Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ashfaq Ahmad | + 8517 word(s) | 8517 | 2022-01-05 07:24:12 | | | |
| 2 | Vivi Li | Meta information modification | 8517 | 2022-01-13 10:02:01 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ahmad, A. Clinacanthus nutans (Burm. f.) Lindau Leaves. Encyclopedia. Available online: https://encyclopedia.pub/entry/18185 (accessed on 07 February 2026).
Ahmad A. Clinacanthus nutans (Burm. f.) Lindau Leaves. Encyclopedia. Available at: https://encyclopedia.pub/entry/18185. Accessed February 07, 2026.
Ahmad, Ashfaq. "Clinacanthus nutans (Burm. f.) Lindau Leaves" Encyclopedia, https://encyclopedia.pub/entry/18185 (accessed February 07, 2026).
Ahmad, A. (2022, January 13). Clinacanthus nutans (Burm. f.) Lindau Leaves. In Encyclopedia. https://encyclopedia.pub/entry/18185
Ahmad, Ashfaq. "Clinacanthus nutans (Burm. f.) Lindau Leaves." Encyclopedia. Web. 13 January, 2022.
Copy Citation
The application of natural products and supplements has expanded tremendously over the past few decades. Clinacanthus nutans (C. nutans), which is affiliated to the Acanthaceae family, has recently caught the interest of researchers from the countries of subtropical Asia due to its medicinal uses in alternative treatment for skin infection conditions due to insect bites, microorganism infections and cancer, as well as for health well-being. A number of bioactive compounds from this plant’s extract, namely phenolic compounds, sulphur containing compounds, sulphur containing glycosides compounds, terpens-tripenoids, terpens-phytosterols and chlorophyll-related compounds possess high antioxidant activities.
C. nutans
medicinal uses
phytochemistry
pharmacology
therapeutic potential
1. Introduction
The application of natural products and supplements has expanded tremendously over the past few decades due to economical and less adverse effects when compared to modern day medicines. Presently, more than 80% of people worldwide rely on them for some part of primary healthcare, especially in underdeveloped nations where drugs are usually pricey and unattainable, which encourages people to adopt traditional remedies. Starting from the late 1980s to recent years, tremendous investigations on herbal plants as alternative therapeutic agents have been used to treat a plethora of ailments due to their inexpensive cost and lower risk of side effects [1]. At present, 350,000 higher plants have been identified and, in relation to this number, only 8000 species are claimed to have medicinal properties [2]. Acanthaceae is one of the advanced and specialized families of 250 genera with approximately 2500 species providing effective traditional remedies against various health conditions [3].
C. nutans, which is affiliated to the Acanthaceae family, has recently caught the interest of researchers from the countries of subtropical Asia because of its medicinal uses. There are various vernacular names of this plant that exists different communities. In Malaysia and Brunei, C. nutans is recognized as “Sabahan snake grass” or “Belalaigajah”; “Dandanggendis” or “Ki tajam” in Indonesia; “Phayayo” or “Saledpangpontuamea” in Thailand and “You dun cao” or “Sha be she cai” in China (Table 1).
Table 1. Common vernacular names of C. nutans.
| Country | Language | Vernacular Names | References |
|---|---|---|---|
| Malaysia, Brunei |
Malay, English |
Pokok stawa ular, Belalai gajah, Sabahan snake grass |
[3] |
| Thailand | Thai | Phaya Plongtong, Phaya Yo, Saled Pangpon Tua Mea |
[4][5] |
| China | Mandarin | E zui hua, Sha be she cao, You dun cao, |
[5][6][7] |
| Indonesia | Jawa | Ki tajam Kijatan Daun dandang gendis |
[3][8][9] |
C. nutans is a scandent shrub with upright branches drooping that is over around 1–3 m tall. Its foliage usually appears as a stalked leaf with lanceolate-ovate, lanceolate to linear-lanceolate about 4–12 cm long by 1–4 cm wide. It has dull red to orange red flowers about 3.2 cm long with a green base borne in dense terminal racemes. The fruits are in the form of a capsule that is 2 cm long with short hair (Figure 1).

Figure 1. C. nutans in (A) plant habitat, (B) stems and leaves and (C) inflorescence (photographs of the plants were taken directly by using Canon, model number 3611C011, Tokyo, Japan).
This plant can be propagated via seed or stem cutting [10]. The ethnobotanical uses of C. nutans are popular in Malaysia, Indonesia, Thailand and China, where this plant is commonly used in folk medicine to treat skin rashes, herpes simplex virus-induced lesions and insect or snake bites, as well as hyperuricemia, gout, urinary complications, diabetes, renal insufficiency, hyperlipidemia and various inflammatory conditions including strains and sprains injuries, hematoma, contusion and rheumatism. Pharmacological research revealed that this plant contains antioxidants and compounds with anti-cancer, anti-viral, anti-inflammatory, antidiarrheal, anti-diabetic and renoprotective activity, as shown in Figure 2. Ever since 2011, there has been a sudden surge in the usage of C. nutans in the folks of Southeast Asia following remarkable news regarding a patient recovered from the final stage of lymph node cancer from Taiping, Malaysia (https://myherbs2017.wordpress.com/category/clinacanthus-nutans, accessed on 15 August 2021). This plant is also utilized as a therapeutic option in menstrual pain, anemia and jaundice, and repairs bone fractures according to some traditional Chinese medicine; however, more attention is required to determine the dosage and form of the plant for which it can be used as medicine [11]. Although the studied data reported the beneficial effects of roots, stem and whole parts of C. nutans, the most frequently used part of the plant was the leaves decoction with water for ingestion and immersed in alcohol for tropical application [12].
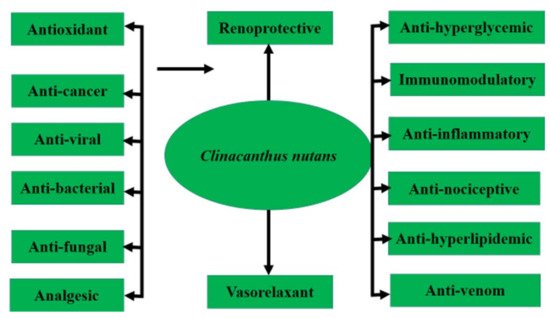
Figure 2. Pharmacological activities of C. nutans.
Due to C. nutans popularity, a wide range of commercial products are formulated in the form of concentrated liquid beverages, tea, soap, essential oil drops, massage oil, ointments, concentrated balms, creams, lotions, capsules and powder [10] (Table 2).
Table 2. A wide variety of commercial products made from C. nutans leaves for traditional and modern uses.
| Formulation | Therapeutic Purposes |
|---|---|
| Tea | Anti-cancer, anti-diabetic, anti-hypertensive and body detoxification |
| Essential oil drop | Relieves oral herpes viral infection and aphthous ulcer |
| Soap or body wash | Treatment of skin problems and blemishes |
| Cream | Treatment of Herpes zoster and Herpes genitalis infection |
| Lotion | Relieve urticarial, itching and rashes |
| Powder | Anti-cancer, anti-hypertensive and anti-diabetic |
| Ointment | Relieve aches, cramps, sprains of muscular and joint, cold, flu and insect bites |
| Balm | Relieve insect bites, skin rashes, inflammation, muscular pain and dizziness |
| Elixir | Anti-cancer, alleviate period pain and diuretic properties for urinary tract and kidney diseases |
| Capsules | General health maintenance, body detoxification, anti-diabetic and anti-hypertensive |
Adapted from: [10].
2. Phytochemistry C. nutans
The phytochemical classes that are present in C. nutans leaves are sulphur containing compounds, sulphur containing glycoside compounds, phenolic compounds, terpenstripenoids and terpens phytosterols compounds [13]. The details of sub-phytochemical compounds are tabulated in (Table 3) and chemical structure is shown in Figure 3
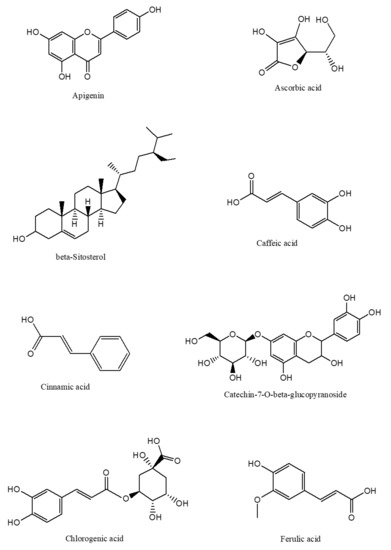
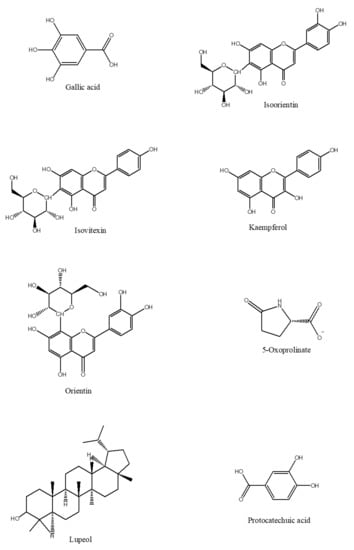
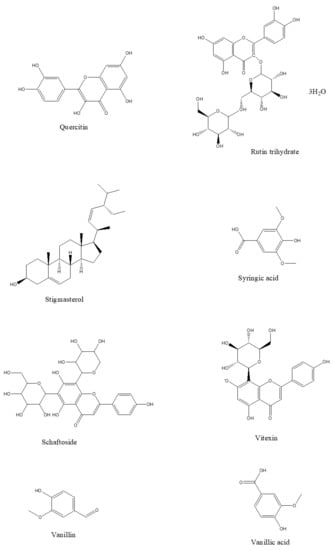
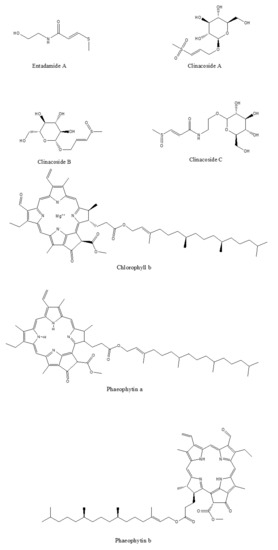
Figure 3. 2D-chemical structure from various phytochemical classes and compounds present in C. nutans leaves (diagram was reconstructed using Chem-Draw ultra-Structure Software).
