Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Cengiz Gokbulut | + 5456 word(s) | 5456 | 2022-01-11 08:42:30 | | | |
| 2 | Catherine Yang | Meta information modification | 5456 | 2022-01-17 08:39:23 | | | | |
| 3 | Panagiota Katikou | Meta information modification | 5456 | 2022-01-17 23:05:48 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gokbulut, C.; Katikou, P. Tetrodotoxin. Encyclopedia. Available online: https://encyclopedia.pub/entry/18325 (accessed on 09 March 2026).
Gokbulut C, Katikou P. Tetrodotoxin. Encyclopedia. Available at: https://encyclopedia.pub/entry/18325. Accessed March 09, 2026.
Gokbulut, Cengiz, Panagiota Katikou. "Tetrodotoxin" Encyclopedia, https://encyclopedia.pub/entry/18325 (accessed March 09, 2026).
Gokbulut, C., & Katikou, P. (2022, January 17). Tetrodotoxin. In Encyclopedia. https://encyclopedia.pub/entry/18325
Gokbulut, Cengiz and Panagiota Katikou. "Tetrodotoxin." Encyclopedia. Web. 17 January, 2022.
Copy Citation
Tetrodotoxin (TTX) is a crystalline, weakly basic, colorless organic substance and is one of the most potent marine toxins known. Although TTX was first isolated from pufferfish, it has been found in numerous other marine organisms and a few terrestrial species.
tetrodotoxin (TTX)
intoxication
treatment
1. Introduction
Tetrodotoxin (TTX) is one of the most potent natural marine toxins, which acts by selectively blocking the action potentials of voltage-gated Na+ channels along nerves, skeletal and cardiac muscle membranes, without changing the resting membrane potentials. TTX was named after the Tetraodontidae pufferfish family, from which it was initially isolated and is considered the most lethal toxin found in the marine environment [1][2]. TTX is both water-soluble and heat stable, therefore not being destroyed by heat processing; on the contrary, it rather increases its toxic effect, whereas there is no known antidote for TTX to date [3][4]. Despite its recognized poisonous, or sometimes lethal effects when ingested by humans at high doses (lethal doses range from 1.5–2.0 mg TTX, equal to blood level 9 ng/mL), when administered at much lower levels, TTX exhibits therapeutic properties, so far mainly targeted to treating cancer-related, neuropathic and/or visceral pain [5].
TTX is a crystalline, weak basic, colorless substance with a molecular formula of C11H17O8N3 (Figure 1). At least 30 structural analogues have been described to date, with varying degrees of toxicity. Depending on the structure, these are classified into three groups: hemilactal, lactone, and 4,9-anhydro types, altogether referred to as tetrodotoxins (TTXs). TTXs are found in various, taxonomically diverse, groups of animals, dwelling in both terrestrial and aquatic (marine, freshwater, and brackish) environments [6][7][8]. TTX is believed to originate from bacteria belonging to the phylum Proteobacteria, comprising Pseudomonas, Pseudoalteromonas, and Vibrio; however, several other bacterial phyla (Actinobacteria, Bacterioides, Firmicutes, Proteobacteria) are occasionally reported as potential TTX sources [1][9]. Such TTX-producing bacteria (i.e., genera Vibrio, Pseudomonas, Aeromonas, Alteromonas, Nocardiopsis, Bacillus, Shewanella, Roseobacter) have been found in the subcutaneous mucus, ovaries, and the gastrointestinal tract of several aquatic species [3][10][11][12], while certain evidence exists the literature suggesting their association to specific dinoflagellate blooms, such as Alexandrium tamarense or Prorocentrum cordatum [13][14][15].
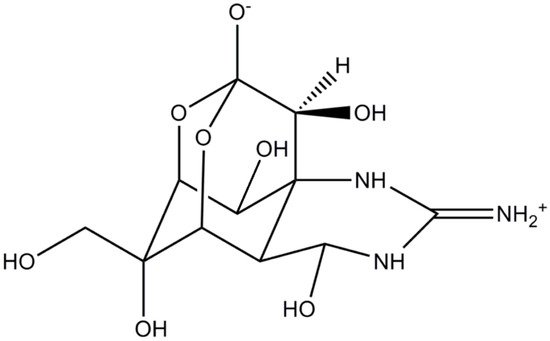
Figure 1. Structure of tetrodotoxin.
TTX has been responsible for numerous—occasionally fatal—human intoxication incidents and typically linked to pufferfish consumption, especially in countries of the Far East (particularly Japan), where they constitute a delicacy known as “fugu” [3]. Until the beginning of the 21st century, TTX was commonly found in tropical waters and was not being reported, or yet perceived as a potential hazard, in temperate areas, such as the Mediterranean Sea and Europe. Starting from 2003, however, a known TTX-vector, the pufferfish species Lagocephalus sceleratus, has been increasingly recorded in eastern Mediterranean coasts, owing to its invasion through the Suez Canal (the so-called Lessepsian migration). By 2007, the species had managed to establish its presence in these habitats, gradually expanding towards Israel, Lebanon, Turkey, Cyprus, and Greece [16][17][18][19][20] and later in the rest of the Mediterranean (Italy, Croatia, Malta, Libya, Algeria, and Tunisia), finally reaching Spain by mid-2014 [21][22][23].
Meanwhile, again, in 2007, the first human TTX-poisoning in Europe was also reported, attributed to the consumption of a TTX contaminated gastropod, the trumpet shell Charonia lampas, initially originating from the south of Portugal, but purchased in Malaga, Spain [24][25]. Later, in 2015, evidence on the occurrence of TTXs in European bivalve mollusks started to appear in the literature. So far, TTXs in shellfish have been reported in a number of European countries (UK, Greece, Netherlands, Portugal, Spain, Italy, France), with this presence detected in samples dating as early as 2006 and in a variety of species, including bivalve mollusks (mussels, oysters, venus clams) and gastropods (Gibbula umbilicalis, Monodonta lineata, and C. lampas) [7][14][26][27][28][29][30][31][32][33][34], but no further human TTX-intoxications were associated with these aquatic organisms. Similarly, there are reports on the presence of TTXs in several bivalve mollusks species from other temperate areas of the world, including New Zealand, China and Japan [8][35][36][37][38], with no relevant intoxications in humans. On the contrary, there are several reports of human poisoning in these “non-traditional” areas related to ingestion of TTX-containing pufferfish, mostly L. sceleratus, specifically in Israel, Lebanon, Palestine (Gaza strip), Turkey, Cyprus, and Greece, with at least three lethal cases in Turkey, and a suspected case of TTX poisoning associated with octopus’ consumption in Malta [17][39][40][41][42][43][44][45][46]. Expectably though, there is potentially significant underreporting of intoxication cases, especially as regards documentation in scientific literature, given that most incidents are generally only clinically diagnosed, without laboratory confirmation of TTX presence, while they are commonly reported in the local daily press. Consequently, important information may also be missed due to language barriers.
The observed increase of TTXs incidences, in terms of both presence in edible marine organisms and human poisoning cases, in countries where they were previously uncommon, has raised concerns about their management from a legislative point of view. So far, Japan remains the only country which has set an official regulatory limit for TTXs at 10 MU/g, equivalent to 2 mg TTX/kg pufferfish tissue [47][48]. The European Union (EU), despite the aforementioned records, has not yet established a maximum permitted level (MPL) for TTXs content in seafood, and this toxin group is not regularly monitored. In fact, as regards TTXs, the current EU legislation only foresees that fishery products derived from poisonous fish of the family Tetraodontidae must not be placed on the market, whereas similar regulatory requirements exist in other non-EU Mediterranean countries, such as Turkey and Egypt [18][49][50][51]. However, as an initial response to the concerns raised by the presence of TTXs in bivalve mollusks and gastropods, the European Commission requested the European Food Safety Authority (EFSA) Panel on “Contaminants in the Food Chain” to deliver a scientific opinion as regards the “risks related to the presence of TTXs in marine bivalves and gastropods”. The opinion was issued in early 2017, proposing a provisional concentration below 44 μg TTX equivalents/kg shellfish meat, which was considered not to result in adverse effects in humans [12]. Nevertheless, the opinion recognized several shortcomings related to availability of epidemiological data and of validated analysis methods, which highlighted the requirement for more solid evidence in order to proceed towards adopting a legislative MPL in the future [47].
2. Treatment of TTX Intoxication in Human
The high fatality rates and lack of any specific antidote are persisting challenges for TTX intoxication cases. Taking necessary precautions against poisoning is the only way to avoid the risks, potentially including death, associated with TTX poisoning cases [52]. In fact, the treatment of human TTX intoxication is largely symptomatic and involves supportive care, including respiratory support measures, until TTX is excreted in the urine. It has been shown indeed that supportive care, including emesis, gastric lavage and respiratory support, and fluid replacement, reduced deaths in TTX intoxication cases [48][53][54][55].
