
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ana Dobrincic | + 3082 word(s) | 3082 | 2021-12-29 03:24:32 | | | |
| 2 | Peter Tang | Meta information modification | 3082 | 2022-01-14 03:16:46 | | |
Video Upload Options
Brown algae are a rich source of bioactive molecules such as proteins, amino acids, polysaccharides, fatty acids, vitamins, minerals, dietary fibre, sterols, pigments, polyphenols etc. which possess a broad spectrum of biological activities (anticoagulant, antithrombotic, anti-viral, anti-cancer, anti-inflammatory and antibacterial). These compounds therefore provide high potential for the application of brown algae extracts in the treatment of arteriosclerosis, rheumatic processes, hypertension, goitre, asthma, ulcers, menstrual disorders, syphilis, skin diseases etc. The extraction process of these polysaccharides includes several complex and time-consuming steps and the correct adjustment of extraction parameters (e.g., time, temperature, power, pressure, solvent and sample to solvent ratio) greatly influences the yield, physical, chemical and biochemical properties as well as their biological activities.
1. Introduction
2. The Chemical Structure and Bioactivity of Polysaccharides from Marine Brown Algae
2.1. Laminarin

2.2. Alginates
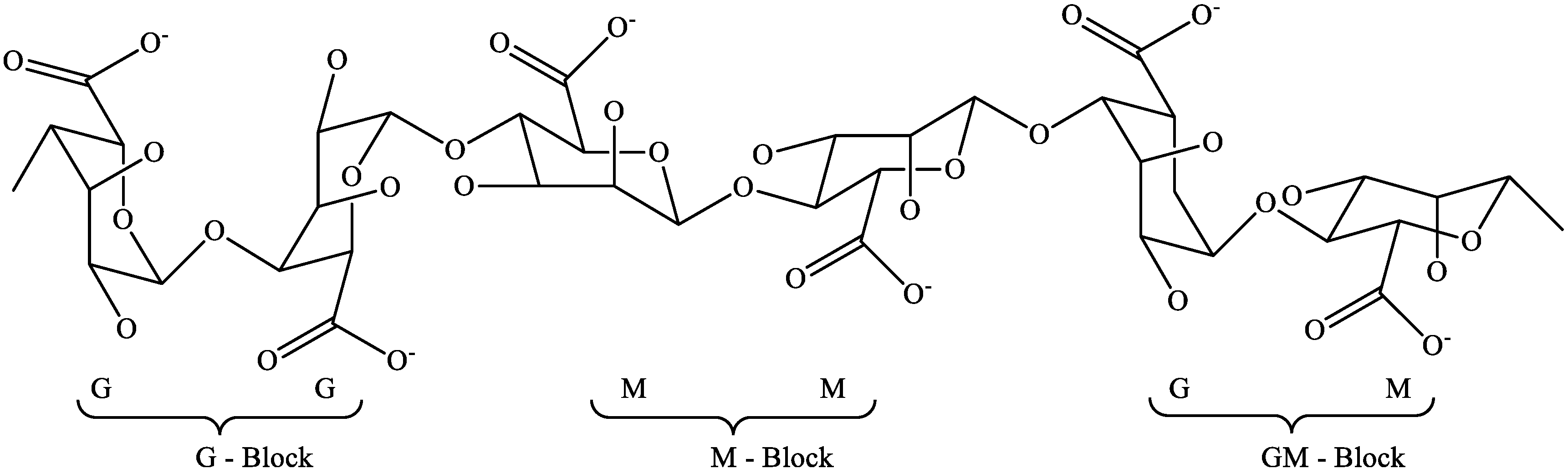
2.3. Fucoidan
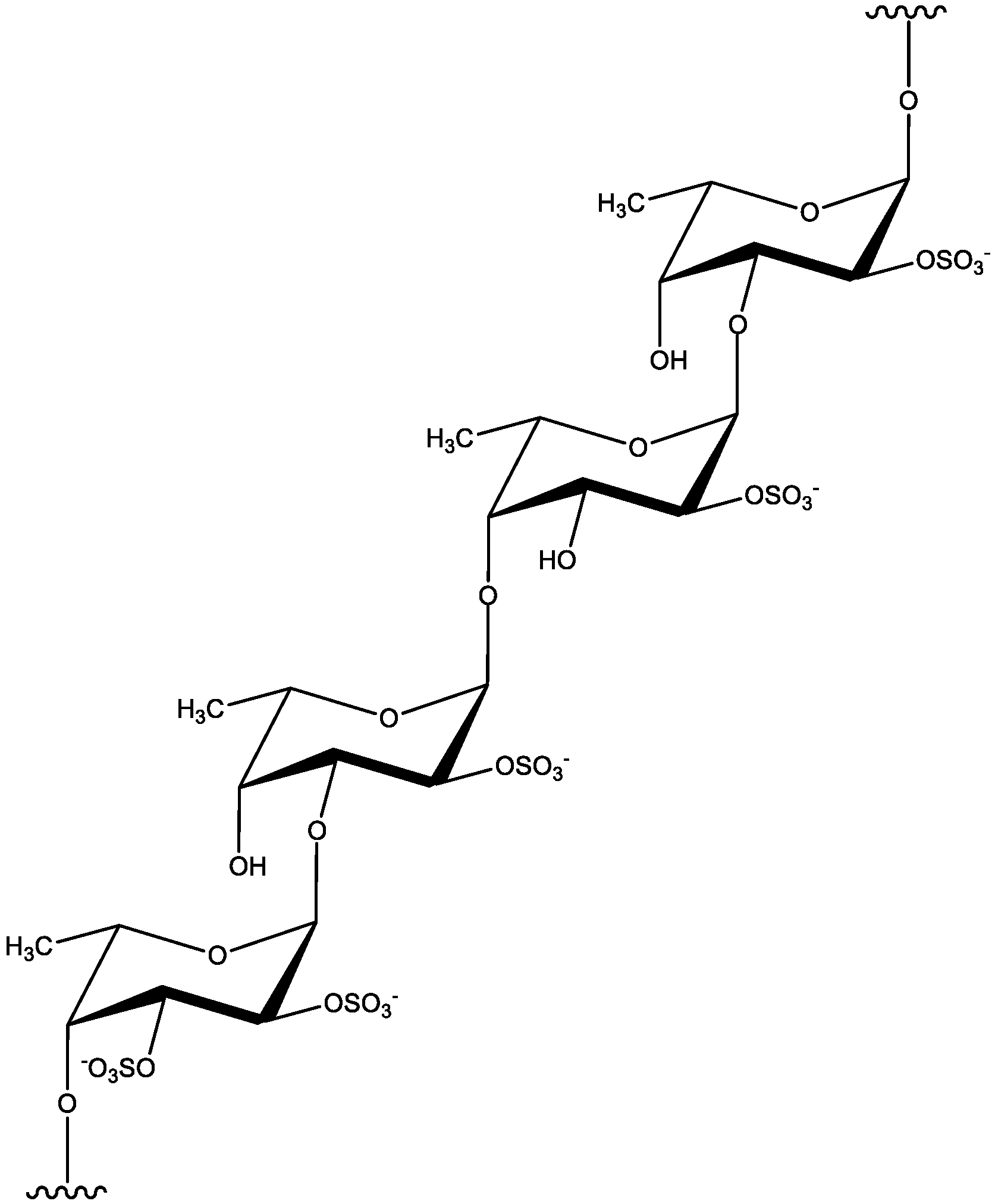
3. The Perspective of Advanced Technologies for Polysaccharide Extraction from Marine Brown Algae
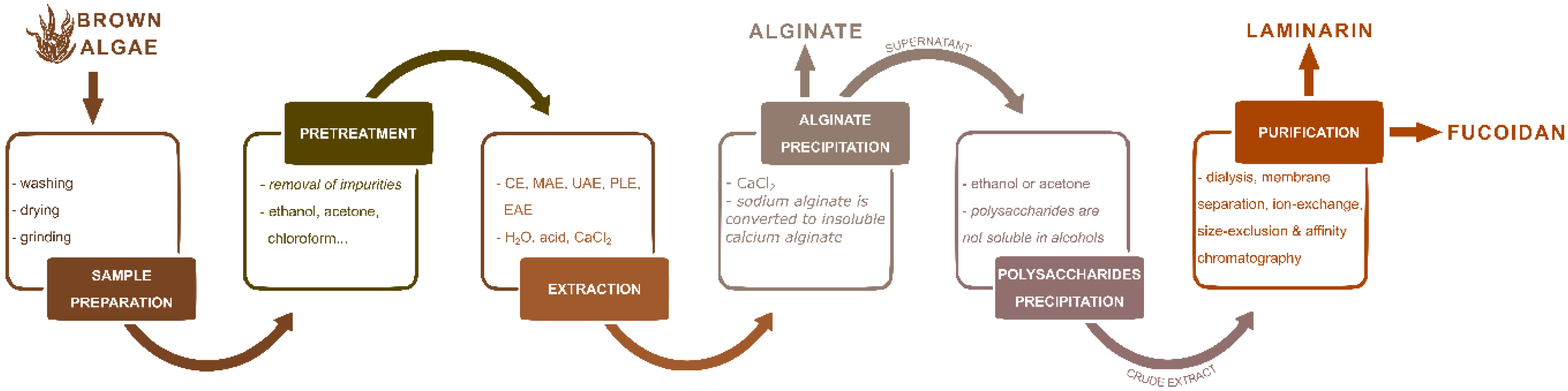
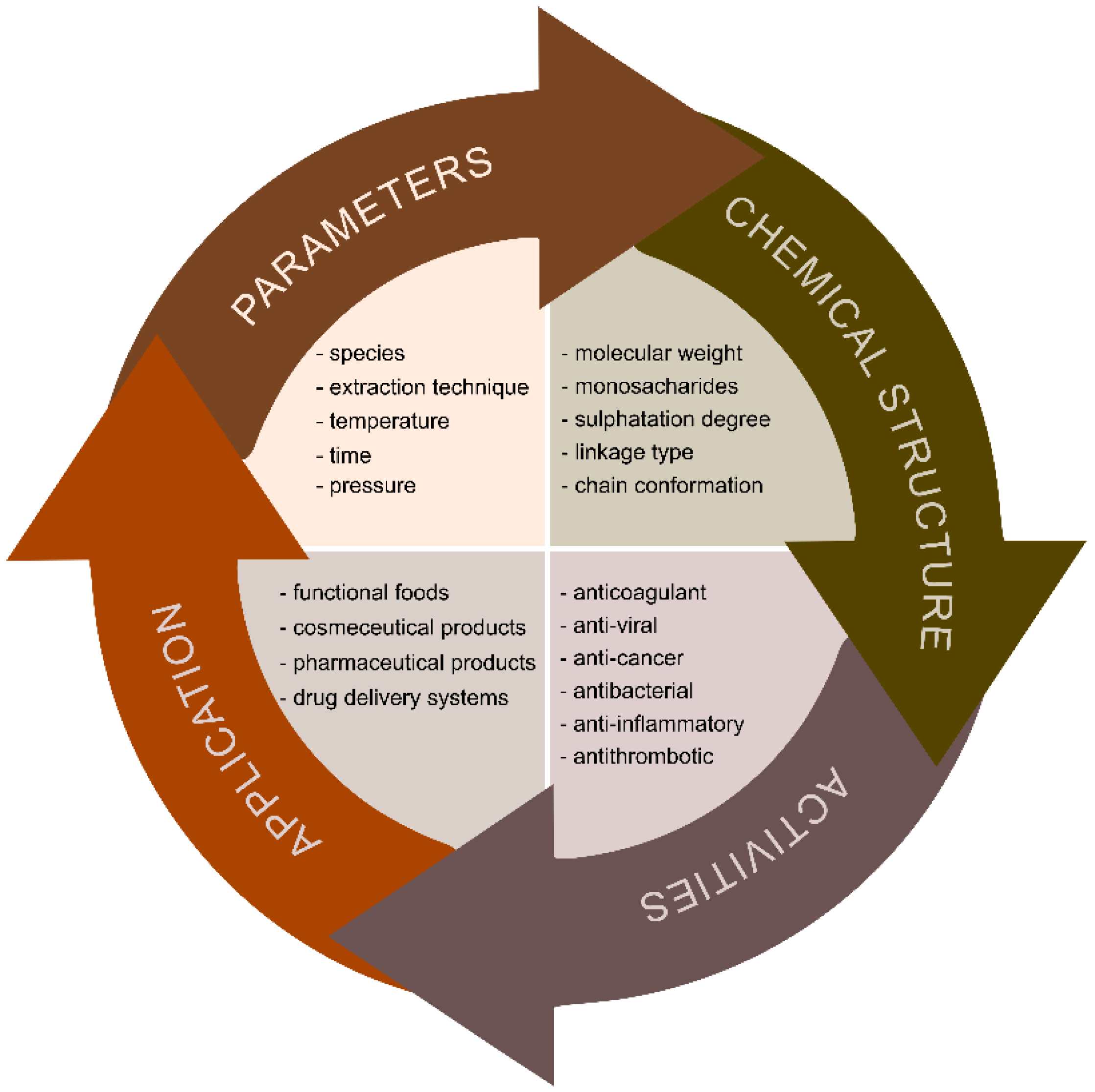
3.1. Pre-treatment of Marine Brown Algae
3.2. Extraction Techniques
3.2.1. Conventional Extraction Technique (CE)
|
Algae |
Polysaccharide |
PRETREATMENT |
EXTRACTION |
Purification |
Yield |
References |
|---|---|---|---|---|---|---|
|
Solvent; Time; Temperature |
Solvent; Time; Temperature |
|||||
|
S. henslowianum |
fucoidan |
95% EtOH; 2 × 12 h |
H2O; 3 × 2 h; reflux |
EtOH precipitation; dialysis (12000 Da) |
5.1% |
[46] |
|
S. fusiforme |
fucoidan |
95% EtOH; 24 h; 30 °C |
H2O; 3 h; 80 °C |
EtOH precipitation; dialysis (3,5 kDa) |
3.94–11.24% |
[50] |
|
1.0M HCl; 6 h; 25 °C |
||||||
|
2% CaCl2; 3 h; 50 °C |
||||||
|
E. maxima L. pallida S. rugosum |
fucoidan |
/ |
H2O; 24 h; 70 °C |
/ |
[43] |
|
|
0.15M HCl; 2 h; 65 °C |
EtOH precipitation |
|||||
|
methanol-chloroform-H2O (4:2:1); overnight; room temp. |
2% CaCl2; 5 h; 85 °C |
10% CTAB |
||||
|
C. barbata |
laminarin |
acetone-methanol (7:3); 2 × 24 h; 30 °C chloroform; 2 × 24 h; 30 °C |
0.1M HCl; 2 × 2 h; 60 °C |
