
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maria Armanda Rodrigues | + 2530 word(s) | 2530 | 2022-01-12 04:25:41 |
Video Upload Options
Leishmania infantum is a parasite that causes zoonotic visceral leishmaniasis, a disease that affects humans, wild and domestic animals, mainly domestic dogs. Macrophages are cells of the immune system, existing in the peripheral blood and associated with different tissues in the mammal body, having the task to protect against microbiological threats. Interestingly, Leishmania can manipulate the macrophages into a non-active ghost-like state, allowing the parasite to stay in the host. The liver, which is a vital organ and a target for the parasite, has a resident population of macrophages designated as Kupfer cells. Therefore, a better understanding of the immune mechanisms exhibited by the macrophages when facing Leishmania parasite is needed to improve control strategies.
1. Introduction
2. L. infantum Can Infect KCs
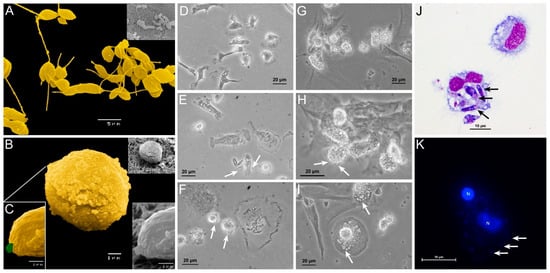
3. L. infantum Infected Blood-MØs Have Increased Lifespan
4. Infected Blood-MØs Display a Peak of PRR Gene Expression in Response to L. infantum
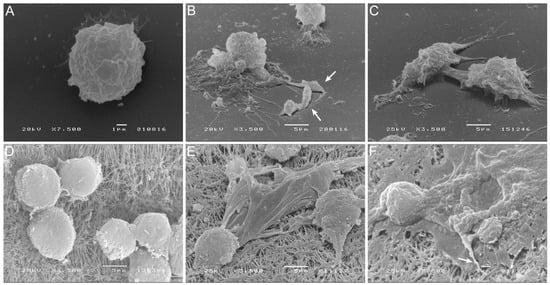
5. Blood-MØs Phagocyte Parasites While KCs Release Extracellular Traps
6. L. infantum Primes Blood-MØs for M2 Phenotype
7. Proposed model
Based on accumulated experimental pieces of evidence, it can be proposed a model of Leishmania-MØs interaction for blood-MØs and KCs (Figure 3). Blood-MØs, are “ready for action” cells by nature, with a myriad of immune tools, and are rapidly activated in the presence of L. infantum, upregulating TLRs and NODs, particularly TLR4 and NOD1, and release NO. However, shortly after parasite phagocytosis and internalization, blood-MØs became anergic and immune-dormant. These M2 primed cells upregulate anti-inflammatory cytokines (IL-4 and IL-10) and support parasite survival, evidencing a long parasite-host adaptation process, since L. infantum can subvert the immune activation state of the blood-MØs. In the liver, KCs are primed by the microenvironment towards immune tolerance and exhibit lower immune activation when encountering L. infantum amastigotes [15]. However, KCs can sense the L. infantum amastigotes by cell’s innate immune receptors, particularly transmembrane TLR2 and TLR4 and intracellular NOD1, generating low amounts of IL-12 and TNF-α, produce NO, and emit METs, favoring the availability of parasite antigens in the liver milieu which can lead to the recruitment of other leukocytes, especially CD8+T cells and natural killer cells that can initiate the formation of a granuloma response. Granuloma is a typical liver structure associated with parasite restriction and local control of the infection. However, the liver is a complex organ and the orchestration of the anti-Leishmania immune response seems derived from a multifaceted interaction between several types of cells. Hepatocytes represent the majority of the liver cells and are normally associated with metabolic functions. Yet, hepatocytes have been recently implicated in the orchestration of the liver’s anti-Leishmania immune response, by playing a potentially key role in the crosstalk between liver cells, as hepatocytes are also able to sense and react to the parasite, upregulating PRRs as well as generating immune mediators [[16][17]], therefore potentially amplifying the immune activation signals emitted by KCs.
