
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Victor Dyakin | + 2635 word(s) | 2635 | 2022-01-04 09:17:40 | | | |
| 2 | Bruce Ren | Meta information modification | 2635 | 2022-01-17 02:13:22 | | |
Video Upload Options
In humans, biological aging (age-associated degrading changes), widely observed in molecular and cellular processes, underly the time-dependent decline in spatial navigation, time perception, cognitive and psychological abilities, and memory. Cross-talk of biological, cognitive, and psychological clocks provides an integrative contribution to healthy and advanced aging. At the molecular level, genome, proteome, and lipidome instability are widely recognized as the primary causal factors in aging.
Through stress response systems (SRS), the environmental and psychological stressors contribute to the age-associated “collapse” of protein homochirality. The role of prevalent protein chirality and entropy of protein folding in biological aging is mainly overlooked. In a more generalized context, the time-dependent shift from enzymatic to the nonenzymatic transformation of biochirality might represent an important and yet underappreciated hallmark of aging. We provide the experimental arguments in support of the racemization theory of aging.
1. Introduction
“The roots of stress research lie in the belief that stress can accelerate biological aging”[1]
2. Aging, Entropy, and Aging Defense System
“It is by avoiding the rapid decay into the inert state of ‘equilibrium’ that an organism appears so enigmatic”[9]
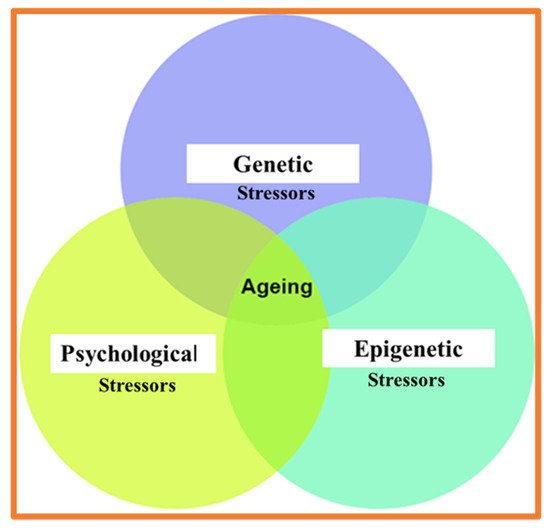
3. Psychological and Physical Stressor
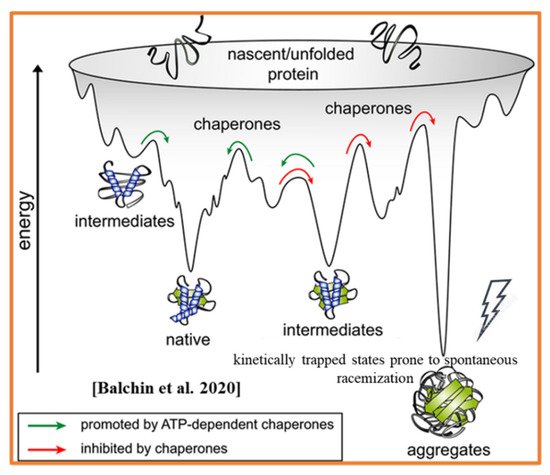
4. Biochirality, Spontaneous Reactions and Aging
“Chirality is a fundamental, persistent, but often overlooked feature of all living organisms on the molecular level as well as on the macroscopic scale.”
References
- Entringer, S.; Epel, E.S. The stress field ages: A close look into cellular aging processes. Psychoneuroendocrinology 2020, 113, 104537.
- Moreno-Villanueva, M.; Bürkle, A. Molecular consequences of psychological stress in human aging. Exp. Gerontol. 2015, 68, 39–42.
- Lavretsky, H.; Newhouse, P.A. Stress, Inflammation, and Aging. Am. J. Geriatr. Psychiatry 2012, 20, 729–733.
- Guillot, M. Thinking of oneself as the thinker: The concept of self and the phenomenology of intellection. Conscious Thinking and Cognitive Phenomenology. Philos. Explor. 2016, 19, 138–160.