EtOH precipitation; ultrafiltration (50, 10 & 1 kDa) |
7.27% |
[42] |
|
Cystoseira compressa |
sodium alginate |
acetone; 2 × 24 h; 25 °C methanol; 2 × 24 h; 25 °C |
0.1M HCl; 2 h; 60 °C 3% Na2CO3; 2 h; 60 °C |
EtOH precipitation; dialysis (3,5 kDa) |
fucoidan—5.2% |
[16] |
|
Dictyopteris divaricata |
polysaccharides |
/ |
H2O; 5–7 h; 80–100 °C; water to solid ratio 90–110 mL/g |
EtOH precipitation |
3.05% |
[51] |
|
Sargassum latifolium |
sodium alginate |
/ |
2% citric acid; 2 h; room temperature 3% Na2CO3; 1–3 h; 25–45 °C |
EtOH precipitation |
18.89–40.43% |
[52] |
|
Fucus serratus F. vesiculosus A. nodosum |
fucoidan |
85% EtOH; overnight; room temp. |
0.1M HCl; 4 h; 80 °C 1% CaCl2; overnight; 4 °C |
EtOH precipitation |
F. serratus—4.2–7.5% F. vesiculosus—8.1–12.2% A. nodosum—6.5–8.9% |
[53] |
|
D. Membranaceae P. Pavonica |
sodium alginate |
methanol-dichloromethane (1:1); 3x48h; room temp. petroleum ether; soxhlet acetone; soxhlet |
2% CaCl2; 3 × 3h 1M Na2CO3; 2 h |
EtOH precipitation |
D. Membranaceae - 18.93% P. Pavonica—66.72% |
[45] |
|
Cystoseira sedoides |
fucoidan sodium alginate |
acetone; 24 h; 25 °C 80% EtOH; 24 h; 25 °C 80% EtOH; 24 h; 78 °C |
2% CaCl2; 7 h; 70°C 2% Na2CO3; 70°C |
dialysis (7 kDa) |
fucoidan—4.2% alginate—11% |