3. Pharmacological and Medicinal Properties
Many plants have abundant active secondary metabolites that exhibit certain pharmacological effects in humans, and the investigation of these phytochemical constituents in medicinal plants has caught the attention of researchers worldwide. This is due to that the isolated bioactive compounds have the greatest contribution in nutraceutical and pharmaceutical industries. It has also been recognized that C. nutans has several promising therapeutic potentials, and the Thai Ministry of Public Health had shortlisted this plant into the “Thai Herbal National Essential Drug List” as one of the medicinal plants for their public healthcare policy on anti-viral activity [14]. Moreover, a non-scientific and unpublished survey of ethnobotanical applications of medicinal plants has demonstrated that C. nutans rated amongst the top five most commonly used herbs for anti-diabetic, anti-hypertensive, anti-inflammatory and antioxidant properties in other sub-tropical countries such as in Malaysia, Brunei and Singapore. Other pharmacological activities such as anti-venom, anti-cancer, anti-bacterial, anti-fungal and anti-analgesic activities have also been reported [10][15][16].
3.1. Antioxidant and Anti-Cancer Properties
From the biological point of view, antioxidants are compounds which are capable of preventing damage by oxidants or free radicals while the products of the reaction between antioxidant and oxidant should not be toxic and not a branch of the radical reaction [17]. In addition to these, the half-life of an effective antioxidant must be long enough to counteract the oxidant. Thus, as a potential antioxidant, it must always remain in sufficient concentration especially during disease prevention circumstances [18]. C. nutans leaves possess diverse medicinal potential in conventional applications. A study reported by Nik Abd Rahman and his team investigated the antioxidant effects of C. nutans extracts using bone marrow smearing, clonogenic and splenocyte immunotype analysis with two different concentrations; 200 mg/kg and 1000 mg/kg methanolic leaf extracts in a 4 -T1 tumor-bearing mice model. They reported that methanol extract from C. nutans leaves at 200 mg/kg and 1000 mg/kg significantly attenuated the nitric oxide (NO) and malondialdehyde (MDA) levels in the blood. Similarly, C. nutans extract from leaves at 1000 mg/kg decreased the number of mitotic cells, tumour weight and tumour volume. From this study, no inflammatory or adverse reactions related to splenocytes activities were found in all treated groups of mice. Moreover, the concentration of both C. nutans leaf extracts has also reduced the number of carcinogenic colonies formed in the liver and lungs. This shows that C. nutans leaf extracts exerted an antioxidant activity in the 4-T1 mouse breast model [19]. Likewise, a study lead by [20] has used C. nutans leaves extracted with 80% methanol and further fractionated with n-hexane, dichloromethane, chloroform, n-butanol and aqueous residue ranging between 125 and 4000 µg/mL, whereas the total flavonoid content, total phenolic content and total antioxidant scavenging activity on breast cancer (Michigan Cancer Foundation-7 [MCF7]) and normal breast (Michigan Cancer Foundation-10A [MCF 10A]) cell lines were measured using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging method and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radical cation decolourization assay. Based on the findings, the total phenolic content in C. nutans leaf extracts was higher than total flavonoid content. On the contrary, the n-hexane fraction had the lowest antioxidant activity; however, the crude fraction had the highest antioxidant activity according to the EC50 value. On the other hand, [21] also reported the anti-proliferative activity of C. nutans leaves extracts against the HeLa cell line, and the dichloromethane fraction had the lowest IC50 value of 70 µg/mL post 48 h incubation period; this indicated that the HeLa cell line, when exposed to the dichloromethane fraction, exhibited remarkable morphological features of apoptosis to the HeLa cancer cell line. On the contrary, [14] reported that the crude methanol extract of C. nutans leaves had the lowest scavenging activity as compared to ethyl acetate and n-butanol fractions of the methanol extract. This contradictory finding attested that the phytochemical content of C. nutans leaves is largely influenced by environmental conditions, i.e., variations in pH and nutrients in soil, temperature, humidity and water variability. Moreover, environmental factors which interact with the genetics of the C. nutans plants may lead to genetic variations that affect the phytochemical contents [22] (Table 3a).
Table 3. Pharmacological effect of C. nutans leaves.
| Pharmacological Activity | Extract/Fraction | Dose Tested/ Test Method |
Animals/ Cell line Culture (In Vivo/In Vitro) |
Experimental Model/ Clinical Trial |
Result | References |
|---|---|---|---|---|---|---|
| (a) Antioxidant and Anti-cancer Properties | ||||||
| Antioxidant, protection against oxidative stress and anti-tumor | 80% methanolic leaves extracts |
200 mg/kg and 1000 mg/kg methanolic extracts |
Murine mammary carcinoma cell line, 4-T1 cells (In-vitro); Female BALB/c mice (In-vivo) |
Antitumor and antioxidant in 4-T1 tumor bearing mice |
Significant decrease in NO and MDA levels in the blood. High dose (1000 mg/kg) extracts significant decrease the number of mitotic cells, tumor weight, and tumor volume |
[19] |
| Antioxidant scavenging activity, and anti-proliferative effects on breast cancer cells |
80% methanolic leaves extracts, and further fractionated sequentially with different solvents (hexane, dichloromethane, chloroform, n-butanol, and aqueous residue) |
MCF 10A cells started with 500 µg/mL (CN-crude and CN-aqueous) and 120 µg/mL (CN-hexane, CN-dichloromethane, CN-chloroform, and CN-butanol fraction extracts |
Breast cancer (Michigan Cancer Foundation-7 [MC ]) and normal breast (Michigan Cancer Foundation-10A [MCF 10A]) cells (In-vitro) |
Molecular docking simulation of the major compounds from C. nutans leaves extract was conducted |
Total phenolic content of C. nutans leaves extract was higher than that of total flavonoid content. CN-dichloromethane extract had the strongest anti-proliferative effect thatinhibited MC cell growth and less toxic towards MCF 10A cells |
[20] |
| Anti-proliferative activity of extracts of C. nutans leaves against human cervical cancer (HeLa) cells |
80% methanol or water extract. The methanol extract was further extracted to obtain hexane, dichloromethane and aqueous fraction |
4,000 µg/mL (water, 80% methanol, and its aqueous fraction) or 250 µg/mL (hexane and dichloromethane fractions of the methanol extract |
HeLa cells (ATCC®CCL-2™) (In-vitro) |
HeLa cells using the Sulforhodamine B (SRB) assay |
Extracts wereanti-proliferative against HeLa cells, and the dichloromethane fraction had the lowest IC50 value of 70 µg/mL at 48 h. Microscopic studies showed that HeLa cells exposed to the DCM fraction exhibited marked morphological features of apoptosis |
[20] |
| Antioxidant and α-glucosidase inhibitory activity, with a subsequent analysis of total phenolic and total flavonoid content of methanol extract |
Liquid-liquid partition chromatography in a separating funnel using hexane, methanol, and water (13:2:5) |
Total phenolic content determined spectrophotometrically using Folin-Ciocalteu method, total flavonoid content estimated according to the based on the formation of aluminum-flavonoid complexes. DPPH for free radical scavenging capacity and FRAP method for total antioxidant capacity |
Antioxidant and α-glucosidase inhibitory activities of methanol extract and its different fractions from C. nutans leaves using biochemical assays (In–vitro) |
Identified the various chemical constituents of the extract and fractions by GC Q-TOF MS, in addition to bioactivity correlation | Ethyl acetate and butanol fractions of the methanol extract had the highest antioxidant and α-glucosidase inhibitory activity which showed a significant correlation with the total phenolic and total flavonoid contents of the fractions |
[14] |
| (b) Anti-viral Properties | ||||||
| Study compounds from C. nutans leaves on dengue virus type 2 infection |
hexane and chloroform leaves extract |
20 µL of leaves extract in MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide. Incubate 3 h at 37 °C | A549 cell monolayers grown in 24- well tissue culture plates were adsorbed with the 0.01 MOI of treated dengue virus serotype-2 (In-vitro) |
Anti-viral activity in pre-incubation vs. post-incubation period and tested using ELISA and RT-PCR |
Phaeophorbide- a methyl ester compound was identified in the extracts could inhibit the dengue virus serotypes-2 replication in post-incubation study |
[23] |
| Anti-herpes simplex virus activities of monogalactosyl diglyceride and digalactosyl diglyceride from C. nutans leaves | chloroform leavesextract | 100 mL of Vero cells at concentration 2.5 × 105 cell/mL seeded into culture medium at 37 °C with 5% CO2 for 1 day with differentconcentrations of chloroform crude extract (20 mL) using MTT assay |
Vero cells (African green monkey kidney cells) cultured with Dulbecco’s modified Eagle medium, supplemented with 5% fetal bovine serum. (In-vitro) |
Cytotoxicity of viral activities was evaluated by the MTT assay samples were measured at 490 nm via spectrophotometry |
100% inhibition of herpes simplex virus type 1 replication at the post step of infection with IC50 values of 36.00 and 40.00 mg/mL, and herpes simplex virus type 2 at 41.00 and 43.20 mg/mL respectively |
[24] |
| Anti-papillomavirus infectivity of C. nutans compounds | 136B, 136C and 136D of C. nutans compounds dissolve in DMSO and heparin solution |
The amount of viable cells was determined by adding 20 µL of 5 mg/mL MTT solution using 293FT cells dissolved with 100 µL of DMSO using an ELISA reader |
Human Papillomavirus 16 PsVs co-transfection of P16 shell and pfwB into 293FT cells (In-vitro) |
