Gueneau, V. (2022, January 17). Environmental Biofilms in Livestock Buildings. In Encyclopedia. https://encyclopedia.pub/entry/18343
Gueneau, Virgile. "Environmental Biofilms in Livestock Buildings." Encyclopedia. Web. 17 January, 2022.
Copy Citation
Biofilms are three-dimensional biological structures composed of microbial communities embedded in cohesive self-produced extracellular polymeric substances (EPS). EPS can drastically vary between biofilms but is generally composed of water and a complex mixture of polysaccharides, extracellular DNA (eDNA), proteins, and amyloid fibers. The presence of EPS, along with spatial organization and specific signaling systems, triggers a diversification of cell types and emerging community functions, including a fantastic adaptation to environmental fluctuations and the action of antimicrobials, in comparison to their free planktonic homologs.
biofilm
sampling
livestock building
surfaces
diversity
1. Introduction
Current knowledge about surface-associated microbial communities in livestock buildings is still very limited, while of prime importance to decipher microbial pathogens dynamics in these environments and their interactions with animals. These questions enter in the One Health context by pinpointing the flow of pathogens between the environment, animals, and humans. Furthermore, national and international regulations are evolving to limit the use of antimicrobials such as antibiotics (to prevent antibiotic resistance) and disinfectants (to prevent environmental pollution) [1]. The development of innovative and alternative strategies are hence explored and are needed to create scientific knowledge and methodologies to analyze complex multispecies biofilms on livestock building surfaces and their dynamics under perturbations [2][3].
In farms, most pathogenic microorganisms responsible for zoonosis can be associated and protected within environmental biofilms. Holobiont of animals is in constant interplay with the biofilms from the farm environment. These biofilms may constitute an environmental route for animal and human contamination [4]. Moreover, because of spatial proximity and high cell density, biofilms are considered as hot spots for the spreading of antibiotic resistance genes and virulence factors by horizontal gene transfer [5][6][7]. Quantification of undesirable species such as bacterial pathogens is hence carried out in livestock buildings regularly to monitor its hygienic status and to comply with eventual national or international regulations. The sampling methodologies used are principally swabs, sponges, and contact plates with specific media [8][9]. Nowadays, there is no chemical or physical methodology able to extract all surface-associated communities from a surface to study it [10][11], and the extracted fraction may not be representative of the initial sessile community [12]. Another strong limitation of these sampling methods is the definitive loss of the biofilm spatial organization that is a major factor of microbial persistence on surfaces.
Indeed, protocols with coupons, defined here as a small surface of a specific material where the biofilm can develop, have been designed and used to capture in-situ and non-invasively the microbial community of interest in other systems [13]. These types of sampling methods were in particular successively applied to describe biofilms in aquatic conditions (biocorrosion in the sea, drinking water distribution system, and wastewater treatment) [14][15][16][17]. Coupons have also been recently used to detect the pathogenic bacteria Listeria monocytogenes in the food processing industry [18]. The coupon-based method allows structural analysis of the microbial community using microscopy techniques that can be combined with microbial diversity and bacterial counts. Moreover, it has been shown that biofilms growing on coupons are representative of the surface of the surroundings and that this method is more robust than classical environmental swabbing with less variability in bacterial counting [19].
In this study, it implemented a coupon-based methodology to capture native biofilms on livestock building surfaces (here a pig farm) along with a set of ex-situ analyses to describe the structural dynamics of these communities over one month. CLSM analysis was put in use to decipher non-invasively the three-dimensional structure of the biofilm, with a special interest in contrasting metabolically active cells. Bacterial plate counting was performed on non-selective media to allow quantification of viable and cultivable species, while an analysis of the bacterial diversity was performed by high-throughput sequencing of the 16S rRNA V3-V4 regions.