[15] |
|
C. myrica |
polysaccharides |
petroleum ether acetone |
H2O; 8 h; 80°C |
EtOH precipitation; 10% CTAB; dialysis |
5.3% |
[44] |
|
Cystoseira crinite C. compressa C. sedoides |
fucoidan |
methanol-dichloromethane (1:1); 3 × 48 h; room temp. |
2% CaCl2; 3 × 3h |
dialysis (30 kDa) |
2.8–3.7% |
[54] |
3.2.2. Advanced Extraction Techniques
3.3. Purification Procedure
References
- Cavalier-Smith, T. Evolution and relationships of algae: Major branches of the tree of life. In Unravelling the Algae the Past, Present, and Future of Algal Systematics; Juliet Brodie, J.L., Ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 21–55. ISBN 0-8493-7989-X.
- Rindi, F. Diversity and Classification of Marine Benthic Algae. Available online: http://marinespecies.org/introduced/wiki/Diversity_and_classification_of_marine_benthic_algae#cite_note-Cavalier-1 (accessed on 24 November 2019).
- Xu, S.Y.; Huang, X.; Cheong, K.L. Recent advances in marine algae polysaccharides: Isolation, structure, and activities. Mar. Drugs 2017, 15, 388.
- Mišurcová, L.; Orsavová, J.; Ambrožová, J.V. Algal Polysaccharides and Health. In Polysaccharides; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–29.