- Maier, S.F.; Watkins, L.R. Cytokines for psychologists: Implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol. Rev. 1998, 105, 83–107.
- Brugada, V.; De Polavieja, G.G.; Román, Á.-C. Toward a Molecular Profile of Self-Representation. Front. Hum. Neurosci. 2016, 10, 602.
- Koseska, A.; Bastiaens, P.I. Cell signaling as a cognitive process. EMBO J. 2017, 36, 568–582.
- Schrödinger, E. What is Life—The Physical Aspect of the Living Cell; Cambridge University Press: Cambridge, UK, 1944; ISBN 978-0-521-42708-1.
- Prigogine, I.; Nicolis, G. On Symmetry-Breaking Instabilities in Dissipative Systems. J. Chem. Phys. 1967, 46, 3542–3550.
- Teschendorff, A.E.; Sollich, P.; Kuehn, R. Signalling entropy: A novel network-theoretical framework for systems analysis and interpretation of functional omic data. Methods 2014, 67, 282–293.
- Gnesotto, F.; Mura, F.; Gladrow, J.; Broedersz, C. Broken detailed balance and non-equilibrium dynamics in living systems: A review. Rep. Prog. Phys. 2018, 81, 066601.
- Michaelian, K. The Dissipative Photochemical Origin of Life: UVC Abiogenesis of Adenine. Entropy 2021, 23, 217.
- Galzitskaya, O.V. Influence of Conformational Entropy on the Protein Folding Rate. Entropy 2010, 12, 961–982.
- Marques, B.S.; Stetz, M.A.; Jorge, C.; Valentine, K.G.; Wand, A.J.; Nucci, N.V. Protein conformational entropy is not slaved to water. Sci. Rep. 2020, 10, 1–8.
- Popov, E.M. Protein folding as a nonlinear nonequilibrium thermodynamic process. Biochem. Mol. Boil. Int. 1999, 47, 443–453.
- Pickett, S.; Sternberg, M. Empirical Scale of Side-Chain Conformational Entropy in Protein Folding. J. Mol. Biol. 1993, 231, 825–839.
- Rajitha Rajeshwar, T.; Saharay, M.; Smith, J.C.; Krishnan, M. Correlated Response of Protein Side-Chain Fluctuations and Conformational Entropy to Ligand Binding. J. Phys. Chem. B 2021, 125, 9641–9651.
- Banik, S.D.; Nandi, N. Chirality and Protein Biosynthesis. Top. Curr. Chem. 2012, 333, 255–305.
- Baxa, M.C.; Haddadian, E.J.; Jumper, J.M.; Freed, K.F.; Sosnick, T.R. Loss of conformational entropy in protein folding calculated using realistic ensembles and its implications for NMR-based calculations. Proc. Natl. Acad. Sci. USA 2014, 111, 15396–15401.
- Trbovic, N.; Cho, J.-H.; Abel, R.; Friesner, R.A.; Rance, M.; Palmer, I.A.G. Protein Side-Chain Dynamics and Residual Conformational Entropy. J. Am. Chem. Soc. 2009, 131, 615–622.
- Faraggi, E.; Dunker, A.K.; Jernigan, R.L.; Kloczkowski, A. Entropy, Fluctuations, and Disordered Proteins. Entropy 2019, 21, 764.
- Bada, J.L. Amino acid racemization dating of fossil bones. Annu. Rev. Earth Planet. Sci. 1985, 13, 241–268.
- Dyakin, V.V.; Lajtha, A.; Dyakina-Fagnano, N.V. Racemization hypothesis of neurodegeneration (RHND). Alzheimer’s Dement. 2020, 16, e047697.
- Balchin, D.; Hayer-Hartl, M.; Hartl, F.U. Recent advances in understanding catalysis of protein folding by molecular chaperones. FEBS Lett. 2020, 594, 2770–2781.