Human Papillomavirus 16 PsVs treated with or without various concentrations of each compound. Human papilloma virus 16 PsVs were adsorbed directly on 293FT cells, infected cells expressing green fluorescent protein and determined under fluorescent microscope |
136B, 136C and 136 D compounds inhibited the early step of infection by direct binding between human papillomavirus particles and host cell receptor and also prevent human papillomavirus 16 PsVs internalization. | [25] |
| Anti-Vera zoster virus infection in oral ulcer | Topical formulation | 4 times daily on infected area and assessed at least 3 times during treatment course | Human oral cavity (In-vivo) |
Recurrent aphthous stomatitis | Reduces pain score and healed the lesion caused by Vera zoster virus | [26] |
| (c) Anti-bacterial Properties | ||||||
| Growth inhibition in all twelve bacteria species: Bacillus subtilis, Enterobacter, Escherichia coli, Enterobacter aerogenes, Enterococcus faecalis, Klebsiella pneumoniae, Proteus vulgaris, Pseudomonas aeruginosa, Staphylococcus aureus, Staphylococcus epidermidis, Staphylococcus saprophyticus | Non-polar and polar C. nutans leaves extract |
C. nutans leaves extract range from 0.25, 0.5, 1, 2, 4, 8, 16, 32 mg/mL | Cell lines in triplicate containing 40µL of bacterial suspension (final conc. of 2–8 × 105 cfu/mL) with 50 µL of test compound inoculated with 10 µL of resazurin. (In-vitro) |
Broth micro dilution method was used to determine the minimum inhibitory concentration (MIC) based on the CLSI M07-A8 guidelines |
Growth inhibition in all 12 bacteria species as extract concentration increased. Non-polar extracts have stronger antibacterial activity than those polar extracts solution in the 32 mg/kg concentration | [27] |
| Anti-biofilm, nitric oxide inhibition and wound healing potential of purpurin-18-phytyl ester (P18PE) isolated from C. nutans leaves | C. nutans leaves -hexane (20.05 g), chloroform (16.2 g) and ethanol (38.34 g) extracts | C. nutans leaves extract range from (5–500 μg/mL) | 1 × 105 cells (RAW 264.7 or HGFs)/well were incubated overnight in 96-well plates. | Murine macrophage RAW 264.7 and HGF cell culture. | Possess anti-inflammatory, in-vitro wound healing, and anti-biofilm activities. | [28] |
| Antibacterial properties of C. nutans leaves extracts against Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans |
100%, 50%, 10% ethanol and 100% chloroformextracts | C. nutans leaves extract range from 12.5, 25, 50 and 100 mg/kg | Disc diffusion agar, minimum inhibitory concentrations (MIC), and minimum bactericidal concentrations (MBC) antibacterial susceptibility tests (In-vitro) |
Disc diffusion agar test |
50% ethanolic extracts have notable antibacterial activity against P. gingivalis and A. actinomycetamcomitans comparable to 0.2% chlorhexidine. Meanwhile, chloroform extract has notable antibacterial activity against P. gingivalis only |
[29] |
| (d) Anti-fungal Properties | ||||||
| In-vitro Anti-Candida Effect of Thai Herbs Supplemented in Tissue Conditioner |
10% aqueous extracts | C. nutans leaves range from 0.354, 0.709, 1.418, 2.836, 5.672, 11.344 µL/mL) in tissue conditioner |
Agar disk diffusion (inhibition zone appearance) and micro-broth dilution (MIC and MFC determination Methods (In-vitro) |
Liquid part of COE-COMFORTTM tissue conditioner | Negative inhibitory activity against Candida albicans |
[30] |
| Light-mediated activities against Candida albicans and Aspergillus fumigatus | 95% ethanolic extracts | C. nutans leaves extract at 5 mg/mL | Agar disk diffusion (In-vitro) |
Disc diffusion agar test |
Extracts were ineffective to exhibit fungicidal effect on both fungus species | [31] |
| In-vitro anti-fungal activities of C. nutans leaves extract and semi-fractions | Crude extracts (0.2 to 10.0 mg/mL) subjected to cold solvent extraction to produce petroleum ether, ethyl acetate and methanol crude extracts, followed by isolation using bioassay-guided fractionation. | C. nutans leaves extract at 2 mg/mL, 4 mg/mL, 6 mg/mL, 8 mg/mL, 10 mg/mL |
HeLa and K-562 cell lines cultured in RPMI1640 and DMEM complete medium (In-vitro) |
Fungal suspensions were streaked on MHA and SDA medium followed by 3-(4,5-dimethylthiazol2-yl)-2,5-diphenyltetrazolium bromide (MTT), minimum inhibitory concentration (MIC) and minimum fungicidal (MFC) assay | A minimal concentration of 1.39 mg/mL of ethyl acetate extract exhibited a fraction of antifungal effect on Candida albicans | [32] |
| (e) Anti-venom Properties | ||||||
| Extracts of C. nutans and Naja naja siamensis venom | 95% alcoholic C. nutans leaves extract | 0.406 mg/mL to 0.706 mg/mL administered orally or intraperitoneally |
Mice (In-vivo) |
Isolated rat phrenic-nerve diaphragm in mice |
Failed to exert the antidote effect against the neurotoxin | [33] |
| C. nutans extract activities against the fibroblast cell lysis | 0, 50 or 90% ethanolic extracts | 0.406 mg/mL to 0.706 mg/mL | Chick embryonic fibroblast cell primary cultures (In-vitro) |
Swiss Webster female mice | Completely negative results as anti-bee venom agents | [34] |
| Extracts of C. nutans Burm. and Naja naja siamensis venom | Water extract | 0.406 mg/mL to 0.706 mg/mL administered orally or intraperitoneally |
Mice (In-vivo) |
Isolated rat phrenic-nerve diaphragm in mice |
Reduced mortality rate by 27%; from 100% to 63 ± 3.34% | [33] |
| Screening of C. nutans containing Naja naja siamensis cobra venom inhibitory activity using modified ELISA technique. | Water extract | extracts at 1:250 pre-incubated for 30 min at 37 °C with 40 g/mL venom in DMEM (test) or without DMEM lacking venom(control) |
Modified ELISA technique (In-vitro) |
Phrenic nerve/ Hemi-diaphragms isolated from adult albino rats, weighing 150 to 200 g |
35% of inhibitory activity and the extract attenuated toxin activity by extending contraction time of diaphragm muscle | [35] |
| Screening of C. nutans plant acting against Heterometrus laoticus scorpion venom activity on fibroblast cell lysis | Water extract | 0.406 mg/mL to 0.706 mg/mL pre-incubated with DMEM (as mock controls), or with 0.2 g/L venom |
Chick embryonic fibroblast cell primary cultures (In-vitro) | Chick embryonic fibroblast cell | Exhibited 46.5% fibroblast cell lysis in Heterometrus laoticus scorpion venom at 0.706 mg/mL but its cytotoxic effect is unsure | [36] |
| (f) Analgesic and Anti-nociceptive Properties | ||||||
| C. nutans leaves extract mediated silver and, gold nanoparticles on muscle relaxant, analgesic activities for pain management | Methanolic extract encoated in silver and gold nanoparticles | 50, 100, 200 mg/kg per body weight in gold and silver nanoparticles; 100, 200, 400 mg/kg per body weight in methanol extract |
Intra-peritoneal injection of extracts on BALB/c mice (In-vivo) |
Twisted wire traction technique for muscle relaxant study and writhing for analgesic study | Extract exerted a very good analgesic and muscle relaxant activities for use in pain management. Gold nanoparticles had most efficient analgesic activity at a small concentration of 50 mg/kg | [paper retracted] |
| Anti-nociceptive activity of petroleum ether fraction obtained from methanolic extract of C. nutans leaves via opioid receptors and NO mediated/cGMP-independent pathway |
Petroleum ether fraction from methanolic extract of C. nutans leaves |
100, 250, 500 mg/kg administered intraperitoneally | Adult male ICR mice (In-vivo) |
Acetic acid-induced abdominal constriction test, hot plate test, formalin–induced paw licking test, and motor coordination Rota–rod test |
Petroleum ether C. nutans leaves extract exerted anti-nociceptive activity at peripheral and central levels via the activation of nonselective opioid receptors | [37] |
| (g) Anti-inflammatory and Immunomodulatory Properties | ||||||
| Effects of C. nutans leaves extract on cytokine secretion in PMA-induced U937 macrophage cells | Water and ethanol leaves extract | 0.25, 0.5, 1.0, 2.0, 4.0 and 8.0 mg/mL | Viability of the extract-treated cells using Presto-Blue test; IL-4 and IL-13 secretion tested via ELISA (In-vitro) |
U937 monocyte-derived macrophages |
In-vitro assays on interleukin-4 (IL-4) and interleukin-13 (IL-13) cytokines secretion in PMA-induced U937 macrophage cells showed reduction of cells viability to 87%, CD14 expression was down-regulated by 36% and CD11b expression was up-regulated by 58%. | [38] |
| Anti-Inflammatory and immune-modulating activity in C. nutans leaves extract | 80% ethanol leaves extract |
0.1 to 10 µg/mL ethanolic extract | Anti-inflammatory: MeO-Suc-Ala-Ala-Pro Valp-nitroanilide was used for observing elastase release and superoxide anion production; Immune-modulating: Lactobacillus casei on IgE production, splenocyte obtained from ovalbumin (OVA)-primed BALB/c mice (In-vivo) |
Ovalbumin (OVA)-primed BALB/c mice | 68.33% inhibition on the generation of superoxide anion and the elastase release by activated neutrophils by 10 µg/mL ethanolic extract; 0.1 μg/mL of 80% ethanol extract led to up-regulation of IFN-γ | [39] |
| (h) Anti-hyperglycemic Properties | ||||||
| Aqueous leaf extract of C. nutans improved metabolic indices and sorbitol-related complications in type II diabetic rats | Hot water extraction method where leaves are mixed with water in a 1:10 ratio (w/v) for 3 h at 100 °C | 100, 200 mg/kg/day of water extract |
Male Sprague Dawley rats (In-vivo) |
Streptozotocin induced diabetic rat model | Improved glycemic control and complications. In fact at higher doses (200 mg/kg), C. nutans leaves extract showed better results |
[40] |
| C. nutans leaves extract reverts endothelial dysfunction in type-2 diabetes rats by improving protein expression of eNOS |
Methanolic extract from leaves | 300, 500 mg/kg/day of methanolic extract |
Male Sprague Dawley rats (In-vivo) |
Intraperitoneal injection of low-dose streptozotocin to rats fed with high-fat diet |
Improved endothelium-dependent relaxation, reduced endothelium -dependent and endothelium-independent contraction in the aorta of diabetic rats |
[41] |
| Characterization of α-glucosidase inhibitors from C. nutans Leaves by Gas Chromatography-Mass Spectrometry-based metabolomics and molecular docking simulation |
80% methanol using the sample to solvent ratio of 1:3 (w/v) for 3 days where the solvent was changed each consecutive day. i.e., hexane; hexane: ethyl acetate; ethyl acetate; ethyl acetate: methanol |