Firstly, if spontaneous vomiting has not already occurred, the toxin should be expelled from the body by inducing vomiting by emetic agents, such as apomorphine, to reduce exposure to unabsorbed TTX [54]. Gastric decontamination should ideally be done if the patient is brought to the hospital within 60 min after TTX poisoning. For this purpose, gastric lavage, especially with sodium bicarbonate solution (2%), followed by activated charcoal, is recommended, since TTX is less stable in an alkaline medium [54]. In the early stages of TTX intoxication, activated charcoal can be administered orally to victims to prevent the gastric absorption of the toxin [56]. Secondly, as the two main causes of death are respiratory arrest and severe hypotension, respiration should be secured, and oxygenation should be provided. In cases of respiratory distress or failure, oxygen and other respiratory support, including endotracheal intubation, are often required to maintain cardiovascular function until the toxin is completely eliminated from the body [57]. As such, in the Thailand TTX poisoning outbreak, patients were treated with endotracheal intubation and mechanical ventilation. As a result, 239 (97.5%) of 245 patients recovered completely, 1 patient (0.4%) had brain damage, and 5 patients (2%) died due to the intoxication [58]. Similarly, in Israel, TTX intoxication cases recovered within 4 days by providing respiratory support during the poisoning outbreak [17]. Further treatment including fluid and electrolyte replacement could be used to reduce resulting fluid loss, to induce urinary excretion of the toxin and to enhance cardiac output and systemic vascular resistance [57]. In addition, hemodialysis may be useful, especially in patients with renal disease or dysfunction. Considering the clinical signs of various seafood poisonings, PSTs intoxication is the most difficult to distinguish from TTX poisoning [59]. Detailed and accurate information for the history of exposure is critical for differential diagnosis since the organisms harboring other neurotoxins are usually different from TTX-containing species [60]. A complete physical examination, including a comprehensive neurological examination, electrophysiological studies, and analytical techniques could be useful in making a differential diagnosis of TTX poisoning; routine laboratory tests are not helpful [61].
An experimental animal study showed that amphetamine, phenylephrine, and norepinephrine are the most effective agents for the treatment of serious hypotension in TTX intoxication, possibly due to their direct or indirect adrenergic effects [62]. However, some investigators suggested the administration of dopamine as the first-line inotropic agent [54]. Some researchers have also proposed using atropine in patients with bradycardia, but its clinical effect is controversial [63][57]. Atropine is not routinely required, as bradycardia is not a common serious problem in human TTX intoxication [64].
Moreover, certain drugs could be useful in relieving the various symptoms, including restoring motor activity associated with TTX intoxication. Some researchers report that administration of the anticholinesterases, such as edrophonium and neostigmine, enhance the recovery of motor power and markedly reduce paresthesia and numbness by increasing the acetylcholine level at the neuromuscular junction [39][57][65][66]. However, other investigators did not support these reports [67][68][63] since TTX probably does not act on the motor end-plate until its concentration reaches a high level [69], indicating that anticholinesterase drugs are not likely to be useful for the treatment of TTX intoxication [67]. The efficacy of neostigmine in 37 TTX intoxication cases has been reviewed by Liu et al., who concluded that the current literature was insufficient to provide an evidence base for or against the use of neostigmine in patients with TTX-associated respiratory failure [70].
Hemodialysis could be a potentially useful approach, especially in patients with renal disease or renal dysfunction. However, there is very limited information in the literature regarding the effectiveness of dialysis in the treatment of human TTX intoxications. It has been reported that hemodialysis applied 21 h after TTX exposure was effective in a uremic patient with serious neurologic dysfunction [71][72]. Recently, hemodialysis following TTX poisoning 12 h after exposure was reviewed in two patients from Oman, who recovered, but the report did not provide enough evidence to support this therapeutic approach in human TTX intoxication cases [73]. In the same report, it was suggested that, to prove the efficiency of hemodialysis, the toxin in the removal of ultrafiltrate could be determined. However, other investigators have indicated that hemodialysis may not be an effective treatment since the toxin has low water solubility [67][74].
For a long time, several experimental studies have been carried out to find a specific antidote and to develop effective treatments against human TTX intoxication. Antiserum and monoclonal antibodies against TTX have been developed and tested successfully in experimental animals [75][76]. Various monoclonal antibodies have been developed against TTX [77][78] and tetrodonic acid [79]; however, none of these were shown to be effective in vivo following TTX exposure. It has been also reported that a polyclonal rabbit anti-TTX antibody was effective for protecting mice from lethal TTX exposure [80]. In another study, monoclonal antibodies protected the animals from TTX intoxication by neutralizing the toxin, resulting in 100% survival [81]. Furthermore, Rivera et al. developed a specific monoclonal antibody against TTX and reported that this antibody was effective for protecting mice from lethal TTX exposure [76]. On the other hand, an effective TTX-specific vaccine was developed, and it was demonstrated to successfully protect animals from haptenic TTX by enhancing humoral immune response [82][83][84]. Although these products have a therapeutic potential for the treatment of TTX intoxication, further investigations are needed to provide enough evidence for their efficacy in real human cases.
3. Therapeutic Use of TTX in Medicine
Various marine natural compounds have the potential to be used as medicines in the treatment of various diseases, further to their use as experimental tools or food supplements [2][85][86][87][88][89]. In this context, potent marine toxins, such as TTX, have received particular attention from researchers in the last three decades and gained importance as experimental tools because of their specific targets and mode of their pharmacological activity [90]. Due to the particular significance of pufferfish in Japanese culture, scientific research on the pharmacological and toxicological effects of pufferfish or TTX have been carried out mostly by Japanese researchers for a long period of time.
Following the discovery of the TTX molecule’s ability to block Na+ ion channels in nerve cells, scientific studies mostly focused on the cellular and molecular mechanisms of TTX [91]. As such, despite its potent neurotoxicity, TTX was considered possible be used in medicine as an analgesic to treat various types of pain, due to its blocking of specific Na+ ion channels and paralyzing effect. TTX targets specific Na+ ion channels and has been used mainly as a popular chemical tool or a product in various scientific studies of neurophysiological and pharmacological processes mediated by those ion channels [2][3][92]. The limitations for its medical use are related to its toxic effects; nevertheless, its potent pharmacological activity shown in several clinical trials and experimental animal models supports its rational use for therapeutic purposes. Although there are many investigations on the medical use of TTX in the literature, most of these studies focused on its analgesic and local anesthetic properties, due to its blocking of very specific Na+ ion channels.
Therapeutic use of TTX is mainly based on blockade of very specific voltage-gated Na+ ion channels with a high degree of selectivity and, thus, suppresses action potentials in axons and reduces ectopic peripheral nerve activity [93]. TTX displays analgesic activity by inhibiting the initiation and conduction of action potentials and, consequently, blocking nerve transmission in the peripheral nervous system [94]. TTX was used as an analgesic agent for the treatment of neuropathic and rheumatic pains in the early 20th century in Japan [95]. TTX has also been used as an analgesic agent in terminal cancer patients in China [90]. Furthermore, in various studies, summarized in Table 1, researchers have conducted some preclinical and clinical studies for the use of subtoxic doses of TTX as an effective analgesic agent in the treatment of various intense pains, such as in severe cancer patients [96][97][98][99]. In addition, Campos-Ríos et al. [100] recently emphasized that TTX is a potential analgesic that can be used to treat visceral pain, especially painful gastrointestinal conditions.
A Canadian pharmaceutical company (WEX Pharmaceuticals, Inc., Vancouver, Canada) has developed a pharmaceutical form containing TTX for subcutaneous injection (Halneuron; Tectin; Tetrodin; Tocudin) as an analgesic in advanced cancer patients to reduce the intense pain as an alternative to narcotics and opioid pain medication and the treatment of opiate addiction. An earlier open-label, multi-center clinic trial indicated that two or three times daily TTX administration for 4 days caused a clinically significant reduction in pain intensity, and relief of pain persisted for up to two weeks in 17 out of 31 treatments in patients with severe cancer-related pain [101]. In another multi-center clinic trial performed in Canada, TTX was administered at a dose of 30 μg intramuscularly twice daily for 4 days to cancer patients [99]. In this trial, according to only pain score assessment, TTX did not provide clinically significant analgesia in a heavily pre-treated cohort of cancer patients with moderate to severe pain. However, according to an analysis of secondary endpoints, and an exploratory post hoc analysis, the authors suggested that TTX may potentially relieve moderate to severe pain in cancer patients, and often for prolonged periods following treatment, with mild side effects. In a further clinic trial for evaluation of long-term safety and efficacy properties of TTX, a sustained analgesic effect with usually acceptable toxicity was observed in patients with cancer-related pain following subcutaneous administration of 30 μg TTX twice daily for 4 days [99]. Similarly, later clinical trials also indicated that TTX may provide clinically meaningful analgesia with acceptable side effects at the same dose and treatment course in cancer patients [96][97]. Besides these, in vivo and in vitro genotoxicity assays indicated that TTX did not have any genotoxic potential for patients [102], and this could be an advantage for its use as an analgesic agent in medicine [2].