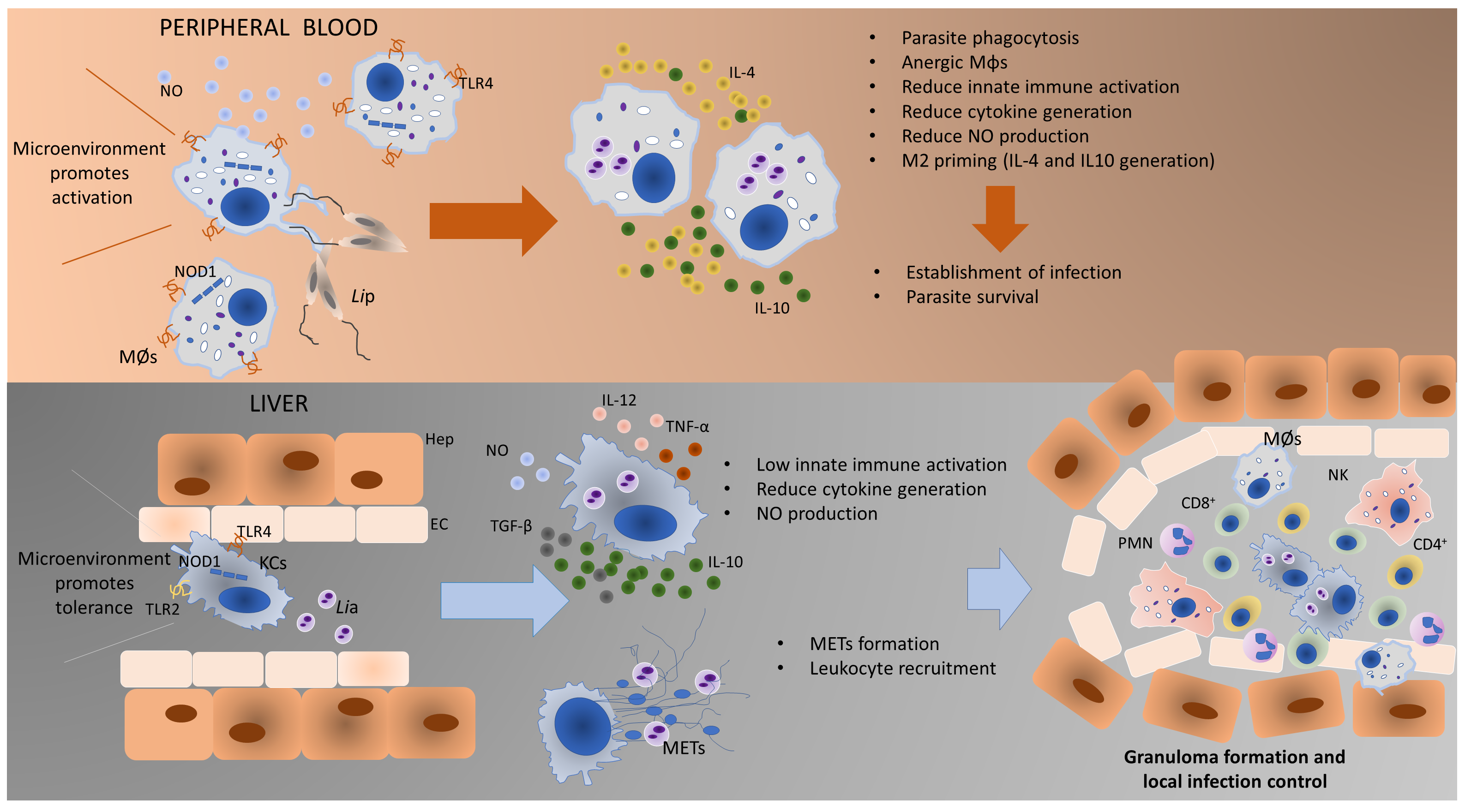 Figure 3. Proposed model of Leishmania-MØs interaction in blood and liver. Blood-MØs are rapidly activated in the presence of L. infantum, upregulating innate sensors (PRRs) and immune mediators (cytokines). However, shortly after parasite internalization blood-MØs became anergic and permissive to parasite survival. In the liver, KCs are primed by the microenvironment towards immune tolerance and exhibit low immune activation towards L. infantum. However, these cells can generate low levels of pro-inflammatory cytokines, release NO and emit METs in the presence of amastigotes, which will recruit other leukocytes and may initiate the constitution of a granuloma. The formation of granuloma is a typical hepatic structure associated with parasite restriction and local control of the infection. MØs-blood macrophages; Lip–L. infantum promastigotes; KCs-Kupffer cells; Lia–L. infantum amastigotes; METs-macrophage extracellular traps; TLR4-Toll-like receptor 4; TLR2- Toll-like receptor 2; NO-nitric oxide; NOD1-nucleotide-binding oligomerization domain-like (NOD) receptor 1; Hep-Hepatocyte; EC-Endothelial Cell; CD8+-cytotoxic T lymphocytes; CD4+-helper T lymphocytes; NK-Natural Killer cells; PMNs-Polymorphonuclear leukocytes.