- Zhao, C.; Yang, C.; Liu, B.; Lin, L.; Sarker, S.D.; Nahar, L.; Yu, H.; Cao, H.; Xiao, J. Bioactive compounds from marine macroalgae and their hypoglycemic benefits. Trends Food Sci. Technol. 2018, 72, 1–12.
- Costa, L.S.; Fidelis, G.P.; Cordeiro, S.L.; Oliveira, R.M.; Sabry, D.A.; Câmara, R.B.G.; Nobre, L.T.D.B.; Costa, M.S.S.P.; Almeida-Lima, J.; Farias, E.H.C.; et al. Biological activities of sulfated polysaccharides from tropical seaweeds. Biomed. Pharmacother. 2010, 64, 21–28.
- Pereira, L. Seaweeds as source of bioactive substances and skin care therapy-Cosmeceuticals, algotheraphy, and thalassotherapy. Cosmetics 2018, 5, 68.
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–233.
- Garcia-Vaquero, M.; Rajauria, G.; O’Doherty, J.V.; Sweeney, T. Polysaccharides from macroalgae: Recent advances, innovative technologies and challenges in extraction and purification. Food Res. Int. 2017, 99, 1011–1020.
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important determinants for fucoidan bioactivity: A critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs 2011, 9, 2106–2130.
- Praveen, M.A.; Parvathy, K.R.K.; Balasubramanian, P.; Jayabalan, R. An overview of extraction and purification techniques of seaweed dietary fibers for immunomodulation on gut microbiota. Trends Food Sci. Technol. 2019, 92, 46–64.