Table 1. Therapeutic use of TTX in clinical treatments.
| Study Direction |
Number of Participants | Study Design | Dose and Exposure | Outcome and Results | Reference |
|---|---|---|---|---|---|
| Cancer related pain | 24 | An open-label, multi-dose study | TTX 15 to 90 μg daily, administered intramuscularly in divided doses, over four days | TTX was overall safe. It effectively relieved severe, treatment-resistant cancer pain in the majority of patients and often for prolonged periods after treatment | [101] |
| 77 | A randomized, double blind, parallel design multi-center study | TTX (30 μg, bid) was administered subcutaneously for 4 days | This study suggested TTX may potentially relieve moderate to severe, treatment-resistant cancer pain in a large proportion of patients, and often for prolonged periods following treatment, but further study is warranted using a composite primary endpoint | [98] | |
| 41 | A multi-center open-label longitudinal and efficacy trial | TTX (30 μg) was administered subcutaneously twice daily for 4 days | Long-term treatment with TTX is associated with acceptable toxicity and, in a substantial minority of patients, resulted in a sustained analgesic effect | [99] | |
| 149 | A multi-center, randomized, double-blind, placebo-controlled, parallel-design trial | TTX (30 μg) was administered subcutaneously twice daily for four consecutive days | TTX may provide clinically meaningful analgesia for patients who have persistent moderate to severe cancer pain despite best analgesic care | [97] | |
| 125 | A randomized, double blind, placebo controlled, parallel dose comparison study | TTX (7.5, 15, and 30 μg/kg BID and 30 μg/kg QD) administered as subcutaneous injections for 4 days | This study suggests the TTX 30 µg b.i.d. regimen is well tolerated with promising early efficacy data |
[96] | |
| Heroin dependence | 45 | Double blind, placebo-controlled |
TTX (5 µg or 10 µg) administered intramuscularly | Low-dose TTX is acutely effective in reducing cue-induced increases in heroin craving and associated anxiety | [103] |
| 216 | A multi-center, randomized, double-blind, placebo-controlled | TTX (5 µg or 10 µg) administered intramuscularly | TTX significantly reduced withdrawal symptoms by day 3 compared with placebo, and there was no significant difference in the incidence of adverse events in study groups |
[104] |
Moreover, the potential effects of TTX against drug addiction behaviors have been investigated in human patients. Intramuscular administration of low dose TTX (5 µg or 10 µg) was found to be effective in reducing cue-induced increases in heroin craving and associated anxiety with no sign of systemic side effects [103]. In addition, a significant reduction of heroin withdrawal symptoms by TTX has been shown in patients with a diagnosis of heroin dependence at dosages of 5 and 10 μg three times a day [104]. In addition, it has been indicated that the intramuscular pre-treatment of TTX substantially prevented morphine withdrawal symptoms in mice and rats without any systemic adverse effects [105]. Although its mechanism of action has not yet been fully elucidated, it was suggested that TTX may be an alternative drug in the treatment of opiate addiction [103].
The potential analgesic effects of TTX have been investigated in various experimental studies at subtoxic doses in rodent models, summarized in Table 2. Nieto et al. [106] reported that the subcutaneous injection of low doses TTX (1, 3, or 6 µg/kg) could be useful to prevent and treat paclitaxel-induced neuropathic pain in mice. Besides, the analgesic effects of subcutaneous TTX injection have been indicated by the formalin test and the partial ligation of the sciatic nerve (Seltzer’s model) in rats without causing any adverse effects [107]. Kayser et al. [108] reported that subcutaneous injection of TTX (0.3, 1, 3, or 6 μg/kg,) into the back displayed antihyperalgesic effects and decreased pain-related behaviors in rats with injured sciatic nerve through mechanisms that involve complex interactions with endogenous opioid system. In addition, it was shown that local injection of TTX (0.03–1 μg) into the gastrocnemius muscle provided effective analgesia in rats with persistent muscle pain produced by carrageenan injection [109]. Gonzalez-Cano et al. [110] concluded that subcutaneous administration of TTX decreased pain-related behaviors and reversed the mechanical hyperalgesia in the colon and peritoneum induced by capsaicin and cyclophosphamide injections in viscera-specific mouse models, respectively. Furthermore, TTX decreased thermal hyperalgesia and mechanical allodynia using a full-thickness thermal injury model in rats following subcutaneous administration at a dose of 8 μg/kg [111]. These animal studies demonstrated that subtoxic doses of TTX could be used as an analgesic drug in neuropathic and inflammatory pain with lower adverse effects. STX displays a similar mode of action and analgesic properties to TTX and induces anesthesia or prolongs the anesthetic effect of local anesthetics in combination treatments [112]. However, its systemic toxicity limits its clinical use as a therapeutic agent. To prevent its toxicity for the treatment of joint pain and intractable localized pain, the preparation of STX by means of microencapsulation in liposomes has been proposed [113]. It has been indicated that this liposomal formulation produced a prolonged nerve blockade in a neuropathic pain model in rats without any toxicity [114]. Moreover, other studies showed that an N-1 hydroxylated STX analogue, neoSTX, displayed efficacy for both acute and chronic pain treatment in rats [115] and in patients with somatic [116] and visceral pain [117] without any adverse effects.
Table 2. Summary of the experimental animal studies on analgesic effects of TTX.
| Experimental animals | Model-Technique | Dose and Exposure |
Outcome and Results | Reference |
|---|---|---|---|---|
| Wistar rats Swiss and Webster mice | The formalin test and to partial ligation of the sciatic nerve (Seltzer’s model) | TTX (0.3, 1, 3, or 6 μg/kg) administered subcutaneously 30 min before the formalin test | TTX decreased pain behavior in the formalin test at the highest dose and in the writhing test at 3 and 6 mg/kg. It also reduced mechanical allodynia and thermal hyperalgesia with an ED50 of 1.08 and 0.62 mg/kg without any adverse effects, respectively |
[107] |
| CD-1 mice | Neuropathic pain induced by paclitaxel | TTX (1, 3, or 6 µg/kg) administered subcutaneously | Low doses of TTX can be useful to prevent and treat paclitaxel-induced neuropathic pain, and that TTX-sensitive subtypes of sodium channels play a role in the pathogenesis of chemotherapy-induced neuropathic pain | [106] |
| Male Sprague–Dawley rats | Chronic unilateral constriction injury to either the sciatic nerve or the infraorbital nerve | TTX (0.3, 1, 3, or 6 μg/kg) administered subcutaneously into the back | TTX alleviates pain-related behaviors in sciatic nerve-lesioned rats through mechanisms that involve complex interactions with endogenous opioid systems | [109] |
| Male Sprague-Dawley rats | Full thickness thermal injury (FTTI) model | TTX (8 μg/kg) administered subcutaneously | TTX reduced thermal hyperalgesia and mechanical allodynia |
[111] |
| Adult male Sprague–Dawley rats | Carrageenan (k-carrageenan, 1% in NaCl 0.9%) was injected into the belly of the gastrocnemius muscle, |
Local injection of TTX (0.03–1 μg) into the gastrocnemius muscle | TTX displays important analgesic effects on rat models of persistent muscle pain, without interfering with the nociceptor function to signal for further potentially harmful stimuli |
[109] |
| Adult wild-type Nav1.7 knockout (KO-Nav1.7) mice | Viscero-specific mouse models of chemical stimulation of the colon (intracolonic instillation of capsaicin and mustard oil) and intraperitoneal cyclophosphamide-induced cystitis | TTX (3 and 6 μg/kg) administered subcutaneously | This study suggests that blockade of TTX-sensitive sodium channels, but not Nav1.7 subtype alone, by systemic administration of TTX might be a potential therapeutic strategy for the treatment of visceral pain |
[110] |
Intensive research has been carried out for many years to develop a local anesthetic or a local anesthetic-sustained release system that can provide long-lasting peripheral nerve blockade with minimal systemic or local toxicity. Various scientific studies have indicated that TTX, a specific Na+ ion channel blocker, produces a potent and long-lasting local anesthesia and causes minimal local and systemic adverse effects at safe doses (Table 3). Topical injections of TTX with epinephrine generated an effective and prolonged local anesthesia of the sciatic nerve in rats and provided reversible blocks that lasted over 13 h at a dose of 11.5 µM [118]. TTX injected with either bupivacaine or epinephrine, resulted in prolonged nerve blockade, with less toxicity compared to bupivacaine administration alone [119]. Besides, co-encapsulation of TTX (75 mg, 0.05%, w/w) in controlled release devices containing dexamethasone and bupivacaine generated effective and long-lasting topical nerve blocks in rats [120]. Similarly, polymer TTX conjugates (1.0–80.0 µg) produced a range of prolonged local anesthesia of nerve block in rats, from several hours to 3 days and causes minimal local and systemic toxic effects [121]. TTX has some advantages, such as not having direct side effects, such as myocardial depression and poorly crossing the blood brain-barrier, compared to conventional local anesthetic agents [122].
The effect of TTX on topical ocular anesthesia has been also investigated experimentally in animal models (Table 3). These studies indicated that TTX applied topically in the eye provided an effective and prolonged topical anesthesia for pain control in surgery procedures in rabbits [123][124]. Besides, Green et al. [125] showed that topical corneal application of TTX (1 mM, 10 μL) in 0.9% saline significantly alleviated photophobia in rats with corneal injury. They suggested that TTX could be used as an effective therapeutic option to reduce the symptoms of photophobia that occurs after ocular surgery and other clinical diseases. It has also been demonstrated that TTX was effective in mitigating ischemic damages caused by occlusion of hippocampus vessels [126] and those caused by exposure to veratridine in neurons of the cerebrum and hippocampus [127] in rats. In addition, previous experimental studies (Table 3) in rats showed that focal injection of TTX was effective in reducing damage at the injury site and attenuated neurological deficits and tissue loss following spinal cord injury [19][128][129]. Moreover, the inhibition of amyloid beta (A4) precursor protein (APP) by TTX (1 μM) has been demonstrated by a western blot technique in rat hippocampal slices to investigate neuronal activity for regulation processing in the mammalian brain [130]. Furthermore, an in vitro study indicated that stimulus train-evoked seizures were blocked after local injection of 50 μM TTX in rat hippocampal slices [131]. TTX blocked the electrographic seizures when applied in the perfusion medium with lower concentrations of (5, 10, or 20 nM). TTX was also found effective to prevent post-traumatic epileptogenesis [132]. Evoked epileptiform field potentials were observed in the injured cortex, and thin sheets of Elvax polymer containing TTX implanted over lesions were effective in decreasing evoked epileptiform potentials. Furthermore, Kitamura et al. [133] showed that TTX decreased the expression of activity-dependent three genes that are likely to be key factors in the regulation of synaptic plasticity in cerebral cortical cells from E18 rat embryos.