10 µL from each sample extracts |
gas chromatography-mass spectrometrybased metabolomics and molecular docking simulation (In-silico) |
α-glucosidase inhibitory potential of C. nutans using the gas chromatography tandem with mass spectrometry (GC-MS) | α-glucosidase inhibitors were identified in C. nutans leaves, indicating the plant’s therapeutic effect to manage hyperglycemia |
[42] |
| (i) Anti-hyperlipidemia properties | ||||||
| Effects of methanolic leaf extract of C. nutans on fatty acid composition and gene expression in male obese mice |
Methanolic leaves extract | 500, 1000, 1500 mg/kg of leaves extract | Male ICR mice (In-vivo) |
High fat diet induced obesity mice | Reduced the body weight, visceral fat and muscle saturated fatty acid compositions and down-regulated the levels of HSL, PPAR α and PPAR γ and SCD gene expressions with 1500 mg/kg had optimum efficacy | [43] |
| Methanolic Extract of C. nutans Leaves can alter adipocyte Cellularity activity in male obese mice |
Methanolic leaves extract |
19.5, 39.0 and 58.5 mg/mL of leaves extract |
Male ICR mice (In-vivo) |
High fat diet induced obesity mice | Lowered adipocyte area, size, and diameter and reduced plasma total cholesterol in mice but had no effect on plasma lipid profile | [44] |
| Effects of phenolic-rich extracts of C. nutans on high fat and high cholesterol diet-induced insulin resistance |
Water and 80% aqueous methanol leaves extract |
oral gavage of 125, 250 or 500 mg/kg/day of leaves extract |
Male Sprague-Dawley rats (In-vivo) |
High fat and high cholesterol rat | Slowed the rate of weight gain induced by high fat-high cholesterol diet |
[45] |
| (j) Vasorelaxation Properties | ||||||
| Anti-hypertensive and vasodilatory effects C. nutans leaves extract |
Water extract, 50% ethanol extract and 95% ethanol extract from leaves | 100 µL of herbal extracts added cumulatively to the organ bath at concentrations from 0.125 mg/mL to 128 mg/mL (equivalent to 0.00125–1.28 mg/mL in organ bath) |
Male Sprague-Dawley rats (In-vitro) |
Pre-contracted aortic rings from rat thoracic aorta | Prominent vasorelaxant activities with highest Rmax values of 95% ethanol extracts (72.67 ± 1.61%) > 50% ethanol extracts (73.57 ± 2.99%) > water extracts (55.85 ± 2.35%) | [11] |
| (k) Renoprotective Properties | ||||||
| Nephroprotective effect of C. nutans leaves against cisplatin-induced nephrotoxicity |
Aqueous extracts | 100, 200 or 400 mg/kg/day for 90 days by oral gavage | Male and female Sprague-Dawley rats (In-vivo) |
Cisplatin-induced renal toxicity in rats | Attenuated the renal toxicity and further increase the glomerular filtration rate, serum electrolytes, and urine creatinine excretion | [unpublished data of same lab] |
| The nephroprotective effect of C. nutans in cisplatin-induced nephrotoxicity in the in vitro condition |
Water and ethanol extracts |
Extractions using different ratios of ethanol to water (0 to 100%, with 20% increment) |
Cell viability assay MTT assay, Lactate dehydrogenase (LDH) assay and NMR analysis of cell extract and corresponding culture Media (In-vitro) |
Rat renal proximal tubular cells (NRK-52E) line | 1000 μg/mL of C. nutans leaves extract had the most potential nephroprotective effect against cisplatin toxicity on NRK-52E cell lines at 89% of viability |
[46] |
| Nephroprotective effect of C. nutans leaves extract against cisplatin-induced nephrotoxic humankidney cells | Water and ethanol extracts |
Extractions using different ratios of ethanol to water (0 to 100%, with 20% increment) |
Cell viability assay MTT assay, lactate dehydrogenase (LDH) assay (In-vitro) |
human kidney cell (PCS-400–010) culture |
Improved the % of cell viability in mitochondrial dehydrogenase activity (MTT) and lactate dehydrogenese (LDH) assay after 24 h pretreatment of the extract | [47] |
3.2. Anti-Viral Properties
C. nutans leaves have long been utilized in Thailand as an alternative traditional medicine for the treatment of herpes simplex virus, varicella-zoster virus, mosquito borne virus and many more. In general, the modes of action of anti-viral properties from C. nutans leaves were demonstrated with three different stages of treatment, i.e., direct inactivation, pre-infection and post-infection methods. An experiment on anti-mosquitoes borne virus was demonstrated using dengue viruses serotypes-2 strain 16681 by immunofluorescence technique and reverse transcriptase polymerase chain reaction. Here, the dengue virus serotypes-2 was treated by incubating the dengue virus either in the absence or presence of the C. nutans leaf compounds in a sub-cytotoxic concentration at 37 °C for two days via pre-incubation and post-incubation techniques. The results showed that a phaeophorbide-a methyl ester compound identified in extracts could inhibit the dengue virus serotypes-2’s replication in a post-incubation study, which indicated that phaeophorbide-a could inhibit the production of viral RNA as well as viral the protein when the virus serotypes-2 infected cells were cultured in the compound [23]. In the treatment of herpes simplex virus (HSV), monogalactosyl diglyceride and digalactosyl diglyceride compound extracted from C. nutans leaves were tested using a plaque reduction assay method for their in-vitro anti-viral activities against herpes simplex virus type 1 and type 2. The result had demonstrated that the monogalactosyl diglyceride and digalactosyl diglyceride compounds which were present in the C. nutans leaves inhibited the replication of HSV type 1 post step of infection by 100% at non-cytotoxic concentration with IC50 values of 36.00 and 40.00 mg/mL, whereas the herpes simplex virus type 2 was at 41.00 and 43.20 mg/mL, respectively. This finding illustrated the inhibitory activity of C. nutans leaves extract against both herpes simplex virus serotypes could be probably via the inhibition of the late stage of viral multiplication, suggesting their promising use as anti-HSV agents [25]. The anti-papillomavirus infectivity of C. nutans leaves was evaluated using human papillomavirus 16 PsVs infection on the 293FT cell line. Based on the in-vitro study, DMSO and heparin extract of C. nutans leaves showed a potential anti-human papillomavirus 16 PsV infections effect by preventing the early step of infection between the direct bindings of human papillomavirus particles to the host cell receptor, while also preventing human papillomavirus 16 PsVs internalization [25]. On the other hand, a clinical evaluation of the anti-vera zoster virus in an aphthous stomatitis experiment was reported by [26], where a double blind controlled trial was undertaken to evaluate the efficacy of orabase C. nutans leaves extract in recurrent aphthous stomatitis patients. Patients were subjected to topical formulation of C. nutans leaves extract in the ulcers site and it was found that application or a base four times a day successfully reduced pain score and healed the Vera zoster virus lesion. The findings suggest the potential role of the C. nutans leaf compounds on the prevention of human papillomavirus infection and Vera Zoster virus infections (Table 3b).
3.3. Anti-Bacterial Properties
Antimicrobial resistance is a global health and development threat in the current century which requires urgent multi-sectorial actions in order to achieve Sustainable Development Goals. A lack of clean water, sanitation and inadequate infection prevention control further promotes the spread of microbes in some of poor countries, some of which can be resistant to antimicrobial treatment. Moreover, misuse and overuse of anti-microbials are the main drivers in the development of drug-resistant pathogens. For example, the rate of resistance to ciprofloxacin, an antibiotic commonly used to treat urinary tract infections, varied from 8.4% to 92.9% for Escherichia coli and from 4.1% to 79.4% for Klebsiella pneumoniae in those countries reporting to the Global Antimicrobial Resistance and Use Surveillance System. With the rise of these phenomena, scientists have changed focus to natural compounds in medicinal plants to identify potential new anti-bacterial compounds and, hence, the anti-bacterial effects of C. nutans leaves have been tested in microbial strains. Lim and his team have reported that the extracts from non-polar and polar C. nutans leaf extracts showed growth inhibition in all 12 bacteria species: Bacillus subtilis, Enterobacter, Escherichia coli, Enterobacter aerogenes, Enterococcus faecalis, Klebsiella pneumoniae, Proteus vulgaris, Pseudomonas aeruginosa, Staphylococcus aureus, Staphylococcus epidermidis and Staphylococcus saprophyticus; as the extracts concentration increased, the results revealed that non-polar C. nutans leaf extracts have a stronger antibacterial activity than those polar extract solutions at 32 mg/kg concentration, whereas the gram-negative bacteria were more sensitive to the extracts compared to gram-positive bacteria [27]. On the other hand, [28] reported that purpurin-18-phytyl-ester compound extracted from C. nutans leaves possesses in-vitro anti-biofilm wound healing activities in RAW 264.7 or the HGFs cell line. In addition to that, the anti-bacterial properties from ethanolic and chloroform fraction of C. nutans leaves were also reported against Porphyromonas gingivalis and Aggregatibacter actinomycet emcomitans using disc diffusion agar, minimum inhibitory concentrations (MIC) and minimum bactericidal concentrations (MBC) antibacterial susceptibility tests done in-vitro. Fifty percent ethanolic C. nutans leaves extract was found to have a notable antibacterial activity against Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans, comparable to 0.2% chlorhexidine. Meanwhile, chloroform C. nutans leaves extract was found to have notable anti-bacterial activity against Porphyromonas gingivalis only [29]. On the whole, this multiplicity of findings suggested that the anti-bacterial effects from C. nutans leaves extract could be selective for only particular strains of microorganisms, and, thus, the exact mode of action of C. nutans leaves extract on bactericidal effects still requires further extensive investigations and re-definition (Table 3c).