- Lester, A.W.; Moffat, S.D.; Wiener, J.; Barnes, C.A.; Wolbers, T. The Aging Navigational System. Neuron 2017, 95, 1019–1035.
- Yu, S.; Boone, A.P.; He, C.; Davis, R.C.; Hegarty, M.; Chrastil, E.R.; Jacobs, E.G. Age-Related Changes in Spatial Navigation Are Evident by Midlife and Differ by Sex. Psychol. Sci. 2021, 32, 692–704.
- Eturgeon, M.; Elustig, C.; Meck, W.H. Cognitive Aging and Time Perception: Roles of Bayesian Optimization and Degeneracy. Front. Aging Neurosci. 2016, 8, 102.
- Adamo, D.E.; Briceño, E.M.; Sindone, J.A.; Alexander, N.B.; Moffat, S.D. Age differences in virtual environment and real world path integration. Front. Aging Neurosci. 2012, 4, 26.
- Flatt, T.; Schmidt, P.S. Integrating evolutionary and molecular genetics of aging. Biochim. Biophys. Acta (BBA) Gen. Subj. 2009, 1790, 951–962.
- Flatt, T.; Partridge, L. Horizons in the evolution of aging. BMC Biol. 2018, 16, 1–13.
- Johnson, A.A.; Shokhirev, M.N.; Shoshitaishvili, B. Revamping the evolutionary theories of aging. Ageing Res. Rev. 2019, 55, 100947.
- Turan, Z.G.; Parvizi, P.; Dönertaş, H.M.; Tung, J.; Khaitovich, P.; Somel, M. Molecular footprint of Medawar’s mutation accumulation process in mammalian aging. Aging Cell 2019, 18, e12965.
- Medawar, P. An Unsolved Problem of Biology; H.K Lewis: London, UK, 1952.
- Williams, G.C. Pleiotropy, natural selection, and the evolution of senescence. Sci. Aging Knowl. Environ. 1957, 11, 398–411.
- Graham, J.E.; Christian, L.; Kiecolt-Glaser, J.K. Stress, Age, and Immune Function: Toward a Lifespan Approach. J. Behav. Med. 2006, 29, 389–400.
- Uhart, M.; Oswald, L.; McCaul, M.E.; Chong, R.; Wand, G.S. Hormonal Responses to Psychological Stress and Family History of Alcoholism. Neuropsychopharmacology 2006, 31, 2255–2263.
- Clark, B.L.; Thomas, P.G. A Cell for the Ages: Human γδ T Cells across the Lifespan. Int. J. Mol. Sci. 2020, 21, 8903.
- Riboni, F.V.; Belzung, C. Stress and psychiatric disorders: From categorical to dimensional approaches. Curr. Opin. Behav. Sci. 2017, 14, 72–77.
- Esch, T.; Stefano, G.B.; Fricchione, G.L.; Benson, H. The role of stress in neurodegenerative diseases and mental disorders. Neuro Endocrinol. Lett. 2002, 23, 199–208.
- Dayas, C.V.; Buller, K.M.; Crane, J.; Xu, Y.; Day, T. Stressor categorization: Acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. Eur. J. Neurosci. 2001, 14, 1143–1152.
- Carter, J.R.; Goldstein, D.S. Sympathoneural and Adrenomedullary Responses to Mental Stress. Compr. Physiol. 2014, 5, 119–146.
- Godoy, L.D.; Rossignoli, M.T.; Delfino-Pereira, P.; Garcia-Cairasco, N.; de Lima Umeoka, E.H. A Comprehensive Overview on Stress Neurobiology: Basic Concepts and Clinical Implications. Front. Behav. Neurosci. 2018, 12, 127.
- Shonkoff, J.P.; Boyce, W.T.; McEwen, B.S. Neuroscience, Molecular Biology, and the Childhood Roots of Health Disparities: Building a new framework for health promotion and disease prevention. JAMA 2009, 301, 2252–2259.
- Miller, G.E.; Chen, E.; Parker, K.J. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychol. Bull. 2011, 137, 959–997.