The antitumor effect of TTX has been investigated in experimental animals with tumor and in the in vitro studies with cancer cell lines summarized in Table 3 and Table 4, respectively. It has been reported that TTX obtained from the skin of the masked pufferfish (Arothron diadematus) was applied to mice with Ehrlich Ascite Carcinoma, resulting in an increase in survival and a decrease in the number of tumor cells [134][135]. Besides, the inhibitory effect of TTX has been shown on prostate cancer using in vitro cell culture model with human glioma cell lines ((HTB-138) or MAT-LyLu and the AT-2 cell lines [136][137][138]. The extracts of three strains of TTX-producing bacteria (Bacillus sp., Kytococcus sedentarius, and Cellulomonas fimi) isolated from Arothron hispidus type pufferfish caught from the southeast coast of India were intraperitoneally injected into mice with leukemia, and growth inhibitory effects were observed on the muscle and leukemia cell lines [139]. Similarly, the anticancer activities of TTX, obtained from 3 three species of TTX-producing bacteria (Vibrio alginolyticus, Microbacterium arabinogalactanolyticum, and Serratia marcescens) were investigated in vitro using SW480 and SW620 colorectal carcinoma cell lines. The results of the study indicated that TTX has a substantial inhibitory effect on both cell lines [140]. The researchers suggested that TTX-producing bacteria isolated from pufferfish can be used to develop potential anti-tumor compounds.
Table 3. Summary of the experimental animal studies on other therapeutic effects of TTX other than its analgesic effect.
| Experimental Animals | Pharmacological Activity | Model- Technique |
Dose and Exposure |
Outcome and Results | Reference |
|---|---|---|---|---|---|
| Adult male Sprague-Dawley rats | Local anesthesia | Sciatic Blockade Technique | Co-administration of capsaicin and TTX-loaded liposomes | The combined delivery of capsaicin and TTX using a sustained-release system can achieve prolonged duration local anesthesia without detectable toxicity | [141] |
| Sciatic Blockade Technique | TTX (15.95 mg/L) and bupivacaine (4442 mg/L) or epinephrine (10.08 mg/L) | TTX injected with either bupivacaine or epinephrine, results in prolonged nerve blockade, with myotoxicity that is no worse and perhaps less than that from bupivacaine | [119] | ||
| Sciatic Blockade Technique Modified hotplate test Weight-bearing test |
Polymer-TTX conjugates (1–80 μg) | 1.0–80.0 µg of TTX released from these polymers produced a range of durations of nerve block, from several h to 3 days, with minimal systemic or local toxicity | [121] | ||
| Sciatic Blockade Technique | Rats received sciatic nerve blocks with 75 mg of microspheres containing 0.05% TTX, 50% bupivacaine and/or 0.05% dexamethasone | Co-encapsulation of TTX in controlled release devices containing bupivacaine and dexamethasone resulted in very prolonged nerve blocks | [120] | ||
| Sciatic Blockade Technique | TTX [0.3 mL, 10 μM-20 μM (3.19 6.38 mg/L)] with and without epinephrine (10.08 mg/L) or bupivacaine (4442 mg/L) | Bupivacaine increased the local anesthetic potency of tetrodotoxin, reduced its systemic toxicity, and, when co-injected subcutaneously, increased the median lethal dose from 13.95 to 15.23 μg/kg | [118] | ||
| Sciatic Blockade Technique and Neurobehavioral Assessment of Nerve Blockade | TTX [0.3 mL of 3.19 mg/L (0.96 μg dose)] with bupivacaine 0.25% (2.5 mg/mL) with or without epinephrine 5 µg/mL | Blocks containing bupivacaine 0.25% with TTX 3.19 mg/L and epinephrine 5 µg/mL were prolonged by roughly 3-fold compared to blocks with bupivacaine 0.25% plain (P < 0.001) or bupivacaine 0.25% with epinephrine 5 µg/mL (P < 0.001) |
[122] | ||
| New Zealand White rabbit | Ocular local anesthesia | TTX was applied into the inferior conjunctival cul-de-sac of the right eye | A 40 TTX (32, 319 or 3190 mg/L) or proparacaine 0.5% |
TTX is a long-acting topical anesthetic in the rabbit cornea (At a dose of 3190 mg/L, TTX produced an anesthesia up to 8 h) | [123] |
| Adult male Sprague Dawley rats | Attenuation of photophobia | Photophobia by corneal de-epithelialization injury | Topical corneal application of TTX (319 mg/L, 10 μL) in 0.9% saline | TTX markedly attenuated photophobia in rats with corneal injury TTX may be an effective therapeutic option to reduce the symptoms of photophobia that occurs after photorefractive keratectomy and other clinical diseases | [125] |
| Female Sprague-Dawley rats | Ameliorate effect by local blockade on spinal cord injury | A laminectomy was performed | Microinjection of TTX (0.5 μL of 95.7 mg/L–47.8 ng dose) | The results demonstrate that TTX preserves axons from loss after spinal cord injury | [19] |
| A laminectomy at the T8 level exposed a 2.8-mm-diameter circle of dura | TTX (47.85-319 ng/L) 0.5 to 2 μL microinjected into the injury site | The TTX group exhibited a significantly enhanced recovery of coordinated hindlimb functions, more normal hindlimb reflexes, and earlier establishment of a reflex bladder | [129] | ||
| Male Sprague–Dawley rats | Anticonvulsant | Cortical injury in a model of chronic epileptogenesis TTX-impregnated Elvax Neocortical slices Behavioral observations |
TTX/Elvax 20 mg/g. The slices incubated with 1.6–15.96 µg and 319 µg/L TTX | The findings indicated that TTX prevents posttraumatic epileptogenesis in rats in a model of chronic epileptogenesis | [132] |
| Adult female Swiss albino mice injected tumor cell line | Anticancer | Ehrlich ascites carcinoma-EAC) | TTX (1/20 of the LD50) administrated intraperitoneally after 10 days into EAC mice | Treatment with TTX caused a significant decrease in the mean tumor weight and an increase in the cumulative mean survival time when compared with EAC group | [134] |
| Adult Swiss female albino mice | Ehrlich Ascite Carcinoma (EAC) | TTX extracted from the fish skin and applied as a dose of 1/10 and the 1/20 of the LD50 | Exposure to TTX caused the rate of cell division to be reduced greatly, especially in the first 6 days post-treatment. The authors suggested that the reduction in cell number is probably due to increased apoptosis | [135] | |
| Male albino mice | Mouse muscle cell line (L929) and leukemia cell line | TTX intraperitoneally administered with 0.25, 0.50, 0.75, and 1.0 mL and dissolved at 5 mg/ml | TTX inhibited the growing of the muscle and leukemia cell lines. It was suggested that TTX can be used to develop anti-tumor compounds | [139] |
Recently, Law et al. [142] showed that TTX is a potent active compound against SARS-CoV-2 according to a ligad-based approach using a Ligand Scout 4.3 software and ligand-based pharmacophore model generator. However, further in vivo and in vitro studies would be needed to confirm this possible antiviral activity.
Table 4. Summary of the in vitro studies on the therapeutic effects of TTX.
| Study Technique |
Pharmacological Activity |
Model-Technique | Dose and Exposure | Outcome and Results | Reference |
|---|---|---|---|---|---|
| Cell culture | Anticancer | Human glioma cell lines (HTB-138) | Cell cultures exposed with TTX concentrations of 3.19 and 6.38 mg/L for a period of 24 and 48 h | TTX exert the inhibitory effect on the invasion of metastatic prostate cancer | [143] |
| Metastatic MAT-LyLu Cell Line of Rat Prostate Cancer | TTX at different concentration between 0.32 µg and 319 µg/L | TTX inhibits (IC50: 5.75 μg/L) invasiveness of metastatic prostate cancer | [144] | ||
| MAT-LyLu and AT-2 prostatic carcinoma cell lines | TTX at 319 μg/L | Migration of the MAT-LyLu cell line was reduced significantly by TTX (at 319 μg/L); in contrast, there was no effect on AT-2 cell motility | [136] | ||
| MAT-LyLu and AT-2 prostatic carcinoma cell lines | Incubation of TTX at 1.91 mg/L for 24 h | TTX produced significant changed the morphology of MAT-LyLu cancer cell | [137] | ||
| Cell culture | Neuroprotective effect | Rat cerebellar neurons | TTX 1.6–31.9 µg/L | TTX protected cultured neurons from veratridine-induced toxicity and could be use in treatment of ischemic neuronal injury by preventing excessive neuronal depolarizations. | [127] |
| Male Sprague-Dawley rat hippocampal slices | Anticonvulsant effect | Hippocampal slices blocked stimulus train-evoked electrographic seizures (EGSs) | Localized injection of TTX | Stimulus train-evoked seizures were blocked after localized injection of 15.95 mg/L TTX in rat hippocampal slices. Low concentrations of TTX (1.6, 3.19, or 6.38 µg/L) in the perfusion medium blocked EGSs without decreasing the amplitude of extracellular responses to single stimuli |
[131] |
| Ligand-based pharmacophore modeling and Ligand Scout 4.3 software. | Antiviral activity against SARS-CoV-2. | Modeling | Structure-based pharmacophore modeling | TTX is a potent active compound against SARS-CoV-2 according to ligand-based approach | [142] |
References
- Jal, S.; Khora, S.S. An overview on the origin and production of tetrodotoxin, a potent neurotoxin. J. Appl. Microbiol. 2015, 119, 907–916.
- Lago, J.; Rodriguez, L.P.; Blanco, L.; Vieites, J.M.; Cabado, A.G. Tetrodotoxin, an extremely potent marine neurotoxin: Distribution, toxicity, origin and therapeutical uses. Mar. Drugs 2015, 13, 6384–6406.
- Bane, V.; Lehane, M.; Dikshit, M.; O’Riordan, A.; Furey, A. Tetrodotoxin: Chemistry, toxicity, source, distribution and detection. Toxins 2014, 6, 693–755.