3.4. Anti-Fungal Properties
C. nutans leaves extract has been widely employed as a traditional medicine for anti-fungal activity in the countries of Southeast Asia. However, to date, there are somewhat limited scientific data available to support the claims that have been made, yet there are still some research findings proven to have positive results. For instance, Choon and his team investigated the inhibitory activity of aqueous C. nutans leaves extract against Candida albicans using agar disk diffusion and the micro-broth dilution technique. The result obtained showed negative inhibitory activity against Candida albicans [30]. The same finding was further supported by [31], who had examined the anti-fungal activity in Candida albicans and Aspergillus fumigatus with 95% ethanol leaf extract at 5 mg/mL. On the contrary, [32] has reported that a minimal concentration of 1.39 mg/mL of ethyl acetate extract exhibited a fraction of an antifungal effect on Candida albicans. Based on the above mixed findings, the polar and non-polar extract exhibited unpromising fungicidal action. There is still substantial room to explore the biological action of C. nutans leaves on anti-fungal activities, which is worthy of further investigation (Table 3d).
3.5. Anti-Venom Properties
Folks have always recognized that one kind of herb can be universally used for relieving symptoms from the venom of many animals or insect species. However, evidence has showed that venom can be neutralized by the body’s defense mechanism with disregard to any effects from herb treatment, which indirectly caused the misunderstanding of this plant as an antidote. Since the major elements that are present in venom are peptides and proteins with very delicate structure, pH or other uncomplicated factors can exert any effect, leading to confusion of their actions. In fact, data from the traditional healers are obvious. However, they prefer to keep this knowledge with themselves for their own profit and tend to end up with their data always lost without any records after they are deceased [34]. Generally, the extracts from C. nutans leaves are used by native and local people from Southeast Asia as the remedy for the envenomation of bites or stings by venomous animals or insects, i.e., snakes, scorpions and bees. Extracts are commonly prepared via direct maceration or using water, with ethanol as an extraction solvent. Previous in-vivo investigations have demonstrated contradictory result indicating that 95% alcoholic C. nutans leaves extract at a concentration of 0.406 mg/mL to 0.706 mg/mL was not sufficient to exert the antidote effect against the neurotoxin disseminated by Naja naja siamensis in isolated pherenic-nerve diaphragm preparations in rats [33]. Similarly, 0.406 mg/mL to 0.706 mg/mL of aqueous ethanolic C. nutans leaves extract was also ineffective against Apis mellifera Linn. In bee’s venom, the viability of fibroblast cell was less than 10% [33]. On the contrary, water extract was able to reduce the mortality rate against the neurotoxin from Naja naja siamensis by 27% [33]. Others have reported effective inhibitory potential of C. nutans leaves extract at a 1:12.5 dilution ratio against the Naja naja siamensis cobra venom using a modified ELISA technique with only 35% of inhibitory activity, indicating that the extract attenuated toxin activity by extending the contraction time of the diaphragm muscle after envenomation and had a potency to protect cellular proteins from venom degradative enzymes [35]. Likewise, an in-vitro study on the effectiveness of water C. nutans leaves extract successfully exhibited 46.5% fibro-blast cell lysis against Heterometrus laoticus scorpion venom at a 0.706 mg/mL concentration [36]. Based on the current available ambivalent results, advanced scientific efforts are necessary to clarify these plant activities (Table 3e).
3.6. Analgesic and Anti-Nociceptive Properties
Pain medication can be defined widely as any medication that relieves pain. Many different pain medicines exist and each has both pros and cons due to the fact that certain pains respond better to some medicines while some do not. Each individual also has a slightly different response to different pain relievers. Currently, the most common medications are over-the-counter medicines such as the non-steroidal anti-inflammatory drugs (NSAIDS) class, which are used for mild to moderate pain and are commonly prescribed for arthritis and musculoskeletal physiotherapy; the opioids class, which includes codeine, morphine and tramadol, are often prescribed for acute pain caused by traumatic injury, such as post-surgery neuropathic pain; anti-epileptic drugs such as pregabalin, gaberpentin and carbamazepine are used for chronic pain, i.e., neuropathic pain; anti-depressants such as amitriptyline and duloxetine are used for chronic pain, i.e., fibromyalgia. These medications often come with some unpleasant side effects, i.e., all NSAIDs come with the risk of gastrointestinal ulceration and bleeding; opioid analgesics commonly cause drowsiness, dizziness and respiratory depression; anti-epileptic drugs cause dizziness, drowsiness and swelling of the lower extremities including dry mouth, difficulty urinating, blurred vision and constipation. Other possible side effects of anti-epileptic drugs include hypotension, tachycardia, palpitations, weight gain and fatigue. The analgesic capabilities of methanolic leaves extract of C. nutans have been investigated to assess their comparative analgesic and muscle relaxant activities in a study conducted on BALB/c mice using gold and silver nanoparticles as the vehicle at a concentration of 50, 100 and 150 mg/kg per body weight and methanolic extract at a concentration of 100, 200 and 400 mg/kg per body weight included under a twisted wire traction technique for the muscle relaxant study, and the analgesic study was assessed by writhing (extension of hind limb, turning of trunk, and contraction of abdomen) that took place during the coming 10 min after treating with acetic acid. The muscle relaxant studies displayed that methanolic leaves extract of C. nutans encoated with silver nanoparticles were comparatively more efficient than methanolic leaves extract of C. nutans encoated with gold nanoparticles in a traction examination. Additionally, the analgesic studies exhibited that those gold nanoparticles, silver nanoparticles and methanolic extracts alone exhibited the maximum percentage reduction in acetic acid induced writhing at the concentrations of 50, 100 and 150 mg/kg per body weight by 48.02, 64.30 and 74.44%; 45.23, 60.00 and 71.50%; 42.30, 58.00 and 69.33% writhing at 100 mg/kg, 200 mg/kg and 400 mg/kg, respectively. These findings indicated that C. nutans leaves extract possesses very good analgesic and muscle relaxant activities for use in pain management. On the other hand, [37] have demonstrated peripherally and centrally mediated anti-nociceptive activity via the modulation of the opioid/NO-mediated pathway using sequentially partitioned to obtain petroleum ether extract from C. nutans leaves, which was subjected to an anti-nociceptive study with the aim of establishing its anti-nociceptive potential by determining the role of opioid receptors and L–arginine/nitric oxide/cyclic-guanosine monophosphate (L-arg/NO/cGMP) pathway in the observed anti-nociceptive activity. In the study, 100, 250 and 500 mg/kg of petroleum ether extract from C. nutans leaves were orally administered and the abdominal constriction, hot plate and formalin-induced paw licking test in mice was investigated. In addition to that, the effect of petroleum ether extract from C. nutans leaves on locomotors activity was also evaluated using the Rota-rod assay. The test outcome showed that petroleum ether extract from C. nutans leaves significantly inhibited the nociceptive effect in all models in a dose-dependent manner; except that the highest dose of petroleum ether extract from C. nutans leaves, 500 mg/kg, did not affect the locomotors activity of treated mice. The authors concluded that the anti-nociceptive activity of petroleum ether extract from C. nutans leaves significantly inhibited all antagonists of μ-, δ- and κ-opioid receptors. In addition, the anti-nociceptive activity of petroleum ether extract from C. nutans leaves was reversed by L-arg, but was somehow insignificantly affected by morphine hydrochloride. This result suggested that petroleum ether extract from C. nutans leaves could exert an anti-nociceptive activity at peripheral and central levels possibly via the activation of nonselective opioid receptors and modulation of the NO-mediated partly via the synergistic action of phenolic compounds presence in the plant extracts (Table 3f).
3.7. Anti-Inflammatory and Immunomodulatory Properties
Anti-inflammatory agents block certain substances in the body that cause inflammation and are used to treat many different disease conditions. Some anti-inflammatory agents are being studied in the prevention and treatment of cancer. On the contrary, an immunomodulatory substance suppresses or stimulates the immune system that helps the body to fight against cancer, infection, or other diseases. Specific immunomodulating agents, i.e., monoclonal antibodies, cytokines and vaccines, affect specific parts of the immune system. Extracts from C. nutans leaves have been adopted to reduce inflammation in viral infection, insect bites and allergic responses in medicine. A few investigations have also reported the effect of C. nutans leaves extract on the immune system. The anti-inflammatory study was assessed by in-vitro assays such as on interleukin-4 (IL-4) and interleukin-13 (IL-13) cytokines secretion in phorbol-12-myristate-13-acetate (PMA)-induced U937 macrophage cells. In this study, a sequential ultrasonic-assisted extraction was carried out using water and ethanol, with a 1:10 ratio of leaves powder to the solvent volume at 0.25, 0.5, 1.0, 2.0, 4.0 and 8.0 mg/mL concentration. Viability of the extract-treated cells using the Presto-Blue test and the IL-4 and IL-13 secretions were assessed with the ELISA technique which caused morphological changes in U937 cells from round-shaped, non-adherent to larger irregular-shaped, adherent cells, and a reduction in cells viability to 87%. Moreover, the CD14 expression was down-regulated by 36% upon PMA stimulation together with the CD11b expression being up-regulated by 58% in PMA-treated cells. ELISA results showed that 1 mg/mL of ethanolic and water extracts stimulated 1200 and 1800 pg/mL IL-4 secretions, respectively, but both extracts caused minimal IL-13 secretion which indicates that aqueous extracts stimulated IL-4 production higher than ethanolic extract in PMA-induced U937 macrophages, suggesting that inflammatory effects could be dampened with such doses [38]. It also reported that 80% ethanolic extract from leaves showed 68.33% inhibition on the generation of superoxide anion and the elastase release by activated neutrophils in 10 µg/mL ethanolic extract. MeO-Suc-Ala-Ala-Pro Valp-nitroanilide was used for observing elastase release and superoxide anion production by detecting the superoxide dismutase-inhibitory reduction from ferricytochorme c complex. On the other hand, the immunomodulating study examined the inhibitory effect of Lactobacillus casei on IgE production, splenocyte obtained from ovalbumin (OVA)-primed BALB/c mice and re-stimulated in-vitro with the same antigen. In this immune-modulating experiment, administration of 0.1 μg/mL of 80% ethanol extract led to up-regulation of IFN-γ exhibiting immune-modulating activity [39] (Table 3g).