2. Coupons Are Colonized by a Densely Clustered Biofilm with Only a Minor Fraction of Cells Metabolically Active
Before being placed in the farm, coupons surface topography was analyzed with the reflection mode of a CLSM (Figure 1). The surface of steel was rougher than PVC with the presence of streaks and holes in abundance, while the PVC was very smooth. To estimate the hydrophilicity of the coupons, contact angles with water were measured [20]. The two side contact angles of 15 drops of 100 µL were measured with the image analysis software ImageJ (1.53 version). The water contact angles show that steel coupons (angle of 51.1° ± 7.4) were more hydrophilic (p < 0.05) than PVC coupons (angle of 84.8° ± 3.2).
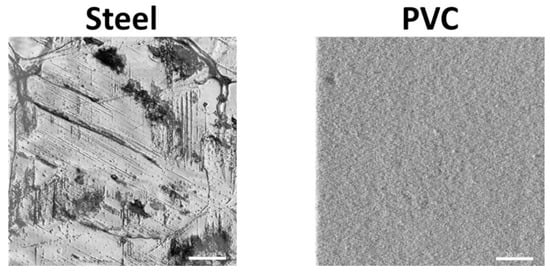
Figure 1. Characterization of the surface of coupons using confocal laser scanning microscopy. A detail of the surface topography of the steel and the PVC coupons by CLSM are shown (scale bar = 30 µm).
Coupons harvested in the building were labeled with SYTO 61 (red) to mark intra- and extracellular nucleic acids in the biofilms and CAM (green) to highlight metabolically active microorganisms inside the community (Figure 3a).
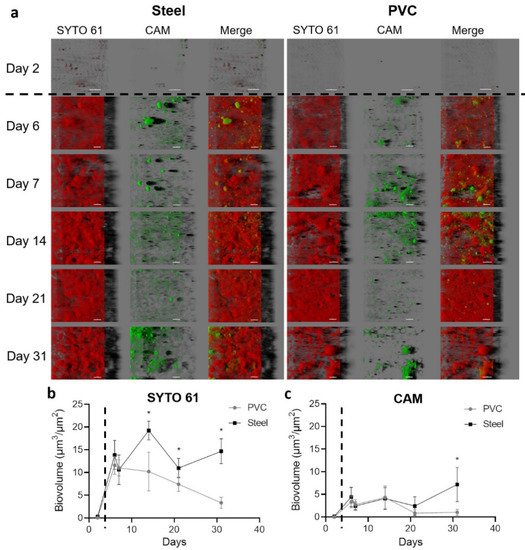
Figure 2. Confocal laser scanning microscopy visualization of biofilms settled on the steel and PVC coupons. Microorganisms and eDNA were labeled in red with SYTO61 and metabolically active cells in green with CAM. The easy 3D representative projections for each time point for steel and PVC coupons are shown (a). Day 2 corresponds to the first sampling, two days after the deposit of the coupons without animals in the farm; the scale bar represents 30 µm for day 2 and 40 µm for the other time points. The biovolume of SYTO 61 (b) and CAM (c) signals were extracted over time on PVC (grey lines) and steel (black lines). The dotted line indicates the animal’s entry into the building. Results represent average ±CI 95% (* p < 0.05).
Images of the first sampling day after the cleaning and disinfection process and before the entry of animals in the farm (day 2) showed only a few sessile scattered microorganisms with the size around the micrometer compatible with bacteria. After animals entered, biovolumes in both channels sharply and significantly increased (p < 0.05) (Figure 2b). From sampling day 6 until the end of the experiment, material contrasted with SYTO 61 was covering all the coupons surfaces. An organization with clusters or compact structures with holes in both materials was visualized. The biovolume of SYTO 61-labeled material was significantly higher on steel than PVC from day 14, and a decrease of the signal was observed from day 7 in PVC (p < 0.05).
Green CAM-labeled clusters corresponding to metabolically active microorganisms were observed in all samples after animal entry. Biovolume of CAM was higher on steel in comparison to PVC on day 31 (p < 0.05), and a decrease of biovolume appeared for days 21 and 31 on PVC (p < 0.05) (Figure 3c).