- De Jesus Raposo, M.F.; De Morais, A.M.B.; De Morais, R.M.S.C. Marine polysaccharides from algae with potential biomedical applications. Mar. Drugs 2015, 13, 2967–3028.
- Hahn, T.; Lang, S.; Ulber, R.; Muffler, K. Novel procedures for the extraction of fucoidan from brown algae. Process Biochem. 2012, 47, 1691–1698.
- Dore, C.M.P.G.; Faustino Alves, M.G.D.C.; Pofírio Will, L.S.E.; Costa, T.G.; Sabry, D.A.; De Souza Rêgo, L.A.R.; Accardo, C.M.; Rocha, H.A.O.; Filgueira, L.G.A.; Leite, E.L. A sulfated polysaccharide, fucans, isolated from brown algae Sargassum vulgare with anticoagulant, antithrombotic, antioxidant and anti-inflammatory effects. Carbohydr. Polym. 2013, 91, 467–475.
- Hadj Ammar, H.; Hafsa, J.; Le Cerf, D.; Bouraoui, A.; Majdoub, H. Antioxidant and gastroprotective activities of polysaccharides from the Tunisian brown algae (Cystoseira sedoides). J. Tunis. Chem. Soc. 2016, 18, 80–88.
- Hentati, F.; Delattre, C.; Ursu, A.V.; Desbrières, J.; Le Cerf, D.; Gardarin, C.; Abdelkafi, S.; Michaud, P.; Pierre, G. Structural characterization and antioxidant activity of water-soluble polysaccharides from the Tunisian brown seaweed Cystoseira compressa. Carbohydr. Polym. 2018, 198, 589–600.
- Mzibra, A.; Meftah Kadmiri, I.; El Arroussi, H. Enzymatic Technologies for Marine Polysaccharides; Trincone, A., Ed.; CRS PRESS: Boca Raton, FL, USA, 2019.
- Chaminda Lakmal, H.H.; Lee, J.-H.; Jeon, Y.-J. Enzyme-assisted extraction of a marine algal polysaccharide, fucoidan and bioactivities. In Polysaccharides: Bioactivity and Biotechnology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–2241. ISBN 9783319162980.
- Lim, S.J.; Wan Aida, W.M. Extraction of sulfated polysaccharides (fucoidan) from brown seaweed. In Seaweed Polysaccharides; Elsevier: Amsterdam, The Netherlands, 2017; pp. 27–46. ISBN 9780128098172.
- Nisizawa, K.; Yamaguchi, T.; Handa, N.; Maeda, M.; Yamazaki, H. Chemical nature of a uronic acid-containing polysaccharide in the peritrophic membrane of the silkworm. J. Biochem. 1963, 54, 419–426.
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Extraction, structure and biofunctional activities of laminarin from brown algae. Int. J. Food Sci. Technol. 2015, 50, 24–31.
- Quillet, M. Glucide metabolism of brown algae. Presence of small quantities of Laminarin in numerous new species, distributed over the entire group of Phaeophyceae. Comptes Rendus l’Académie Sci. 1958, 246, 812–815.
- Chizhov, A.O.; Dell, A.; Morris, H.R.; Reason, A.J.; Haslam, S.M.; McDowell, R.A.; Chizhov, O.S.; Usov, A.I. Structural analysis of laminarans by MALDI and FAB mass spectrometry. Carbohydr. Res. 1998, 310, 203–210.
- Menshova, R.V.; Ermakova, S.P.; Anastyuk, S.D.; Isakov, V.V.; Dubrovskaya, Y.V.; Kusaykin, M.I.; Um, B.H.; Zvyagintseva, T.N. Structure, enzymatic transformation and anticancer activity of branched high molecular weight laminaran from brown alga Eisenia bicyclis. Carbohydr. Polym. 2014, 99, 101–109.
- Miao, H.Q.; Elkin, M.; Aingorn, E.; Ishai-Michaeli, R.; Stein, C.A.; Vlodavsky, I. Inhibition of heparanase activity and tumor metastasis by laminarin sulfate and synthetic phosphorothioate oligodeoxynucleotides. Int. J. Cancer 1999, 83, 424–431.