3.8. Anti-Hyperglycemic Properties
Large numbers of studies have provided evidence for the significant role of oxidative stress in diabetes, obesity and some form of metabolic syndromes. Oxidative stress occurs due to an imbalance between endogenous antioxidant systems and the generation of reactive oxygen species (ROS). ROS overproduction has been reported to be an important trigger of insulin resistance and a contributing factor in the development of type-2 diabetes [48]. Diabetes is a chronic disease that occurs when the pancreas does not produce enough insulin. The common effect of uncontrolled diabetes over time leads to serious damage to many of the body’s systems, especially the nerves and blood vessels which are responsible for the development of cardiovascular disease, with approximately 80% of cardiovascular mortality and morbidity linked to vascular complications. According to the statistical report from the World Health Organization, the number of personnel with diabetes rose from 108 million in 1980 to 422 million in 2014. Prevalence has been rising more rapidly in low- and middle-income countries than in high-income countries. At present, it has been estimated that up to one-third of personnel suffering from diabetes mellitus adopted some form of ethnomedical applications. One of the medicinal plants that caught the attention of diabetic patients for its perceived anti-diabetic properties is C. nutans. The anti-hyperglycemic effect was demonstrated via aqueous leaf extract on serum metabolic indices, sorbitol production and aldose reductase enzyme activities in the kidneys, ocular lens and sciatic nerve of type-2 diabetic (T2D) rats at a concentration of 100 and 200 mg/kg/day p.o., potentially lowering the fasting blood glucose levels post-intervention by 14.2 and 14.0 mmol/L, respectively, at week four, compared with the untreated group 22.1 mmol/L. In addition to that, C. nutans leaves extract also attenuated the oxidative stress marker, namely F2-isoprostane, with an enhancement of aldose reductase enzyme activity increased by 64 and 99%. These findings indicated that C. nutans leaves extract has the potential to attenuate type-2 diabetic-induced metabolic perturbations and complications [40]. Moreover, [41] have also reported that 500 mg/kg/daily of C. nutans leaves extract reverts endothelial dysfunction in type-2 diabetes rats by improving protein expression of endothelial nitric oxide synthase (eNOS) enzyme with respect to 300 mg/kg/daily of metformin. Treatment of both diabetic groups with C. nutans leaves extract or metformin improved the impairment of endothelium-dependent vasorelaxation associated with up-regulated expression of aortic eNOS protein. Moreover, C. nutans leaves extract and metformin also reduced aortic endothelium-dependent and aortic endothelium-independent contractions in diabetic rats. Both of these diabetic-treated groups had reduced blood glucose levels and increased body weight compared to the untreated diabetic group. This finding indicated that C. nutans leaves extract could be a potential anti-diabetic therapy in the future as it displayed a similar therapeutic outcome as compared to metformin. The anti-diabetic potential of C. nutans leaves extract was also studied in-silico via the characterization of α-glucosidase inhibitors by gas chromatography-mass spectrometry-based metabolomics and molecular docking simulation using 80% methanolic dried leave samples. GC-MS data analysis discovered 11 bioactive compounds including palmitic acid, phytol, hexadecanoic acid, 1-monopalmitin, stigmast-5-ene, pentadecanoic acid, heptadecanoic acid, 1-linolenoylglycerol, glycerol monostearate, alpha-tocospiro B and stigmasterol. Some of the potential inhibitor compounds were identified from the leaves extract and the molecular interactions of the inhibitors identified with the protein were predominantly hydrogen bonding-involving residues, namely LYS156, THR310, PRO312, LEU313, GLU411, ASN415, PHE314 and ARG315 residues with hydrophobic interaction. This finding supported scientific evidences of the potential of C. nutans leaves in α-glucosidase enzyme inhibition, ideal for the development either on medicinal preparations, nutraceutical and novel therapeutic or preventive agents for future anti-diabetic treatment [42] (Table 3h).
3.9. Anti-Hyperlipidemia Properties
Globally, there are now more people who are obese, and this trend is observed in every region over the world. It is suggested that, by the year of 2030, the population experiencing overweight and obesity in adults will reach 2.16 billion worldwide [49]. Obesity can be defined as abnormal or excessive fat accumulation that may impair the body’s health state and elevated body mass index is a major risk factor for many non-communicable diseases such as: increases cardiovascular risk factors via increased fasting plasma triglycerides, elevated low density lipoprotein levels cholesterol, lowered high density lipoprotein cholesterol, elevated blood glucose and insulin levels and high blood pressure [50]. One of the plants with medicinal properties that includes crude extracts and isolated compounds which are effective for controlling and reducing weight gain is from C. nutans leaves. Treatment of high fat diet induced obese mice with methanolic leaf extract of C. nutans at 500, 1000 and 1500 mg/kg for 21 days reduced the body weight gained, visceral fat and muscle saturated fatty acid compositions. Moreover, the levels of HSL, PPAR α and PPAR γ and SCD gene expressions in the obese mice treated with 1500 mg/kg methanolic leaf extract of C. nutans were downregulated [43]. A similar finding was also reported where 39.0 and 58.5 mg/mL of methanolic leaves extract significantly lowered the area, size, and diameter of adipocyte. Although supplementation of C. nutans methanolic leaf extract could reduce plasma total cholesterol in mice, it was somehow not effective on other plasma lipid profile regulations [44]. In addition to that, C. nutans was able to slow down the rate of weight gain induced by high fat-high cholesterol diet in insulin resistance in rats, and also improved the antioxidant capacity in the obese rats. This anti-hyperlipidemic effect was mediated by the up-regulation of gene coding for phosphatidylinositol-3-phosphate, insulin receptor substrate, leptin and adiponectin receptors [45] (Table 3i).
3.10. Vasorelaxation Properties
As stated in Hypertension Clinical Practice Guidelines of Malaysia, thiazide, diuretics, β-blockers, CCBs, ACEIs and ARBs were selected as first line mono-therapeutic agents; however, several adverse side effects such as dizziness, fatigue, joint pain and stomach upset, constipation, dehydration, erectile dysfunction and low effectivity were always being reported. For this reason, the discovery of the new drugs to control blood pressure enchanted a number of researchers. Previously, C. nutans leaves showed limited data reports as antihypertensive agents. The prevalence of diabetes, dyslipidemia and hypertension are always responsible for a substantial risk of cardiovascular diseases. Reduced nitric oxide bioavailability may lead to endothelial dysfunction and hypertension which is thought to be related to loss of eNOS cofactor such as tetrahydrobiopterin, further substantiating oxidative stress to induce vascular pathogenesis [51]. It has been reported that extracts from C. nutans leaves contain several active ingredients which can undergo multiple vasorelaxation-mediated signaling pathways and decrease the time taken to achieve the targeted blood pressure with less concomitant adverse effects. Reference [11] has demonstrated a preliminary test to screen for their antihypertensive and vasorelaxant activities of C. nutans leaves using Fourier transform IR (FTIR), second-derivative IR (SD-IR) and two-dimensional correlation IR (2D-correlation IR) analyses to determine the main constituents and the fingerprints from this herb. In addition to that, water extracts, 50% ethanol extracts and 90% ethanol extracts from C. nutans leaves were used to determine the contractile forces on the pre-contracted aortic rings measured with a GRASS Force-Displacement Transducer FT03C on adult male Sprague–Dawley rats. Based on their findings, the vasorelaxant activities were prominent with the highest Rmax values of 95% ethanol extracts (72.67 ± 1.61%) vs. 50% ethanol extracts (73.57 ± 2.99%) vs. water extracts (55.85 ± 2.35%). This outcome revealed that the flavonoid content obtained from this herb possesses a potential vasorelaxant activity (Table 3j).
3.11. Renoprotective Properties
C. nutans has also been evaluated for its renoprotective activities. The renoprotective effect of C. nutans has been demonstrated by several in-vivo and in-vitro studies [44][46][47]. In 2017, the nephroprotective effect of C. nutans leaves against cisplatin-induced nephrotoxicity and the safety assessment of C. nutans leaves has been demonstrated by [44]. The study has demonstrated that cisplatin-induced renal toxicity caused rapid loss of glomerular filtration, polyuria, hyperkalemia, hypernatremia and azotemia in animal models. Their protective activities on renal tubular cells (NRK52-E) were evaluated for cellular viability (MTT assay) and apoptosis (Hoechst and Rhodamine 123 staining). In vivo studies of C. nutans leaves were administered via oral gavage at doses of 100, 200 or 400 mg/kg for 90 days, while receiving weekly doses of cisplatin (1 mg/kg). Simultaneous treatment with cisplatin and C. nutans leaves extract significantly attenuated the renal toxicity manifested by decreased levels of serum creatinine and proteins, blood urea nitrogen, urine electrolytes and urine volume when compared to the cisplatin group. Furthermore, an increase in the glomerular filtration rate, serum electrolytes and urine creatinine excretion were demonstrated. Collectively, these findings highlighted the potential use of C. nutans leaves extract in the management and treatment of cisplatin-induced nephrotoxicity. Meanwhile, [46] has also reported the nephroprotective effect of C. nutans in cisplatin-induced nephrotoxicity under an in-vitro condition using the Proton Nuclear Magnetic Resonance (1H NMR) and Liquid Chromatography Mass Spectroscopy (LCMS) techniques coupled with multivariate data analysis to characterize the metabolic variations in intracellular metabolites and the compositional changes of the corresponding culture media in rat renal proximal tubular cells (NRK-52E). Investigations of this study have highlighted the altered pathways perturbed by cisplatin induced nephrotoxic on NRK-52E cells which involved changes in amino acid metabolism, lipid metabolism and glycolysis such that choline, creatinine, phosphocholine, valine, acetic acid, phenylalanine, leucine, glutamic acid, threonine, uridine and proline as the main metabolites which differentiated the cisplatin induced group of NRK-52E from control cells extract while the corresponding media exhibited lactic acid, glutamine, glutamic acid and glucose-1-phosphate as the varied metabolites. C. nutans aqueous leaves extract at 1000 μg/mL exhibited the highest potential for a nephroprotective effect against cisplatin toxicity on NRK-52E cell lines at 89% of viability where the protective effect of C. nutans aqueous leaves extract could be discerned by the changes in the metabolites such as choline, alanine and valine in the pre-treated samples with those of the cisplatin-induced group [46]. Moreover, the nephroprotective effect of C. nutans against cisplatin-induced nephrotoxic human kidney cells was also reported by the same group using 8 different solvent extracts from C. nutans leaves. The aqueous extract showed a protective effect against the induced cell line based on the improvement of the percentage viability in mitochondrial dehydrogenase activity (MTT) and lactate dehydrogenase (LDH) assay pretreated with the extract after 24 h [47] (Table 3k). Renoprotective data by C. nutans showed that many areas need to be unfolded and extensive research is required for bench work to clinical practice.