3. Enumeration of Aerobic Cultivable Bacteria from Coupons
Enumeration with TSA plates as a non-selective media to quantify total aerobic bacteria on coupons was performed. In addition, heat treatment of 10 min at 80 °C was carried out before TSA plating to select spores from the same samples (Figure 3).
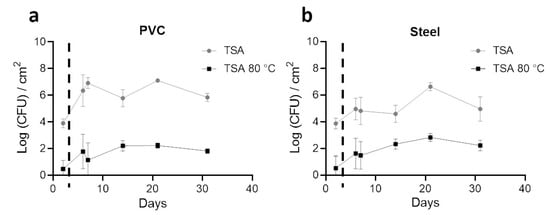
Figure 3. Enumeration profiles (Log CFU/cm2) of the total aerobic bacteria (TSA) and spores (TSA, 80ºC, 10min) on steel and PVC coupons during the trial. Biofilms on coupons were removed mechanically by pipetting and by a round trip with saline solution. After successive dilutions, bacteria inside samples were enumerated on TSA (grey lines) and on TSA after a 10 min at 80 °C treatment to select spores (black lines). Results are average ±CI 95% of 2 enumeration profiles for PVC (a) and Steel (b) coupons. The dotted line indicates the animal’s entry into the building.
Before the entry of animals and 2 days after coupon placement (day 2), 4 logs (CFU/cm2) of total bacteria were enumerated in both materials. After animals entered, a significant increase of total aerobic cultivable bacteria was obtained on PVC in comparison to steel (p < 0.05) for all days, except at day 21. A stabilization of non-selective counting was obtained after animals entered with a value around 6 logs (CFU/cm2) for PVC and 5 logs (CFU/cm2) for steel. A peak was observed in both materials on day 21. With heat treatment, less than 1 log (CFU/cm2) of spores were enumerated on both materials on the first sampling. Values increased on PVC and steel after day 2 to reach more than 2 logs (CFU/cm2) of spores on day 21; no significant differences were observed on both materials for each time point. A higher number of total aerobic cultivable bacteria were counted significantly on PVC compared to steel (p < 0.05), however, with a tendency to have fewer spores counted.
4. 16S rRNA High-Throughput Sequencing Analysis to Decipher the Dynamic of Biofilm Bacterial Diversity
A total of 60 samples from PCR targeting the 16S rRNA coding gene were successfully sequenced. After error filtering, alignment, and chimera removal, 1,146,915 reads were generated, corresponding to 19,115 ± 6505 sequences per sample. Taxonomic analysis shows that Firmicutes phylum was the most represented in all samples, followed by Proteobacteria, Actinobacteria, and Bacteroidetes. Lactobacillales, Clostridiales, Bacillales, Actinomycetales, Pseudomonadales, Bacteroidales, and Enterobacteriales were the most dominant orders (Figure 4).
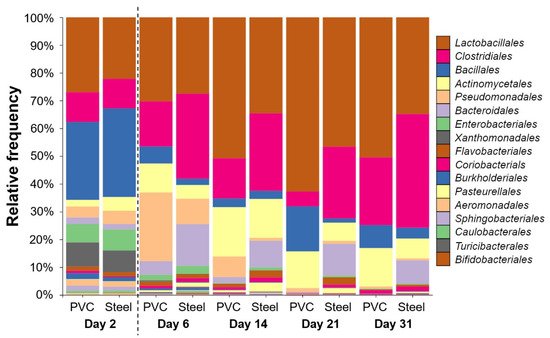
Figure 4. Taxonomic order profile of bacterial communities on the pig farm surface determined using PVC and steel coupons. Each color bar represents the relative frequency of one bacterial order inside the total bacterial community. For each day, PVC and steel taxonomic profiles are shown. The dotted line indicates the animal’s entry into the building.