Figure 3. Proposed model of Leishmania-MØs interaction in blood and liver. Blood-MØs are rapidly activated in the presence of L. infantum, upregulating innate sensors (PRRs) and immune mediators (cytokines). However, shortly after parasite internalization blood-MØs became anergic and permissive to parasite survival. In the liver, KCs are primed by the microenvironment towards immune tolerance and exhibit low immune activation towards L. infantum. However, these cells can generate low levels of pro-inflammatory cytokines, release NO and emit METs in the presence of amastigotes, which will recruit other leukocytes and may initiate the constitution of a granuloma. The formation of granuloma is a typical hepatic structure associated with parasite restriction and local control of the infection. MØs-blood macrophages; Lip–L. infantum promastigotes; KCs-Kupffer cells; Lia–L. infantum amastigotes; METs-macrophage extracellular traps; TLR4-Toll-like receptor 4; TLR2- Toll-like receptor 2; NO-nitric oxide; NOD1-nucleotide-binding oligomerization domain-like (NOD) receptor 1; Hep-Hepatocyte; EC-Endothelial Cell; CD8+-cytotoxic T lymphocytes; CD4+-helper T lymphocytes; NK-Natural Killer cells; PMNs-Polymorphonuclear leukocytes.
8. Conclusions
The immune activation potential of two different lineages of MØs in the context of CanL is demonstrated and highlighted the close evolution of Leishmania and dog macrophage. L. infantum can take advantage of the natural predisposition of blood-MØs to be activated and phagocyte pathogens and subvert the cell’s immune activation mechanisms, which allow parasite replication and dissemination, establishing infection in the host and assuring the completion of the parasite life cycle. On the other hand, liver KCs, primed for immune tolerance, are not extensively activated by the presence of this pathogen, allowing L. infantum to establish and replicate. However, KCs can activate other mechanisms, such as NO production and METs release, to ensure parasite antigen exposition, launching a cascade of leukocyte recruitment and activation to locally control the infection. Altogether KCs reveal a different response pattern from blood-MØs when facing L. infantum. In addition, KCs response appears to be more efficient in controlling this parasite, thus contributing to the ability of the liver to naturally control parasite dissemination. Understanding the mechanism of liver resistance to L. infantum infection may lead to a new paradigm in CanL epidemiology regarding the liver as a possible parasite-reservoir organ.
References
- Van Furth, R.; Beekhuizen, H. Monocytes. In Encyclopedia of Immunology; Elsevier: Amsterdam, The Netherlands, 1998; pp. 1750–1754.
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969.
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801.
- Zhou, L.; Cao, X.; Fang, J.; Li, Y.; Fan, M. Macrophages polarization is mediated by the combination of PRR ligands and distinct inflammatory cytokines. Int. J. Clin. Exp. Pathol. 2015, 8, 10964–10974.
- Shaw, M.H.; Reimer, T.; Kim, Y.G.; Nuñez, G. NOD-like receptors (NLRs): Bona fide intracellular microbial sensors. Curr. Opin. Immunol. 2008, 20, 377–382.