3.12. Toxicology Studies
Toxicity testing provides the knowledge regarding some of the risks that may be associated with use of herbs, therefore avoiding potential harmful effects when used for therapeutic purposes. Generally, toxicological studies could be divided into acute, sub-acute and chronic phases, depending on the exposure duration of animals to any drugs but also depending on the dose of the substance and also on the toxic properties of the substance. The relationships between these two factors are crucial in the evaluation of therapeutic dosage in pharmacology and herbalism [52]. In an acute in-vivo study, rats treated with 5000 mg/kg survived throughout the 14-day observation period. Neither death nor signs of toxicity-related changes were reported on skin and fur, eyes, mucous membranes, respiratory pattern, autonomic or behavior patterns such as convulsions, salivation, diarrhea or lethargy including changes in the body weight, water and food consumption in animals. The authors also reported that single dose administration of aqueous C. nutans leaves extract over 14 days showed no early or late morbidity, mortality or apparent signs of toxicity [unpublished data]. As in hematological parameters, the serum eosinophil level in rats treated with 500 mg/kg of aqueous C. nutans leaves extract was elevated by 1.9 times as compared to their control counterparts; however, the authors claimed that the variation was within the normal physiological range (0 < 2.5 < 3%) for rats. In addition to that, rats treated with 2000 mg/kg aqueous C. nutans leaves extract for 90 days showed increases in the activated partial thromboplastin time by 3.7 times compared to the normal control group. Their finding suggested that 2000 mg/kg of aqueous C. nutans leaves extract could potentially act as an anti-inflammatory therapeutic and anticoagulant agent [53]. In another study by [54], acute oral toxicity of methanolic extracts treated to male Swiss albino mice at 900 and 1800 mg/kg for 14 days did not exhibit any mortality cases and side effects on kidney, liver, lungs, spleen and heart. While, for sub-chronic toxicity study, the no-observed-adverse-effect level (NOAEL) is greater than 2500 mg/kg/day but its renal creatinine level was elevated at doses of 500 and 2500 mg/kg/day in a sub-chronic toxicity study [55]. In a study reported by [56], 1.3 g/kg of ethanolic C. nutans leaves extract administered subcutaneously, intraperitoneally and orally did not produce any signs of acute toxicity in rats. On the contrary, a recent study had claimed that sub-acute administration of the extracts once at 2000 mg/kg induced mild hepatic and renal histological alterations in mice. Similarly, repeated daily oral administration of C. nutans leaves extract for 28 days induced mild to moderate hepatic degeneration at 500 mg/kg and renal necrosis at 1000 mg/kg in female ICR mice [57][58]. These polarized findings could be due to insufficient scientific studies being conducted previously; moreover, the majority of those experiments were done as preliminary and fundamentally oriented. Therefore, further sophisticated investigation still needs to be done, due to lack of data obtained from biological investigations that are associated with other phytochemical bioactive fractions from this plant still leading to toxicity incidents in laboratory animals. This further pinpoint that the phytochemical compositions that are present in this plant extract used for toxicity studies were not fully identified via any phytochemical analysis in order to compare the biological studies. Farsi also simulated the use of this extract on a human equivalent dose, based on the results obtained from oral toxicological studies using the body surface area (BSA) normalization method, illustrating the human equivalent dose of aqueous C. nutans leaves extract is equal to 324.32 mg/kg [44]. However, the acceptable daily intake on the non-observable adverse effect level value obtained from the animal study is 9 mg/kg in humans with reference to the guidelines from Food and Agriculture Material Inspection Center [10][59]. Hence, a well-designed clinical study is still needed to assess its chronic toxicity to affirm a specific safety dose to be adopted for human consumption to avoid any potential adverse side effects.
References
- Bernardini, S.; Tiezzi, A.; Laghezza Masci, V.; Ovidi, E. Natural products for human health: An historical overview of the drug discovery approaches. Nat. Prod. Res. 2018, 32, 1926–1950.
- Beyene, B.; Beyene, B.; Deribe, H. Review on application and management of medicinal plants for the livelihood of the local community. Int. J. Hum. Resour. Manag. 2016, 22, 33–39.
- Haida, Z.; Hakiman, M. A review of therapeutic potentials of Clinacanthus nutans as source for alternative medicines. Sains Malaysiana 2019, 48, 2683–2691.
- Mat Yusuf, S.N.A.; Che Mood, C.N.A.; Ahmad, N.H.; Sandai, D.; Lee, C.K.; Lim, V. Optimization of biogenic synthesis of silver nanoparticles from flavonoid-rich Clinacanthus nutans leaf and stem aqueous extracts. R. Soc. Open Sci. 2020, 7, 200065.
- Aslam, M.S.; Ahmad, M.S.; Mamat, A.S. A review on phytochemical constituents and pharmacological activities of Clinacanthus nutans. Int. J. Pharm. 2015, 7, 4.
- Quattrocchi, U. Clinacanthus Nees Acanthaceae.“ CRC World Dict Med. Poisonous Plants Common Names, Sci Names, Eponyms, Synonyms, Etymology, 1st ed.; CRC Press: Boca Raton, FL, USA, 2012.
- Kuo, X.; Herr, D.R.; Ong, W.-Y. Anti-inflammatory and Cytoprotective Effect of Clinacanthus nutans Leaf But Not Stem Extracts on 7-Ketocholesterol Induced Brain Endothelial Cell Injury. Neuromolecular Med. 2021, 23, 176–183.
- Kosai, P.; Sirisidthi, K.; Jiraungkoorskul, W. Evaluation of total phenolic compound and cytotoxic activity of Clinacanthus nutans. Indian, J. Pharm. Sci. 2016, 78, 283–286.
- Andasari, S.D.; Mustofa, C.H. Standarisasi Spesifik Dan Non Spesifik Ekstrak Etil Asetat Daun Dandang Gendis (Clinacanthus Nutans). MOTORIK Jurnal Ilmu Kesehatan 2020, 15, 70–75.
- Zulkipli, I.N.; Rajabalaya, R.; Idris, A.; Sulaiman, N.A.; David, S.R. Clinacanthus nutans: A review on ethnomedicinal uses, chemical constituents and pharmacological properties. Pharm. Biol. 2017, 55, 1093–1113.
- Ch’ng, Y.S.; Tan, C.S.; Loh, Y.C.; Ahmad, M.; Asmawi, M.Z.; Yam, M.F. Vasorelaxation study and tri-step infrared spectroscopy analysis of Malaysian local herbs. J. Pharmacopuncture. 2016, 19, 145.
- Yahaya, R.; Dash, G.K.; Abdullah, M.S.; Mathews, A. Clinacanthus nutans (burm. F.) Lindau: An useful medicinal plant of south-east Asia. Int. J. Pharmacogn. Phytochem. 2015, 7, 1244–1250.
- Kobun, R. Recent methods for extraction and identification of Clinacanthus Nutans. Biology 2019, 1–25.
- Alam, M.A.; Zaidul, I.; Ghafoor, K.; Sahena, F.; Hakim, M.; Rafii, M.; Abir, H.; Bostanudin, M.; Perumal, V.; Khatib, A. In vitro antioxidant and, α-glucosidase inhibitory activities and comprehensive metabolite profiling of methanol extract and its fractions from Clinacanthus nutans. BMC Complement Altern. Med. 2017, 17, 1–10.
- Yeo, B.S.; Yap, Y.J.; Koh, R.Y.; Ng, K.Y.; Chye, S.M. Medicinal properties of Clinacanthus nutans: A review. Trop. J. Pharm. Res. 2018, 17, 375–382.
- Tan, L.T.-H.; Khaw, K.Y.; Ong, Y.S.; Khan, T.M.; Lee, L.-H.; Lee, W.-L.; Goh, B.-H. Clinacanthus nutans (Burm. f.) Lindau as a Medicinal Plant with Diverse Pharmacological Values. In Plant-derived Bioactives; Swamy, M., Ed.; Springer: Singapore, 2020; pp. 461–491.
- Ďuračková, Z.; Gvozdjáková, A. Mitochondrial Medicine; Gvozdjáková, A., Ed.; Springer: Amsterdam, The Netherlands, 2008; pp. 19–54.
- Irshad, M.; Chaudhuri, P.S. Oxidant-antioxidant system: Role and significance in human body. Indian J Exp Biol. 2002, 40, 1233–1239.
- Abd Rahman, N.M.A.N.; Nurliyana, M.; Afiqah, M.N.N.; Osman, M.A.; Hamid, M.; Lila, M.A.M. Antitumor and antioxidant effects of Clinacanthus nutans Lindau in 4 T1 tumor-bearing mice. BMC Complement Altern. Med. 2019, 19, 1–9.
- Ismail, N.Z.; Md Toha, Z.; Muhamad, M.; Nik Mohamed Kamal, N.N.S.; Mohamad Zain, N.N.; Arsad, H. Antioxidant effects, antiproliferative effects, and molecular docking of Clinacanthus nutans leaf extracts. Molecules 2020, 25, 2067.
- Haron, N.H.; Toha, Z.M.; Abas, R.; Hamdan, M.R.; Azman, N.; Khairuddean, M.; Arsad, H. In vitro cytotoxic activity of Clinacanthus nutans leaf extracts against HeLa cells. Asian Pac. J. Cancer Prev. 2019, 20, 601.
- Ismail, N.Z.; Arsad, H.; Samian, M.R.; Ab Majid, A.H.; Hamdan, M.R. Evaluation of genetic diversity of Clinacanthus nutans (Acanthaceaea) using RAPD, ISSR and RAMP markers. Physiol. Mol. Biol. Plants. 2016, 22, 523–534.