Lactobacillales was the most represented order in both materials when samples were compiled for days with significantly more relative frequency on PVC (40%) compared to steel (31%). The trends are reversed with Clostridiales that is the second most represented significantly on steel (25%) compared to PVC (14%) (p < 0.05). Other orders were also significantly different per material: Bacillales, Actinomycetales, and Pseudomonadales on PVC and Bacteroidales, Rhodocyclales, Enterobacteriales, Coriobacteriales, and Flavobacteriales on steel (p < 0.05). The trends stated above are true for each time point except for day 2 (Figure 3). On day 2, after C&D procedures and before the animals enter the building, taxonomic profiles are different, with the larger abundance corresponding to Bacillales order on both materials (28–32%). Just after animals enter at day 6, Pseudomonadales order proportion increases to reach 25% of relative frequency on the PVC in comparison to a less increase on the steel of 9%. Bacteroidales are more detectable on steel than PVC for every time point except for day 2, which is at the same level. The level of Enterobacteriales decreased from day 2 to the end of the experiment (Figure 4).
Shannon indexes were used to compare α-diversity between steel and PVC materials during the experiment (Figure 5). On day 2, before animal entry, the diversity was the same on both materials (6.3 and 6.2). The Shannon index on PVC decreased on day 6 until day 21, in comparison to a significantly higher and stable diversity level on steel for all the breeding duration. Except for the first sampling on day 2, a significantly lower level of diversity is observed on PVC compared to steel (p < 0.05), corresponding to a decrease of richness and evenness in the bacterial population. Similar results were found for observed ASVs from 285 at day 2 to a peak on day 6 with 341–431, reaching 170–290 ASVs at the end of the trial, for PVC and steel, respectively. Weighted UniFrac distances of beta diversity showed significant differences between the compositional 16S of both materials per day (p < 0.05).
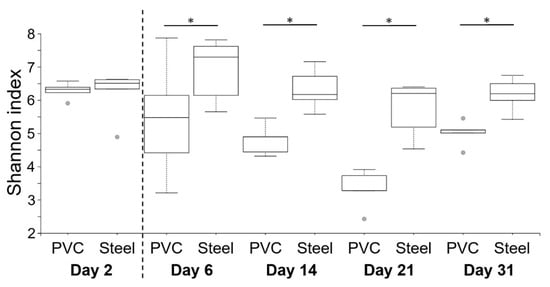
Figure 5. Comparison of α-diversity on PVC and steel coupons during the experiment. α-diversity was determined using the Shannon index value. The dotted line indicates the animal’s entry into the building. Pairwise comparisons between materials per day are shown with an asterisk (* p < 0.05).
References
- Taylor, K. Ten years of REACH—An animal protection perspective. Altern. Lab. Anim. 2018, 46, 347–373.
- McEwen, S.A.; Collignon, P.J. Antimicrobial resistance: A one health perspective. Microbiol. Spectr. 2018, 6, ARBA-0009-2017.
- Cross, A.R.; Baldwin, V.M.; Roy, S.; Essex-Lopresti, A.E.; Prior, J.L.; Harmer, N.J. Zoonoses under our noses. Microbes Infect. 2019, 21, 10–19.
- Chlebicz, A.; Śliżewska, K. Campylobacteriosis, salmonellosis, yersiniosis, and listeriosis as zoonotic foodborne diseases: A review. Int. J. Environ. Res. Public Health 2018, 15, 863.
- Lerminiaux, N.A.; Cameron, A.D.S. Horizontal transfer of antibiotic resistance genes in clinical environments. Can. J. Microbiol. 2019, 65, 34–44.
- Soucy, S.M.; Huang, J.; Gogarten, J.P. Horizontal gene transfer: Building the web of life. Nat. Rev. Genet. 2015, 16, 472–482.
- Madsen, J.S.; Burmølle, M.; Hansen, L.H.; Sørensen, S.J. The interconnection between biofilm formation and horizontal gene transfer. FEMS Immunol. Med. Microbiol. 2012, 65, 183–195.