- Saoudi, M.; Rabeh, F.B.; Jammoussi, K.; Abdelmouleh, A.; Belbahri, L.; Feki, A.E. Biochemical and physiological responses in Wistar rat after administration of puffer fish (Lagocephalus lagocephalus) flesh. J. Food Agric. Environ. 2007, 5, 107–111.
- Bucciarelli, G.M.; Lechner, M.; Fontes, A.; Kats, L.B.; Eisthen, H.L.; Shaffer, H.B. From poison to promise: The evolution of tetrodotoxin and its potential as a therapeutic. Toxins 2021, 13, 517.
- Reverté, L.; De La Iglesia, P.; Del Río, V.; Campbell, K.; Elliott, C.T.; Kawatsu, K.; Katikou, P.; Diogène, J.; Campàs, M. Detection of Tetrodotoxins in Puffer Fish by a Self-Assembled Monolayer-Based Immunoassay and Comparison with Surface Plasmon Resonance, LC-MS/MS, and Mouse Bioassay. Anal. Chem. 2015, 87, 10839–10847.
- Silva, M.; Azevedo, J.; Rodriguez, P.; Alfonso, A.; Botana, L.M.; Vasconcelos, V. New gastropod vectors and tetrodotoxin potential expansion in temperate waters of the Atlantic Ocean. Mar. Drugs 2012, 10, 712–726.
- Biessy, L.; Boundy, M.J.; Smith, K.F.; Harwood, D.T.; Hawes, I.; Wood, S.A. Tetrodotoxin in marine bivalves and edible gastropods: A mini-review. Chemosphere 2019, 236, 124404.
- Magarlamov, T.Y.; Melnikova, D.I.; Chernyshev, A.V. Tetrodotoxin-producing bacteria: Detection, distribution and migration of the toxin in aquatic systems. Toxins 2017, 9, 166.
- Wu, Z.; Yang, Y.; Xie, L.; Xia, G.; Hu, J.; Wang, S.; Zhang, R. Toxicity and distribution of tetrodotoxin-producing bacteria in puffer fish Fugu rubripes collected from the Bohai Sea of China. Toxicon 2005, 46, 471–476.
- Noguchi, T.; Arakawa, O.; Takatani, T. TTX accumulation in pufferfish. Comp. Biochem. Physiol.-Part D Genom. Proteom. 2006, 1, 145–152.
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; et al. Risks for public health related to the presence of tetrodotoxin (TTX) and TTX analogues in marine bivalves and gastropods. EFSA J. 2017, 15, e04752.
- Kodama, M.; Sato, S.; Sakamoto, S.; Ogata, T. Occurrence of tetrodotoxin in Alexandrium tamarense, a causative dinoflagellate of paralytic shellfish poisoning. Toxicon 1996, 34, 1101–1105.
- Vlamis, A.; Katikou, P.; Rodriguez, I.; Rey, V.; Alfonso, A.; Papazachariou, A.; Zacharaki, T.; Botana, A.M.; Botana, L.M. First detection of tetrodotoxin in greek shellfish by UPLC-MS/MS potentially linked to the presence of the dinoflagellate Prorocentrum minimum. Toxins 2015, 7, 1779–1807.
- Rodríguez, I.; Alfonso, A.; Alonso, E.; Rubiolo, J.A.; Roel, M.; Vlamis, A.; Katikou, P.; Jackson, S.A.; Menon, M.L.; Dobson, A.; et al. The association of bacterial C9-based TTX-like compounds with Prorocentrum minimum opens new uncertainties about shellfish seafood safety. Sci. Rep. 2017, 7, 40880.
- Akyol, O.; Ünal, V.; Ceyhan, T.; Bilecenoglu, M. First confirmed record of Lagocephalus sceleratus (Gmelin, 1789) in the Mediterranean Sea. J. Fish Biol. 2005, 66, 1183–1186.
- Bentur, Y.; Ashkar, J.; Lurie, Y.; Levy, Y.; Azzam, Z.S.; Litmanovich, M.; Golik, M.; Gurevych, B.; Golani, D.; Eisenman, A. Lessepsian migration and tetrodotoxin poisoning due to Lagocephalus sceleratus in the eastern Mediterranean. Toxicon 2008, 52, 964–968.
- Katikou, P.; Georgantelis, D.; Sinouris, N.; Petsi, A.; Fotaras, T. First report on toxicity assessment of the Lessepsian migrant pufferfish Lagocephalus sceleratus (Gmelin, 1789) from European waters (Aegean Sea, Greece). Toxicon 2009, 54, 50–55.
- Rosenberg, L.J.; Wrathall, J.R. Time course studies on the effectiveness of tetrodotoxin in reducing consequences of spinal cord contusion. J. Neurosci. Res. 2001, 66, 191–202.
- Michailidis, N. Study on the lessepsian migrant Lagocephalus sceleratus in Cyprus. In Proceedings of the EastMed, 2010. Report of the Sub-Regional Technical Meeting on the Lessepsian Migration and Its Impact on Eastern Mediterranean Fishery, Nicosia, Cyprus, 7−9 December 2010; GCP/INT/041/EC-GRE-ITA/TD-04; FAO: Athens, Greece, 2010; pp. 74–87. Available online: http://www.faoeastmed.org/pdf/publications/EastMed_TD04.pdf (accessed on 30 November 2021).
- Katikou, P.; Vlamis, A. Tetrodotoxins: Recent advances in analysis methods and prevalence in European waters. Curr. Opin. Food Sci. 2017, 18, 1–6.
- Rambla-Alegre, M.; Reverté, L.; del Río, V.; de la Iglesia, P.; Palacios, O.; Flores, C.; Caixach, J.; Campbell, K.; Elliott, C.T.; Izquierdo-Muñoz, A.; et al. Evaluation of tetrodotoxins in puffer fish caught along the Mediterranean coast of Spain. Toxin profile of Lagocephalus sceleratus. Environ. Res. 2017, 158, 1–6.
- Ujević, I.; Roje-Busatto, R.; Dragičević, B.; Dulčić, J. Tetrodotoxin in Invasive Silver-cheeked Toadfish Lagocephalus sceleratus (Gmelin, 1789) in the Adriatic Sea. In The Handbook of Environmental Chemistry; Joksimović, D., Đurović, M., Zonn, I.S., Kostianoy, A.G., Semenov, A.V., Eds.; The Montenegrin Adriatic Coast; Springer: Cham, Switzerland, 2020; Volume 110, pp. 141–149.
- Fernndez-Ortega, J.F.; Santos, J.M.M.D.L.; Herrera-Gutirrez, M.E.; Fernndez-Snchez, V.; Loureo, P.R.; Rancao, A.A.; Tllez-Andrade, A. Seafood intoxication by tetrodotoxin: First case in europe. J. Emerg. Med. 2010, 39, 612–617.
- Rodriguez, P.; Alfonso, A.; Vale, C.; Alfonso, C.; Vale, P.; Tellez, A.; Botana, L.M. First toxicity report of tetrodotoxin and 5,6,11-trideoxyTTX in the trumpet shell Charonia lampas lampas in Europe. Anal. Chem. 2008, 80, 5622–5629.
- Turner, A.; Dean, K.; Lewis, A.; Jenkins, Z.; Bear, B.; Batista, F.; Ryder, D.; Maskrey, B.; Later, A.; House, C.; et al. ScillyHAB: A Multi-Disciplinary Survey of Harmful Marine Phytoplankton and Shellfish Toxins in the Isles of Scilly, Utilizing Citizen Science in a Remote Offshore U.K. Territory during the COVID-19 Pandemia, 2021. Presentation no. SM-O-15_221 in the 19th International Conference on Harmful Algal Blooms, LaPaz, Mexico, 10–15 October 2021. Available online: https://imsvirtualcenter.com.mx/icha (accessed on 30 November 2021).
- Turner, A.D.; Higgins, C.; Higman, W.; Hungerford, J. Potential threats posed by tetrodotoxins in UK waters: Examination of detection methodology used in their control. Mar. Drugs 2015, 13, 7357–7376.
- Gerssen, A.; Bovee, T.H.F.; Klijnstra, M.D.; Poelman, M.; Portier, L.; Hoogenboom, R.L.A.P. First report on the occurrence of tetrodotoxins in bivalve mollusks in the Netherlands. Toxins 2018, 10, 450.
- Leão, J.M.; Lozano-Leon, A.; Giráldez, J.; Vilariño, Ó.; Gago-Martínez, A. Preliminary results on the evaluation of the occurrence of tetrodotoxin associated to marine Vibrio spp. in bivalves from the Galician Rias (Northwest of Spain). Mar. Drugs 2018, 16, 81.
- Hort, V.; Arnich, N.; Guérin, T.; Lavison-Bompard, G.; Nicolas, M. First detection of tetrodotoxin in bivalves and gastropods from the French mainland coasts. Toxins 2020, 12, 599.
- Bacchiocchi, S.; Campacci, D.; Siracusa, M.; Dubbini, A.; Leoni, F.; Tavoloni, T.; Accoroni, S.; Gorbi, S.; Giuliani, M.E.; Stramenga, A.; et al. Tetrodotoxins (TTXs) and Vibrio alginolyticus in mussels from central Adriatic Sea (Italy): Are they closely related? Mar. Drugs 2021, 19, 304.
- Bordin, P.; Dall’Ara, S.; Tartaglione, L.; Antonelli, P.; Calfapietra, A.; Varriale, F.; Guiatti, D.; Milandri, A.; Dell’Aversano, C.; Arcangeli, G.; et al. First occurrence of tetrodotoxins in bivalve mollusks from Northern Adriatic Sea (Italy). Food Control 2021, 120, 107510.