- Bae, H.; Song, G.; Lee, J.; Hong, T.; Chang, M.; Lim, W. Laminarin-derived from brown algae suppresses the growth of ovarian cancer cells via mitochondrial dysfunction and ER stress. Mar. Drugs 2020, 18, 152.
- Hernández-Carmona, G.; Freile-Pelegrín, Y.; Hernández-Garibay, E. Conventional and Alternative Technologies for the Extraction of Algal Polysaccharides; Woodhead Publishing: Shaston, UK, 2013; ISBN 9780857095121.
- Bixler, H.J.; Porse, H. A decade of change in the seaweed hydrocolloids industry. J. Appl. Phycol. 2011, 23, 321–335.
- Rasmussen, R.S.; Morrissey, M.T. Marine biotechnology for production of food ingredients. Adv. Food Nutr. Res. 2007, 52, 237–292.
- Collado-González, M.; Cristina Ferreri, M.; Freitas, A.R.; Santos, A.C.; Ferreira, N.R.; Carissimi, G.; Sequeira, J.A.D.; Guillermo Díaz Baños, F.; Villora, G.; Veiga, F.; et al. Complex polysaccharide-based nanocomposites for oral insulin delivery. Mar. Drugs 2020, 18, 55.
- Burtin, P. Nutritional value of seaweeds. Electron. J. Environ. Agric. Food Chem. 2003, 2, 498–503.
- Murata, M.; Nakazoe, J. Production and use of marine algae in Japan. Jpn. Agric. Res. Q. 2001, 35, 281–290.
- Kimura, Y.; Watanabe, K.; Okuda, H. Effects of soluble sodium alginate on cholesterol excretion and glucose tolerance in rats. J. Ethnopharmacol. 1996, 54, 47–54.
- Kim, I.H.; Lee, J.H. Antimicrobial activities against methicillin-resistant Staphylococcus aureus from macroalgae. J. Ind. Eng. Chem. 2008, 14, 568–572.
- Chapman, V.J.; Chapman, D.J. Seaweeds and Their Uses, 3rd ed.; Springer: Dordrecht, The Netherlands, 1980.
- Zvyagintseva, T.N.; Shevchenko, N.M.; Chizhov, A.O.; Krupnova, T.N.; Sundukova, E.V.; Isakov, V.V. Water-soluble polysaccharides of some far-eastern brown seaweeds. Distribution, structure, and their dependence on the developmental conditions. J. Exp. Mar. Biol. Ecol. 2003, 294, 1–13.
- Zvyagintseva, T.N.; Shevchenko, N.M.; Popivnich, I.B.; Isakov, V.V.; Scobun, A.S.; Sundukova, E.V.; Elyakova, L.A. A new procedure for the separation of water-soluble polysaccharides from brown seaweeds. Carbohydr. Res. 1999, 322, 32–39.
- Rioux, L.E.; Turgeon, S.L.; Beaulieu, M. Characterization of polysaccharides extracted from brown seaweeds. Carbohydr. Polym. 2007, 69, 530–537.
- Bilan, M.I.; Grachev, A.A.; Ustuzhanina, N.E.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. A highly regular fraction of a fucoidan from the brown seaweed Fucus distichus L. Carbohydr. Res. 2004, 339, 511–517.
- Van Weelden, G.; Bobi, M.; Okła, K.; van Weelden, W.J.; Romano, A.; Pijnenborg, J.M.A. Fucoidan structure and activity in relation to anti-cancer mechanisms. Mar. Drugs 2019, 17, 32.
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695.
- Sellimi, S.; Maalej, H.; Rekik, D.M.; Benslima, A.; Ksouda, G.; Hamdi, M.; Sahnoun, Z.; Li, S.; Nasri, M.; Hajji, M. Antioxidant, antibacterial and in vivo wound healing properties of laminaran purified from Cystoseira barbata seaweed. Int. J. Biol. Macromol. 2018, 119, 633–644.