- Danese, A.; Caspi, A.; Williams, B.; Ambler, A.; Sugden, K.; Mika, J.; Werts, H.; Freeman, J.; Pariante, C.M.; Moffitt, T.; et al. Biological embedding of stress through inflammation processes in childhood. Mol. Psychiatry 2010, 16, 244–246.
- Mifsud, K.R.; Gutièrrez-Mecinas, M.; Trollope, A.F.; Collins, A.; Saunderson, E.A.; Reul, J.M. Epigenetic mechanisms in stress and adaptation. Brain Behav. Immun. 2011, 25, 1305–1315.
- Eachus, H.; Cunliffe, V. Biological Embedding of Psychosocial Stress over the Life Course. Epigenetics Aging Longev. 2018, 14, 251–270.
- Santos, A.C.L.; Muniz, C.R.; Oliveira, L.T.; Souza, J.T. Contributions of a modified electrodynamics to the molecular biochirality. Chirality 2020, 32, 1186–1190.
- Manhas, R.; Rath, P.C. Ribosome, protein synthesis, and aging. In Models, Molecules and Mechanisms in Biogerontology; Springer: Singapore, 2020; pp. 67–87.
- McCudden, C.R.; Kraus, V.B. Biochemistry of amino acid racemization and clinical application to musculoskeletal disease. Clin. Biochem. 2006, 39, 1112–1130.
- Morrow, S.M.; Bissette, A.; Fletcher, S.P. Transmission of chirality through space and across length scales. Nat. Nanotechnol. 2017, 12, 410–419.
- Pályi, G.; Zucchi, C.; Caglioti, L. Advances in BioChirality; Elsevier: Amsterdam, The Netherlands, 1999.
- Inaki, M.; Liu, J.; Matsuno, K. Cell chirality: Its origin and roles in left–right asymmetric development. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150403.
- Dyakin, V.V.; Lucas, J.; Dyakina-Fagnano, N.V.; Posner, E.V. The Chain of Chirality Transfer as Determinant of Brain Functional Laterality. Breaking the Chirality Silence: Search for New Generation of Biomarkers; Relevance to Neurodegenerative Diseases, Cognitive Psychology, and Nutrition Science. Neurol. Neurosci. Res. 2017, 1, 2.
- Liu, B.; Pappas, C.; Ottelé, J.; Schaeffer, G.; Jurissek, C.; Pieters, P.F.; Altay, M.; Marić, I.; Stuart, M.C.A.; Otto, S. Spontaneous Emergence of Self-Replicating Molecules Containing Nucleobases and Amino Acids. J. Am. Chem. Soc. 2020, 142, 4184–4192.
- Plasson, R.; Brandenburg, A. Homochirality and the Need for Energy. Orig. Life Evol. Biosph. 2009, 40, 93–110.
- Lambeth, T.R.; Riggs, D.L.; Talbert, L.E.; Tang, J.; Coburn, E.; Kang, A.S.; Noll, J.; Augello, C.; Ford, B.D.; Julian, R.R. Spontaneous Isomerization of Long-Lived Proteins Provides a Molecular Mechanism for the Lysosomal Failure Observed in Alzheimer’s Disease. ACS Central Sci. 2019, 5, 1387–1395.
- Hinterholzer, A.; Stanojlovic, V.; Regl, C.; Huber, C.G.; Cabrele, C.; Schubert, M. Detecting aspartate isomerization and backbone cleavage after aspartate in intact proteins by NMR spectroscopy. J. Biomol. NMR 2021, 75, 71–82.
- Lehmann, J.; Ye, S. D Amino Acids Highlight the Catalytic Power of the Ribosome. Cell Chem. Biol. 2019, 26, 1639–1641.
- Englander, M.T.; Avins, J.L.; Fleisher, R.; Liu, B.; Effraim, P.R.; Wang, J.; Schulten, K.; Leyh, T.S.; Gonzalez, R.L.; Cornish, V.W. The ribosome can discriminate the chirality of amino acids within its peptidyl-transferase center. Proc. Natl. Acad. Sci. USA 2015, 112, 6038–6043.