- Réveillon, D.; Savar, V.; Schaefer, E.; Chevé, J.; Halm-Lemeille, M.-P.; Hervio-Heath, D.; Travers, M.-A.; Abadie, E.; Rolland, J.-L.; Hess, P. Tetrodotoxins in French Bivalve Mollusks—Analytical Methodology, Environmental Dynamics and Screening of Bacterial Strain Collections. Toxins 2021, 13, 740.
- Dell’Aversano, C.; Tartaglione, L.; Polito, G.; Dean, K.; Giacobbe, M.; Casabianca, S.; Capellacci, S.; Penna, A.; Turner, A.D. First detection of tetrodotoxin and high levels of paralytic shellfish poisoning toxins in shellfish from Sicily (Italy) by three different analytical methods. Chemosphere 2019, 215, 881–895.
- Han, C.; Zhang, X.; Li, L.; Chen, S.; Yan, Z.; Gao, X.; Chang, J. Analysis and Evaluation of Tetrodotoxin in Coastal Aquatic Products of Zhejiang Province. J. Coast. Res. 2018, 83, 380–385.
- Numano, S.; Kudo, Y.; Cho, Y.; Konoki, K.; Yotsu-Yamashita, M. Temporal variation of the profile and concentrations of paralytic shellfish toxins and tetrodotoxin in the scallop, Patinopecten yessoensis, cultured in a bay of East Japan. Mar. Drugs 2019, 17, 653.
- Biessy, L.; Smith, K.F.; Harwood, D.T.; Boundy, M.J.; Hawes, I.; Wood, S.A. Spatial variability and depuration of tetrodotoxin in the bivalve Paphies australis from New Zealand. Toxicon X 2019, 2, 100008.
- Biessy, L.; Pearman, J.K.; Smith, K.F.; Hawes, I.; Wood, S.A. Seasonal and Spatial Variations in Bacterial Communities From Tetrodotoxin-Bearing and Non-tetrodotoxin-Bearing Clams. Front. Microbiol. 2020, 11, 1860.
- Kheifets, J.; Rozhavsky, B.; Girsh Solomonovich, Z.; Marianna, R.; Soroksky, A. Severe Tetrodotoxin Poisoning after Consumption of Lagocephalus sceleratus (Pufferfish, Fugu) Fished in Mediterranean Sea, Treated with Cholinesterase Inhibitor. Case Rep. Crit. Care 2012, 2012, 782507.
- CyprusMail. Mother, Son in Critical Condition after Eating Poisonous Fish (Updated). 2016. Available online: https://cyprus-mail.com/2016/04/14/mother-son-critical-condition-eating-poisonous-fish/ (accessed on 30 November 2021).
- Mifsud, R.; Gouder, C.; Deidun, A.; Cachia, M.J.; Montefort, S. The first documented case of neurotoxicity in two patients following octopus flesh ingestion in the Mediterranean: A case study from the Maltese Islands (Central Mediterranean). J. Black Sea/Mediterr. Environ. 2019, 25, 93–100.
- HaberTürk. This Time It Was Scary! Ate a Pufferfish, Died. 2020. Available online: https://www.haberturk.com/son-dakika-haberi-bu-sefer-korkulan-oldu-balon-baligi-yedi-oldu-2756509 (accessed on 30 November 2021).
- Bédry, R.; de Haro, L.; Bentur, Y.; Senechal, N.; Galil, B.S. Toxicological risks on the human health of populations living around the Mediterranean Sea linked to the invasion of non-indigenous marine species from the Red Sea: A review. Toxicon 2021, 191, 69–82.
- Sputniknews Türkiye. Ship Captain Who Ate Pufferfish Died, 4 Sailors Hospitalized. 2021. Available online: https://tr.sputniknews.com/20210211/balon-baligi-gemi-kaptanini-oldurdu-4-denizci-hastanelik-oldu-1043784529.html (accessed on 30 November 2021).
- Guardone, L.; Maneschi, A.; Meucci, V.; Gasperetti, L.; Nucera, D.; Armani, A. A Global Retrospective Study on Human Cases of Tetrodotoxin (TTX) Poisoning after Seafood Consumption. Food Rev. Int. 2020, 36, 645–667.
- Abd Rabou, A.F.N. On the occurrence and health risks of the silver-cheeked Toadfish (Lagocephalus sceleratus Gmelin, 1789) in the marine ecosystem of the Gaza Strip, Palestine. Biodiversitas 2019, 20, 2620–2627.
- Katikou, P. Public health risks associated with tetrodotoxin and its analogues in European waters: Recent advances after the EFSA scientific opinion. Toxins 2019, 11, 240.
- Noguchi, T.; Ebesu, J.S.M. Puffer poisoning: Epidemiology and treatment. J. Toxicol.-Toxin Rev. 2001, 20, 1–10.
- European Union. Corrigendum to Regulation (EC) no 853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for food of animal origin. Off. J. Eur. Union 2004, 226, 22–82.
- Nader, M.; Indary, S.; Boustany, L. FAO EastMed the Puffer Fish Lagocephalus sceleratus (Gmelin, 1789) in the Eastern Mediterranean. GCP/INT/041/EC–GRE –ITA/TD-10. Available online: http://www.fao.org/3/ap967e/ap967e.pdf (accessed on 30 November 2021).
- Kosker, A.R.; Özogul, F.; Ayas, D.; Durmus, M.; Ucar, Y.; Regenstein, J.M.; Özogul, Y. Tetrodotoxin levels of three pufferfish species (Lagocephalus sp.) caught in the North-Eastern Mediterranean sea. Chemosphere 2019, 219, 95–99.
- Saoudi, M.; Messarah, M.; Boumendjel, A.; Abdelmouleh, A.; Kammoun, W.; Jamoussi, K.; Feki, A. El Extracted tetrodotoxin from puffer fish Lagocephalus lagocephalus induced hepatotoxicity and nephrotoxicity to wistar rats. Afr. J. Biotechnol. 2011, 10, 8140–8145.
- Arakawa, O.; Hwang, D.F.; Taniyama, S.; Takayani, T. Toxins of Pufferfish That Cause Human Intoxications. In Coastal Environmental and Ecosystem Issues of the East China Sea; Lie, A., Ishimatsu, H.-J., Eds.; Terrapub and Nagasaki University: Nagasaki, Japan, 2010; pp. 227–244.
- Sims, J.K.; Ostman, D.C. Pufferfish poisoning: Emergency diagnosis and management of mild human tetrodotoxication. Ann. Emerg. Med. 1986, 15, 1094–1098.
- Ahasan, H.A.M.N.; Mamun, A.A.; Karim, S.R.; Bakar, M.A.; Gazi, E.A.; Bala, C.S. Paralytic Complications of Puffer Fish (Tetrodotoxin) Poisoning. Singap. Med. J. 2004, 45, 73–74.
- Clark, R.F.; Williams, S.R.; Nordt, S.P.; Manoguerra, A.S. A review of selected seafood poisonings. Undersea Hyperb. Med. 1999, 26, 175–184.
- Torda, T.A.; Sinclair, E.; Ulyatt, D.B. Puffer fish (tetrodotoxin) poisoning: Clinical record and suggested management. Med. J. Aust. 1973, 1, 599–602.
- Kanchanapongkul, J. Tetrodotoxin poisoning following ingestion of the toxic eggs of the horseshoe crab Carcinoscorpius rotundicauda, a case series from 1994 through 2006. Southeast Asian J. Trop. Med. Public Health 2008, 39, 303–306.
- Lange, W.R. Puffer fish poisoning. Am. Fam. Physician 1990, 42, 1029–1033.
- Sims, J.K. A theoretical discourse on the pharmacology of toxic marine ingestions. Ann. Emerg. Med. 1987, 16, 1006–1015.
- Yang, C.C. Tetrodotoxin. In Critical Care Toxicology: Diagnosis and Management of the Critically Poisoned Patient; Brent, J., Burkhart, K., Dargan, P., Hatten, B., Megarbane, B., Palmer, R., White, J., Eds.; Springer: Cham, Switzerland, 2017; pp. 2085–2099.
- Flachsenberger, W.A. Respiratory failure and lethal hypotension due to blue-ringed octopus and tetrodotoxin envenomation observed and counteracted in animal models. Clin. Toxicol. 1986, 24, 485–502.
- Tibballs, J. Severe tetrodotoxic fish poisoning. Anaesth. Intensive Care 1988, 16, 215–217.
- Bernstein, M.E. Pharmacologic effects of tetrodotoxin: Cardivascular and antiarrhythmic activities. Toxicon 1969, 7, 287–302.
- Chew, S.K.; Chew, L.S.; Wang, K.W.; Mah, P.K.; Tan, B.Y. Anticholinesterase Drugs in the Treatment of Tetrodotoxin Poisoning. Lancet 1984, 324, 108.
- Chew, S.K.; Goh, G.H.; Wang, K.W.; Mah, P.K.; Tan, B.Y. Puffer fish (tetrodotoxin) poisoning: Clinical report and role of anti-cholinesterase drugs in therapy. Singap. Med. J. 1983, 24, 168–171.
- Kao, C.Y. Tetrodotoxin, saxitoxin and their significance in the study of excitation phenomena. Pharmacol. Rev. 1966, 18, 997–1049.
- Yang, C.C.; Liao, S.C.; Deng, J.F. Tetrodotoxin poisoning in Taiwan: An analysis of poison center data. Vet. Hum. Toxicol. 1996, 38, 282–286.
- Matsumura, M.; Yamamoto, S. The effect of tetrodotoxin on the neuromuscular junction and peripheral nerve of the toad. Jpn. J. Pharmacol. 1954, 4, 62–68.