- January, G.G.; Naidoo, R.K.; Kirby-McCullough, B.; Bauer, R. Assessing methodologies for fucoidan extraction from South African brown algae. Algal Res. 2019, 40, 101517.
- Sahera, M.F.; Thani, S.M.; Salha, S.Y. Characterization of sulphated polysaccharide with antiviral activity from marine brown alga Cystoseira myrica collected from Jazan coasts, KSA. Int. J. PharmTech Res. 2015, 8, 198–203.
- Deghrigue Abid, M.; Lajili, S.; Hadj Ammar, H.; Cherif, D.; Eltaief, N.; Majdoub, H.; Bouraoui, A. Chemical and biological properties of sodium alginates isolated from tow brown algae Dictyopteris Membranaceae and Padina Pavonica. Trends J. Sci. Res. 2019, 4, 62–67.
- Sun, Q.L.; Li, Y.; Ni, L.Q.; Li, Y.X.; Cui, Y.S.; Jiang, S.L.; Xie, E.Y.; Du, J.; Deng, F.; Dong, C.X. Structural characterization and antiviral activity of two fucoidans from the brown algae Sargassum henslowianum. Carbohydr. Polym. 2020, 229, 115487.
- Zhao, G.; Chen, X.; Wang, L.; Zhou, S.; Feng, H.; Chen, W.N.; Lau, R. Ultrasound assisted extraction of carbohydrates from microalgae as feedstock for yeast fermentation. Bioresour. Technol. 2013, 128, 337–344.
- Harun, R.; Yip, J.W.S.; Thiruvenkadam, S.; Ghani, W.A.W.A.K.; Cherrington, T.; Danquah, M.K. Algal biomass conversion to bioethanol-a step-by-step assessment. Biotechnol. J. 2014, 9, 73–86.
- Kadam, S.U.; Tiwari, B.K.; O’Connell, S.; O’Donnell, C.P. Effect of ultrasound pretreatment on the extraction kinetics of bioactives from brown seaweed (Ascophyllum nodosum). Sep. Sci. Technol. 2015, 50, 670–675.
- Liu, J.; Wu, S.-Y.; Chen, L.; Li, Q.-J.; Shen, Y.-Z.; Jin, L.; Zhang, X.; Chen, P.-C.; Wu, M.-J.; Choi, J.; et al. Different extraction methods bring about distinct physicochemical properties and antioxidant activities of Sargassum fusiforme fucoidans. Int. J. Biol. Macromol. 2019.
- Cui, Y.; Liu, X.; Li, S.; Hao, L.; Du, J.; Gao, D.H.; Kang, Q.; Lu, J. Extraction, characterization and biological activity of sulfated polysaccharides from seaweed Dictyopteris divaricata. Int. J. Biol. Macromol. 2018, 117, 256–263.
- Fawzy, M.A.; Gomaa, M.; Hifney, A.F.; Abdel-Gawad, K.M. Optimization of alginate alkaline extraction technology from Sargassum latifolium and its potential antioxidant and emulsifying properties. Carbohydr. Polym. 2017, 157, 1903–1912.
- Fletcher, H.R.; Biller, P.; Ross, A.B.; Adams, J.M.M. The seasonal variation of fucoidan within three species of brown macroalgae. Algal Res. 2017, 22, 79–86.
- Yuan, Y.; Macquarrie, D. Microwave assisted extraction of sulfated polysaccharides (fucoidan) from Ascophyllum nodosum and its antioxidant activity. Carbohydr. Polym. 2015, 129, 101–107.
- Scott, J.E. Fractionation by precipitation with quaternary ammonium salts. In Methods in Carbohydrate Chemistry; Whistler, R.L., BeMiller, J.N., Eds.; Academic Press: New York, NY, USA, 1965; pp. 38–44.
- Sosa-Hernández, J.E.; Escobedo-Avellaneda, Z.; Iqbal, H.M.N.; Welti-Chanes, J. State-of-the-art extraction methodologies for bioactive compounds from algal biome to meet bio-economy challenges and opportunities. Molecules 2018, 23, 2953.