- Valentine, N.B.; Butcher, M.G.; Su, Y.-F.; Jarman, K.H.; Matzke, M.; Webb-Robertson, B.-J.; Panisko, E.A.; Seiders, B.A.B.; Wahl, K.L. Evaluation of sampling tools for environmental sampling of bacterial endospores from porous and nonporous surfaces. J. Appl. Microbiol. 2008, 105, 1107–1113.
- Ismaïl, R.; Aviat, F.; Michel, V.; Le Bayon, I.; Gay-Perret, P.; Kutnik, M.; Fédérighi, M. Methods for recovering microorganisms from solid surfaces used in the food industry: A review of the literature. Int. J. Environ. Res. Public Health 2013, 10, 6169–6183.
- Gomes, I.B.; Lemos, M.; Mathieu, L.; Simões, M.; Simões, L.C. The action of chemical and mechanical stresses on single and dual species biofilm removal of drinking water bacteria. Sci. Total Environ. 2018, 631–632, 987–993.
- Stiefel, P.; Mauerhofer, S.; Schneider, J.; Maniura-Weber, K.; Rosenberg, U.; Ren, Q. Enzymes enhance biofilm removal efficiency of cleaners. Antimicrob. Agents Chemother. 2016, 60, 3647–3652.
- Grand, I.; Bellon-Fontaine, M.-N.; Herry, J.-M.; Hilaire, D.; Moriconi, F.-X.; Naïtali, M. Possible Overestimation of Surface Disinfection Efficiency by Assessment Methods Based on Liquid Sampling Procedures as Demonstrated by in Situ Quantification of Spore Viability. Appl. Environ. Microbiol. 2011, 77, 6208–6214.
- Goeres, D.M.; Loetterle, L.R.; Hamilton, M.A.; Murga, R.; Kirby, D.W.; Donlan, R.M. Statistical assessment of a laboratory method for growing biofilms. Microbiology 2005, 151, 757–762.
- Douterelo, I.; Boxall, J.B.; Deines, P.; Sekar, R.; Fish, K.E.; Biggs, C.A. Methodological approaches for studying the microbial ecology of drinking water distribution systems. Water Res. 2014, 65, 134–156.
- Deines, P.; Sekar, R.; Husband, P.S.; Boxall, J.B.; Osborn, A.M.; Biggs, C.A. A new coupon design for simultaneous analysis of in situ microbial biofilm formation and community structure in drinking water distribution systems. Appl. Microbiol. Biotechnol. 2010, 87, 749–756.
- Douterelo, I.; Jackson, M.; Solomon, C.; Boxall, J. Microbial analysis of in situ biofilm formation in drinking water distribution systems: Implications for monitoring and control of drinking water quality. Appl. Microbiol. Biotechnol. 2016, 100, 3301–3311.
- Krishnan, M.; Dahms, H.-U.; Seeni, P.; Gopalan, S.; Sivanandham, V.; Jin-Hyoung, K.; James, R.A. Multi metal assessment on biofilm formation in offshore environment. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 73, 743–755.
- Rodríguez-López, P.; Rodríguez-Herrera, J.J.; Cabo, M.L. Tracking bacteriome variation over time in listeria monocytogenes-positive foci in food industry. Int. J. Food Microbiol. 2020, 315, 108439.
- Moen, B.; Røssvoll, E.; Måge, I.; Møretrø, T.; Langsrud, S. Microbiota formed on attached stainless steel coupons correlates with the natural biofilm of the sink surface in domestic kitchens. Can. J. Microbiol. 2016, 62, 148–160.
- Menees, T.S.; Radhakrishnan, R.; Ramp, L.C.; Burgess, J.O.; Lawson, N.C. Contact angle of unset elastomeric impression materials. J. Prosthet. Dent. 2015, 114, 536–542.
More
Information
Subjects:
Microbiology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
776
Entry Collection:
Environmental Sciences
Revisions:
2 times
(View History)
Update Date:
18 Jan 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