- Liu, S.H.; Tseng, C.Y.; Lin, C.C. Is neostigmine effective in severe pufferfish-associated tetrodotoxin poisoning? Clin. Toxicol. 2015, 53, 13–21.
- Lan, M.Y.; Lai, S.L.; Chen, S.S.; Hwang, D.F. Tetrodotoxin intoxication in a uraemic patient. J. Neurol. Neurosurg. Psychiatry 1999, 67, 127–128.
- Nakashima, R.; Nakata, Y.; Kameoka, M.; Hayashi, N.; Watanabe, K.; Yagi, K. Case of tetrodotoxin intoxication in a uremic patient. Jpn. J. Toxicol. 2007, 20, 141–145.
- Ravaonindrina, N.; Andriamaso, T.H.; Rasolofonirina, N. Puffer fish poisoning in Madagascar: Four case reports. Arch. Inst. Pasteur Madag. 2001, 67, 61–64.
- Evans, M.H. Mechanism of saxitoxin and tetrodotoxin poisoning. Br. Med. Bull. 1969, 25, 263–267.
- Matsumura, K. A monoclonal antibody against tetrodotoxin that reacts to the active group for the toxicity. Eur. J. Pharmacol. Environ. Toxicol. 1995, 293, 41–45.
- Rivera, V.R.; Poli, M.A.; Bignami, G.S. Prophylaxis and treatment with a monoclonal antibody of tetrodotoxin poisoning in mice. Toxicon 1995, 33, 1231–1237.
- Huot, R.I.; Armstrong, D.L.; Chanh, T.C. Protection against nerve toxicity by monoclonal antibodies to the sodium channel blocker tetrodotoxin. Am. Soc. Clin. Investig. 1989, 83, 1821–1826.
- Kaufman, B.; Wright, D.C.; Ballou, W.R.; Monheit, D. Protection against tetrodotoxin and saxitoxin intoxication by a cross-protective rabbit anti-tetrodotoxin antiserum. Toxicon 1991, 29, 581–587.
- Watabe, S.; Sato, Y.; Nakaya, M.; Hashimoto, K.; Enomoto, A.; Kaminogawa, S.; Yamauchi, K. Monoclonal antibody raised against tetrodonic acid, a derivative of tetrodotoxin. Toxicon 1989, 27, 265–268.
- Fukiya, S.; Matsumura, K. Active and passive immunization for tetrodotoxin in mice. Toxicon 1992, 30, 1631–1634.
- Matsumura, K. In vivo neutralization of tetrodotoxin by a monoclonal antibody. Toxicon 1995, 33, 1239–1241.
- Xu, Q.; Huang, K.; Gao, L.; Zhang, H.; Rong, K. Toxicity of tetrodotoxin towards mice and rabbits. Wei Sheng Yan Jiu 2003, 32, 371–374.
- Xu, Q.H.; Wei, C.H.; Huang, K.; Rong, K.T. Toxin-neutralizing effect and activity-quality relationship for mice tetrodotoxin-specific polyclonal antibodies. Toxicology 2005, 206, 439–448.
- Qinhui, X.U.; Wei, C.; Huang, K.K. An experimental vaccine against tetrodotoxin with longer term of validity. Chin. J. Immunol. 1985, 12, 3–6.
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2018, 35, 8–53.
- Hill, R.T.; Fenical William, W. Pharmaceuticals from marine natural products: Surge or ebb? Curr. Opin. Biotechnol. 2010, 21, 777–779.
- Lichota, A.; Gwozdzinski, K. Anticancer activity of natural compounds from plant and marine environment. Int. J. Mol. Sci. 2018, 19, 3533.
- Newman, D.J.; Cragg, G.M. Drugs and Drug Candidates from Marine Sources: An Assessment of the Current “State of Play”. Planta Med. 2016, 82, 775–789.
- von Schwarzenberg, K.; Vollmar, A.M. Targeting apoptosis pathways by natural compounds in cancer: Marine compounds as lead structures and chemical tools for cancer therapy. Cancer Lett. 2013, 332, 295–303.
- Saoudi, M.; Abdelmouleh, A.; El Feki, A. Tetrodotoxin: A potent marine toxin. Toxin Rev. 2010, 29, 60–70.
- Narahashi, T. Mechanism of action of tetrodotoxin and saxitoxin on excitable membranes. Fed. Proc. 1972, 31, 1124–1132.
- Narahashi, T. Tetrodotoxin—A brief history. Proc. Japan Acad. Ser. B 2008, 84, 147–154.
- Omana-Zapata, I.; Khabbaz, M.A.; Hunter, J.C.; Clarke, D.E.; Bley, K.R. Tetrodotoxin inhibits neuropathic ectopic activity in neuromas, dorsal root ganglia and dorsal horn neurons. Pain 1997, 72, 41–49.
- Moczydlowski, E.G. The molecular mystique of tetrodotoxin. Toxicon 2013, 63, 165–183.
- Noguchi, T.; Arakawa, O. Tetrodotoxin—Distribution and accumulation in aquatic organisms, and cases of human intoxication. Mar. Drugs 2008, 6, 220–242.
- Goldlust, S.A.; Kavoosi, M.; Nezzer, J.; Kavoosi, M.; Korz, W.; Deck, K. Tetrodotoxin for Chemotherapy-Induced Neuropathic Pain: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Dose Finding Trial. Toxins 2021, 13, 235.
- Hagen, N.A.; Cantin, L.; Constant, J.; Haller, T.; Blaise, G.; Ong-Lam, M.; Du Souich, P.; Korz, W.; Lapointe, B. Tetrodotoxin for Moderate to Severe Cancer-Related Pain: A Multicentre, Randomized, Double-Blind, Placebo-Controlled, Parallel-Design Trial. Pain Res. Manag. 2017, 2017, 7212713.
- Hagen, N.A.; du Souich, P.; Lapointe, B.; Ong-Lam, M.; Dubuc, B.; Walde, D.; Love, R.; Ngoc, A.H. Tetrodotoxin for Moderate to Severe Cancer Pain: A Randomized, Double Blind, Parallel Design Multicenter Study. J. Pain Symptom Manag. 2008, 35, 420–429.
- Hagen, N.A.; Lapointe, B.; Ong-Lam, M.; Dubuc, B.; Walde, D.; Gagnon, B.; Love, R.; Goel, R.; Hawley, P.; Ngoc, A.H.; et al. A multicentre open-label safety and efficacy study of tetrodotoxin for cancer pain. Curr. Oncol. 2011, 18, 109–116.
- Campos-Ríos, A.; Rueda-Ruzafa, L.; Herrera-Pérez, S.; Rivas-Ramírez, P.; Lamas, J.A. Tetrodotoxin: A new strategy to treat visceral pain? Toxins 2021, 13, 496.
- Hagen, N.A.; Fisher, K.M.; Lapointe, B.; du Souich, P.; Chary, S.; Moulin, D.; Sellers, E.; Ngoc, A.H. An Open-Label, Multi-Dose Efficacy and Safety Study of Intramuscular Tetrodotoxin in Patients with Severe Cancer-Related Pain. J. Pain Symptom Manag. 2007, 34, 171–182.
- Guzmán, A.; de Henestrosa, A.R.F.; Marín, A.P.; Ho, A.; Borroto, J.I.G.; Carasa, I.; Pritchard, L. Evaluation of the genotoxic potential of the natural neurotoxin Tetrodotoxin (TTX) in a battery of in vitro and in vivo genotoxicity assays. Mutat. Res.-Genet. Toxicol. Environ. Mutagen. 2007, 634, 14–24.
- Shi, J.; Liu, T.T.; Wang, X.; Epstein, D.H.; Zhao, L.Y.; Zhang, X.L.; Lu, L. Tetrodotoxin reduces cue-induced drug craving and anxiety in abstinent heroin addicts. Pharmacol. Biochem. Behav. 2009, 92, 603–607.
- Song, H.; li, J.; Lu, C.L.; Kang, L.; Xie, L.; Zhang, Y.Y.; Zhou, X.B.; Zhong, S. Tetrodotoxin alleviates acute heroin withdrawal syndrome: A multicentre, randomized, double-blind, placebo-controlled study. Clin. Exp. Pharmacol. Physiol. 2011, 38, 510–514.
- Chen, S.Q.; Ren, L.M.; Huang, Z.Q. Effects of tetrodotoxin on naloxone-precipitated withdrawal syndrome in morphine-dependent rats and mice. Chin. J. Pharmacol. Toxicol. 2001, 15, 434–440.
- Nieto, F.R.; Entrena, J.M.; Cendán, C.M.; Del Pozo, E.; Vela, J.M.; Baeyens, J.M. Tetrodotoxin inhibits the development and expression of neuropathic pain induced by paclitaxel in mice. Behav. Pharmacol. 2008, 18, S52.
- Marcil, J.; Walczak, J.S.; Guindon, J.; Ngoc, A.H.; Lu, S.; Beaulieu, P. Antinociceptive effects of tetrodotoxin (TTX) in rodents. Br. J. Anaesth. 2006, 96, 761–768.
- Kayser, V.; Viguier, F.; Ioannidi, M.; Bernard, J.F.; Latrémolière, A.; Michot, B.; Vela, J.M.; Buschmann, H.; Hamon, M.; Bourgoin, S. Differential anti-neuropathic pain effects of tetrodotoxin in sciatic nerve-versus infraorbital nerve-ligated rats—Behavioral, pharmacological and immunohistochemical investigations. Neuropharmacology 2010, 58, 474–487.
- Alvarez, P.; Levine, J.D. Antihyperalgesic effect of tetrodotoxin in rat models of persistent muscle pain. Neuroscience 2015, 311, 499–507.