- Yona, S.; Kim, K.W.; Wolf, Y.; Mildner, A.; Varol, D.; Breker, M.; Strauss-Ayali, D.; Viukov, S.; Guilliams, M.; Misharin, A.; et al. Fate Mapping Reveals Origins and Dynamics of Monocytes and Tissue Macrophages under Homeostasis. Immunity 2013, 38, 79–91.
- Ginhoux, F.; Guilliams, M. Tissue-Resident Macrophage Ontogeny and Homeostasis. Immunity 2016, 44, 439–449.
- Epelman, S.; Lavine, K.J.; Randolph, G.J. Origin and Functions of Tissue Macrophages. Immunity 2014, 41, 21–35.
- Jenne, C.N.; Kubes, P. Immune surveillance by the liver. Nat. Immunol. 2013, 14, 996–1006.
- Dixon, L.J.; Barnes, M.; Tang, H.; Pritchard, M.T.; Nagy, L.E. Kupffer cells in the liver. Compr. Physiol. 2013, 3, 785–797.
- Tomiotto-Pellissier, F.; Bortoleti, B.T.d.; Assolini, J.P.; Gonçalves, M.D.; Carloto, A.C.M.; Miranda-Sapla, M.M.; Conchon-Costa, I.; Bordignon, J.; Pavanelli, W.R. Macrophage Polarization in Leishmaniasis: Broadening Horizons. Front. Immunol. 2018, 9, 2529.
- Pereira, M.A.; Alexandre-Pires, G.; Câmara, M.; Santos, M.; Martins, C.; Rodrigues, A.; Adriana, J.; Passero, L.F.D.; da Fonseca, I.P.; Santos-Gomes, G. Canine neutrophils cooperate with macrophages in the early stages of Leishmania infantum in vitro infection. Parasite Immunol. 2019, 41, e12617.
- Olivier, M.; Gregory, D.J.; Forget, G. Subversion mechanisms by which Leishmania parasites can escape the host immune response: A signaling point of view. Clin. Microbiol. Rev. 2005, 18, 293–305.
- Bogdan, C. Macrophages as host, effector and immunoregulatory cells in leishmaniasis: Impact of tissue micro-environment and metabolism. Cytokine X 2020, 2, 100041.
- Rodrigues A., Santos-Mateus D., Alexandre-Pires G., Valério-Bolas A., Rafael-Fernandes M., Pereira MA., Ligeiro D., Jesus J., Alves-Azevedo R., Lopes-Ventura S., Santos M., Tomás A., Pereira da Fonseca I., Santos-Gomes G.; Leishmania infantum exerts immunomodulation in canine Kupffer cells reverted by meglumine antimoniate. Comparative Immunology, Microbiology and Infectious Diseases 2017, 55, 42-52, https://doi.org/10.1016/j.cimid.2017.09.004.
- Rodrigues, A., Alexandre-Pires, G., Valério-Bolas, A., Santos-Mateus, D., Rafael-Fernandes, M., Pereira, M., Ligeiro, D., Nunes, T., Alves-Azevedo R., Lopes-Ventura S., Santos M., Tomás A., Pereira da Fonseca I., Santos-Gomes, G.; Dog hepatocytes are key effector cells in the liver innate immune response to Leishmania infantum.. Parasitology 2019, 146, 753-764, doi:10.1017/S0031182018002068.
- Armanda V. Rodrigues; Graça Alexandre-Pires; Ana Valério-Bolas; David Santos-Mateus; Mariana Rafael-Fernandes; Maria A. Pereira; Dário Ligeiro; Telmo Nunes; Raquel Alves-Azevedo; Marcos Santos; et al.Isabel Pereira Da FonsecaGabriela Santos-Gomes 3D-Hepatocyte Culture Applied to Parasitology: Immune Activation of Canine Hepatic Spheroids Exposed to Leishmania infantum. Biomedicines 2020, 8, 628, 10.3390/biomedicines8120628.