- Huang, P.-Y.; Wang, F.; Narasimhan, K.; Chatman, K.; Aach, J.; Trauger, S.A.; Spoering, R.; Church, G.M. Toward D-Peptide Biosyn-thesis: Elongation Factor P Enables Ribosomal Incorporation f Consecutive D-Amino Acids. bioRxiv 2017, 125930.
- Raghunathan, S.; Yadav, K.; Rojisha, V.C.; Jaganade, T.; Prathyusha, V.; Bikkina, S.; Lourderaj, U.; Priyakum, U.D. Transition between - and -stereoisomers without bond breakng. Phys. Chem. Chem. Phys. 2020, 22, 14983–14991.
- Grassi, L.; Cabrele, C. Susceptibility of protein therapeutics to spontaneous chemical modifications by oxidation, cyclization, and elimination reactions. Amino Acids 2019, 51, 1409–1431.
- Schneiderman, N.; Ironson, G.; Siegel, S.D. Stress and Health: Psychological, Behavioral, and Biological Determinants. Annu. Rev. Clin. Psychol. 2005, 1, 607–628.
- Dziechciaż, M.; Filip, R. Biological psychological and social determinants of old age: Bio-psycho-social aspects of human aging. Ann. Agric. Environ. Med. 2014, 21, 835–838.
- Kohda, M.; Hotta, T.; Takeyama, T.; Awata, S.; Tanaka, H.; Asai, J.-Y.; Jordan, A.L. If a fish can pass the mark test, what are the implications for consciousness and self-awareness testing in animals? PLoS Biol. 2019, 17, e3000021.
- Poplin, L.; Delong, R. Accelerated Aging due to Enzymatic Racemization. Gerontology 1978, 24, 365–368.
- Fujii, N.; Ishibashi, Y.; Satoh, K.; Fujino, M.; Harada, K. Simultaneous racemization and isomerization at specific aspartic acid residues in αB-crystallin from the aged human lens. Biochim. Biophys. Acta (BBA) Protein Struct. Mol. Enzym. 1994, 1204, 157–163.
- Mori, H.; Ishii, K.; Tomiyama, T.; Furiya, Y.; Sahara, N.; Asano, S.; Endo, N.; Shirasawa, T.; Takio, K. Racemization: Its Biological Significance on Neuropathogenesis of Alzheimer’s Disease. Tohoku J. Exp. Med. 1994, 174, 251–262.
- Holloszy, J.O.; Nair, K.S. Muscle Protein Turnover: Methodological Issues and the Effect of Aging. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 1995, 50, 107–112.
- Harding, J.J.; Beswick, H.T.; Ajiboye, R.; Huby, R.; Blakytny, R.; Rixon, K.C. Non-enzymic post-translational modification of proteins in aging. A review. Mech. Ageing Dev. 1989, 50, 7–16.
- Maddox, J.L. The Encyclopedia of Aging: A Comprehensive Resource in Gerontology and Geriatrics, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 568, 854–856, 1117.
- Ritz-Timme, S.; Collins, M.J. Racemization of aspartic acid in human proteins. Ageing Res. Rev. 2002, 1, 43–59.
- Inoue, K.; Hosaka, D.; Mochizuki, N.; Akatsu, H.; Tsutsumiuchi, K.; Hashizume, Y.; Matsukawa, N.; Yamamoto, T.; Toyo’Oka, T. Simultaneous Determination of Post-Translational Racemization and Isomerization of N-Terminal Amyloid-β in Alzheimer’s Brain Tissues by Covalent Chiral Derivatized Ultraperformance Liquid Chromatography Tandem Mass Spectrometry. Anal. Chem. 2013, 86, 797–804.
- Morimoto, R.I.; Cuervo, A.M. Protein Homeostasis and Aging: Taking Care of Proteins from the Cradle to the Grave. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2009, 64, 167–170.