- González-Cano, R.; Tejada, M.Á.; Artacho-Cordón, A.; Nieto, F.R.; Entrena, J.M.; Wood, J.N.; Cendán, C.M. Effects of tetrodotoxin in mouse models of visceral pain. Mar. Drugs 2017, 15, 188.
- Salas, M.M.; McIntyre, M.K.; Petz, L.N.; Korz, W.; Wong, D.; Clifford, J.L. Tetrodotoxin suppresses thermal hyperalgesia and mechanical allodynia in a rat full thickness thermal injury pain model. Neurosci. Lett. 2015, 607, 108–113.
- Adams, H.J.; Blair, M.R.; Takman, B.H. The local anesthetic activity of saxitoxin alone and with vasoconstrictor and local anesthetic agents. Arch. Int. Pharmacodyn. Ther. 1976, 224, 275–282.
- Chorny, M.; Levy, R.J. Site-specific analgesia with sustained release liposomes. Proc. Natl. Acad. Sci. USA 2009, 106, 6891–6892.
- Shankarappa, S.A.; Tsui, J.H.; Kim, K.N.; Reznor, G.; Dohlman, J.C.; Langer, R.; Kohane, D.S. Prolonged nerve blockade delays the onset of neuropathic pain. Proc. Natl. Acad. Sci. USA 2012, 109, 17555–17560.
- Zepeda, R.J.; Candiracci, M.; Lobos, N.; Lux, S.; Miranda, H.F. Chronic Toxicity Study of Neosaxitoxin in Rats. Mar. Drugs 2014, 12, 5055–5071.
- Rodriguez-NaVarro, A.J.; Lagos, N.; Lagos, M.; Braghetto, I.; Csendes, A.; Hamilton, J.; Figueroa, C.; Truan, D.; Garcia, C.; Rojas, A. Neosaxitoxin as a local anesthetic: Preliminary observations from a first human trial. Anesthesiology 2007, 106, 339–345.
- Manriquez, V.; Castro Caperan, D.; Guzman, R.; Naser, M.; Iglesia, V.; Lagos, N. First evidence of neosaxitoxin as a long-acting pain blocker in bladder pain syndrome. Int. Urogynecol. J. 2015, 26, 853–858.
- Kohane, D.S.; Yieh, J.; Lu, N.T.; Langer, R.; Strichartz, G.R.; Berde, C.B. A re-examination of tetrodotoxin for prolonged duration local anesthesia. Anesthesiology 1998, 89, 119–131.
- Padera, R.F.; Tse, J.Y.; Bellas, E.; Kohane, D.S. Tetrodotoxin for prolonged local anesthesia with minimal myotoxicity. Muscle Nerve 2006, 34, 747–753.
- Kohane, D.S.; Smith, S.E.; Louis, D.N.; Colombo, G.; Ghoroghchian, P.; Hunfeld, N.G.M.; Berde, C.B.; Langer, R. Prolonged duration local anesthesia from tetrodotoxin-enhanced local anesthetic microspheres. Pain 2003, 104, 415–421.
- Zhao, C.; Liu, A.; Santamaria, C.M.; Shomorony, A.; Ji, T.; Wei, T.; Gordon, A.; Elofsson, H.; Mehta, M.; Yang, R.; et al. Polymer-tetrodotoxin conjugates to induce prolonged duration local anesthesia with minimal toxicity. Nat. Commun. 2019, 10, 2566.
- Berde, C.B.; Athiraman, U.; Yahalom, B.; Zurakowski, D.; Corfas, G.; Bognet, C. Tetrodotoxin-bupivacaine-epinephrine combinations for prolonged local anesthesia. Mar. Drugs 2011, 9, 2717–2728.
- Schwartz, D.M.; Fields, H.L.; Duncan, K.G.; Duncan, J.L.; Jones, M.R. Experimental study of tetrodotoxin, a long-acting topical anesthetic. Am. J. Ophthalmol. 1998, 125, 481–487.
- Schwartz, D.M.; Duncan, K.G.; Duncan, J.L. Experimental use of tetrodotoxin for corneal pain after excimer laser keratectomy. Cornea 1998, 17, 196–199.
- Green, P.G.; Alvarez, P.; Levine, J.D. Topical Tetrodotoxin Attenuates Photophobia Induced by Corneal Injury in the Rat. J. Pain 2015, 16, 881–886.
- Yamasaki, Y.; Kogure, K.; Hara, H.; Ban, H.; Akaike, N. The possible involvement of tetrodotoxin-sensitive ion channels in ischemic neuronal damage in the rat hippocampus. Neurosci. Lett. 1991, 121, 251–254.
- Lysko, P.G.; Webb, C.L.; Yue, T.L.; Gu, J.L.; Feuerstein, G. Neuroprotective effects of tetrodotoxin as a na+ channel modulator and glutamate release inhibitor in cultured rat cerebellar neurons and in gerbil global brain ischemia. Stroke 1994, 25, 2476–2482.
- Rosenberg, L.J.; Teng, Y.D.; Wrathall, J.R. Effects of the sodium channel blocker tetrodotoxin on acute white matter pathology after experimental contusive spinal cord injury. J. Neurosci. 1999, 19, 6122–6133.
- Teng, Y.D.; Wrathall, J.R. Local blockade of sodium channels by tetrodotoxin ameliorates tissue loss and long-term functional deficits resulting from experimental spinal cord injury. J. Neurosci. 1997, 17, 4359–4366.
- Nitsch, R.M.; Farber, S.A.; Growdon, J.H.; Wurtman, R.J. Release of amyloid β-protein precursor derivatives by electrical depolarization of rat hippocampal slices. Proc. Natl. Acad. Sci. USA 1993, 90, 5191–5193.
- Burack, M.A.; Stasheff, S.F.; Wilson, W.A. Selective suppression of in vitro electrographic seizures by low-dose tetrodotoxin: A novel anticonvulsant effect. Epilepsy Res. 1995, 22, 115–126.
- Graber, K.D.; Prince, D.A. Tetrodotoxin prevents posttraumatic epileptogenesis in rats. Ann. Neurol. 1999, 46, 234–242.
- Kitamura, C.; Takahashi, M.; Kondoh, Y.; Tashiro, H.; Tashiro, T. Identification of synaptic activity-dependent genes by exposure of cultured cortical neurons to tetrodotoxin followed by its withdrawal. J. Neurosci. Res. 2007, 85, 2385–2399.
- El-Dayem, S.M.A.; Fouda, F.M.; Ali, E.H.A.; Motelp, B.A.A. El The antitumor effects of tetrodotoxin and/or doxorubicin on Ehrlich ascites carcinoma-bearing female mice. Toxicol. Ind. Health 2013, 29, 404–417.
- Fouda, F.M. Anti-tumor activity of tetrodotoxin extracted from the Masked Puffer fish Arothron diadematus. Egypt. J. Biol. 2005, 7, 1–13.
- Fraser, S.P.; Salvador, V.; Manning, E.A.; Mizal, J.; Altun, S.; Raza, M.; Berridge, R.J.; Djamgoz, M.B.A. Contribution of functional voltage-gated Na+ channel expression to cell behaviors involved in the metastatic cascade in rat prostate cancer: I. Lateral motility. J. Cell. Physiol. 2003, 195, 479–487.
- Fraser, S.P.; Ding, Y.; Liu, A.; Foster, C.S.; Djamgoz, M.B.A. Tetrodotoxin suppresses morphological enhancement of the metastatic MAT-LyLu rat prostate cancer cell line. Cell Tissue Res. 1999, 295, 505–512.
- Raghavendra Prasad, H.S.; Qi, Z.; Srinivasan, K.N.; Gopalakrishnakone, P. Potential effects of tetrodotoxin exposure to human glial cells postulated using microarray approach. Toxicon 2004, 44, 597–608.
- Bragadeeswaran, S.; Therasa, D.; Prabhu, K.; Kathiresan, K. Biomedical and pharmacological potential of tetrodotoxin-producing bacteria isolated from marine pufferfish arothron hispidus (Muller, 1841). J. Venom. Anim. Toxins Incl. Trop. Dis. 2010, 16, 421–431.
- Xiao, Z.; Bian, Z. Biosynthetic Studies of Tetrodotoxin and Its Anticancer Activities Assessment In Vitro. Master’s Thesis, Hong Kong Baptist University, Hong Kong, China, 2014.
- Shomorony, A.; Santamaria, C.M.; Zhao, C.; Rwei, A.Y.; Mehta, M.; Zurakowski, D.; Kohane, D.S. Prolonged Duration Local Anesthesia by Combined Delivery of Capsaicin- A nd Tetrodotoxin-Loaded Liposomes. Anesth. Analg. 2019, 129, 709–717.
- Law, W.Y.; Asaruddin, M.R.; Bhawani, S.A.; Mohamad, S. Pharmacophore modelling of vanillin derivatives, favipiravir, chloroquine, hydroxychloroquine, monolaurin and tetrodotoxin as MPro inhibitors of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). BMC Res. Notes 2020, 13, 527.
- Dandawate, P.R.; Subramaniam, D.; Anant, S. Targeting Cancer Stem Cells by Functional Foods and Their Constituents. Semin. Cancer Biol. 2016, 40, 192–208.
- Grimes, J.A.; Djamgoz, M.B.A. Electrophysiological characterization of voltage-gated Na+ current expressed in the highly metastatic Mat-LyLu cell line of rat prostate cancer. J. Cell. Physiol. 1998, 175, 50–58.
More
Information
Subjects:
Toxicology; Medicine, Research & Experimental
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
6.9K
Revisions:
3 times
(View History)
Update Date:
17 Jan 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