- Sakdarat, S.; Sittiso, S.; Ekalaksananan, T.; Pientong, C.; Charoensri, N.; Kongyingyoes, B. Study on Effects of compounds from Clinacanthus nutans on dengue virus type 2 infection. SSRN 2017, 16, 1–6.
- Pongmuangmul, S.; Phumiamorn, S.; Sanguansermsri, P.; Wongkattiya, N.; Fraser, I.H.; Sanguansermsri, D. Anti-herpes simplex virus activities of monogalactosyl diglyceride and digalactosyl diglyceride from Clinacanthus nutans, a traditional Thai herbal medicine. Asian Pac. J. Trop. Biomed. 2016, 6, 192–197.
- Sookmai, W.; Ekalaksananan, T.; Pientong, C.; Sakdarat, S.; Kongyingyoes, B. The anti-papillomavirus infectivity of Clinacanthus nutans compounds. Srinagarind Med. J. 2011, 26, 240–243.
- Timpawat, S.; Vajrabhaya, L. Clinical evaluation of Clinacanthus nutans Lindau in orabase in the treatment of recurrent aphthous stomatitis. MDJ 1994, 14, 10–16.
- Lim, S.-H.E.; Almakhmari, M.A.; Alameri, S.I.; Chin, S.-Y.; Abushelaibi, A.; Mai, C.-W.; Lai, K.-S. Antibacterial Activity of Clinacanthus nutans Polar and Non-Polar Leaves and Stem Extracts. Biomed. Pharmacol. J. 2020, 13, 1169–1175.
- Roeslan, M.O.; Ayudhya, T.D.N.; Yingyongnarongkul, B.-E.; Koontongkaew, S. Anti-biofilm, nitric oxide inhibition and wound healing potential of purpurin-18 phytyl ester isolated from Clinacanthus nutans leaves. Biomed. Pharmacother. 2019, 113, 108724.
- Hanafiah, R.M.; Kamaruddin, K.A.C.; Saikin, N.A.A.; WNABWA, A.; Yakop, M.F.; Lim, V.; Ghafar, S.; Azizan, N.; Said, S.M. Antibacterial properties of clinacanthus nutans extracts against porphyromonas gingivalis and aggregatibacter actinomycetemcomitans: An in-vitro study. J. Int. Dent. Med. Res. 2019, 12, 401–404.
- Choonharuangdej, S.; Amornvit, P.; Srithavaj, T.; Alam, M.K. In vitro anti-candida effect of Thai herbs supplemented in tissue conditioner. Int. Med. J. 2014, 21, 331–334.
- Cheeptham, N.; Towers, G. Light-mediated activities of some Thai medicinal plant teas. Fitoterapia 2002, 73, 651–662.
- Arullappan, S.; Rajamanickam, P.; Thevar, N.; Kodimani, C.C. In vitro screening of cytotoxic, antimicrobial and antioxidant activities of Clinacanthus nutans (Acanthaceae) leaf extracts. Trop. J. Pharm. Res. 2014, 13, 1455–1461.
- Cherdchu, C.; Poopyruchpong, N.; Adchariyasucha, R.; Ratanabanangkoon, K. The absence of antagonism between extracts of Clinacanthus nutans Burm. and Naja naja siamensis venom. Southeast Asian J. Trop. Med. Public Health 1977, 8, 249–254.
- Uawonggul, N.; Thammasirirak, S.; Chaveerach, A.; Chuachan, C.; Daduang, J.; Daduang, S. Plant extract activities against the fibroblast cell lysis by honeybee venom. J. Med. Plant Res. 2011, 5, 1978–1986.
- Daduang, S.; Sattayasai, N.; Sattayasai, J.; Tophrom, P.; Thammathaworn, A.; Chaveerach, A.; Konkchaiyaphum, M. Screening of plants containing Naja naja siamensis cobra venom inhibitory activity using modified ELISA technique. Anal. Biochem. 2005, 341, 316–325.
- Uawonggul, N.; Chaveerach, A.; Thammasirirak, S.; Arkaravichien, T.; Chuachan, C.; Daduang, S. Screening of plants acting against Heterometrus laoticus scorpion venom activity on fibroblast cell lysis. J. Ethnopharmacol. 2006, 103, 201–207.
- Zakaria, Z.A.; Rahim, M.H.A.; Sani, M.H.M.; Omar, M.H.; Ching, S.M.; Kadir, A.A.; Ahmed, Q.U. Antinociceptive activity of petroleum ether fraction obtained from methanolic extract of Clinacanthus nutans leaves involves the activation of opioid receptors and NO-mediated/cGMP-independent pathway. BMC Complement. Altern. Med. 2019, 19, 1–14.
- Ooi, S.H.; Noor Mohamed, N.M.; Kalaichelvam, R.K.; Lim, V. Effects of Clinacanthus nutans extracts on cytokine secretion in PMA-induced U937 macrophage cells. RJP 2021, 8, 27–35.
- Tu, S.-F.; Liu, R.H.; Cheng, Y.-B.; Hsu, Y.-M.; Du, Y.-C.; El-Shazly, M.; Wu, Y.-C.; Chang, F.-R. Chemical constituents and bioactivities of Clinacanthus nutans aerial parts. Molecules 2014, 19, 20382–20390.
- Umar Imam, M.; Ismail, M.; George, A.; Chinnappan, S.M.; Yusof, A. Aqueous leaf extract of Clinacanthus nutans improved metabolic indices and sorbitol-related complications in type II diabetic rats (T2D). Food Sci. Nutr. 2019, 7, 1482–1493.
- Azemi, A.K.; Mokhtar, S.S.; Rasool, A.H.G. Clinacanthus nutans Leaves Extract Reverts Endothelial Dysfunction in Type 2 Diabetes Rats by Improving Protein Expression of eNOS. Oxid. Med. Cell. Longev. 2020, 2020.
- Murugesu, S.; Ibrahim, Z.; Ahmed, Q.-U.; Nik Yusoff, N.-I.; Uzir, B.-F.; Perumal, V.; Abas, F.; Saari, K.; El-Seedi, H.; Khatib, A. Characterization of α-glucosidase inhibitors from Clinacanthus nutans Lindau leaves by gas chromatography-mass spectrometry-based metabolomics and molecular docking simulation. Molecules 2018, 23, 2402.
- Abdulwahid, S.J.; Goh, M.Y.; Ebrahimi, M.; Mohtarrudin, N.; Hashim, Z.B. Effects of Methanolic Leaf Extract of Clinacanthus nutans on Fatty Acid Composition and Gene Expression in Male Obese Mice. Preprints 2018, 2018040232.
- Abdulwahid, S.; Ebrahimi, M.; Goh, Y.; Adeyemi, K.; Ismail, H.; Hashim, Z. Methanolic extract of Clinacanthus nutans leaves can alter adipocytes cellularity, inflammation, and acetyl cholinesterase activity in male obese mice. J. Obes. Weight Loss Ther. 2017, 7.
- Sarega, N.; Imam, M.U.; Ooi, D.J.; Chan, K.W.; Md Esa, N.; Zawawi, N.; Ismail, M. Phenolic Rich Extract from Clinacanthus nutans Attenuates Hyperlipidemia-Associated Oxidative Stress in Rats. Oxid. Med. Cell. Longev. 2016, 2016, 4137908.
- Mahmod, I.I.; Ismail, I.S.; Alitheen, N.B.; Normi, Y.M.; Abas, F.; Khatib, A.; Latip, J. NMR and LCMS analytical platforms exhibited the nephroprotective effect of Clinacanthus nutans in cisplatin-induced nephrotoxicity in the in vitro condition. BMC Complement. Altern. Med. 2020, 20, 1–18.
- Ismail, I.; Mahmod Ilya, I. Nephroprotective effect of Clinacanthus nutans against cisplatin-induced human kidney cell (PCS-400-010). Planta Med. Int. 2017, 4.
- Azemi, A.K.; Mokhtar, S.S.; Rasool, A.H.G. Clinacanthus nutans: Its potential against diabetic vascular diseases. Braz. J. Pharm. Sci. 2021, 56, 1–10.
- Popkin, B.M.; Adair, L.S.; Ng, S.W. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev. 2012, 70, 3–21.
- Nelson, R.H. Hyperlipidemia as a risk factor for cardiovascular disease. Prim. Care. 2013, 40, 195–211.
- Li, Q.; Yon, J.-Y.; Cai, H. Mechanisms and consequences of eNOS dysfunction in hypertension. J. Hypertens. 2015, 33, 1128.
- Chanda, S.; Parekh, J.; Vaghasiya, Y.; Dave, R.; Baravalia, Y.; Nair, R. Medicinal plants-from traditional use to toxicity assessment: A review. Int. J. Pharm. Sci. Res. 2015, 6, 2652.
- Thongrakard, V.; Tencomnao, T. Modulatory effects of Thai medicinal plant extract on proinflammatory cytokines-induced apoptosis in human keratinocyte HaCaT cells. Afr. J. Biotechnol. 2010, 9, 4999–5003.
- P’ng, X.W.; Akowuah, G.A.; Chin, J.H. Acute oral toxicity study of Clinacanthus nutans in mice. Int. J. Pharm. Sci. Res. 2012, 3, 4202.
- Abdul Rahim, M.H.; Zakaria, Z.A.; Mohd Sani, M.H.; Omar, M.H.; Yakob, Y.; Cheema, M.S.; Ching, S.M.; Ahmad, Z.; Abdul Kadir, A. Methanolic Extract of Clinacanthus nutans Exerts Antinociceptive Activity via the Opioid/Nitric Oxide-Mediated, but cGMP-Independent, Pathways. Evid. Based Complement. Alternat. Med. 2016, 2016, 1494981.
- Chavalittumrong, P.; Attawish, A.; Rugsamon, P.; Chuntapet, P. Toxicological study of Clinacanthus nutans (Burm. f.) Lindau. Warasan Krom Witthayasat Kan Phaet 1995, 37, 232–338.
- Abdulwahid, S.; Goh, M.; Ebrahimi, M.; Mohtarrudin, N.; Hashim, Z. Sub-acute oral toxicity profiling of the methanolic leaf extract of clinacanthus nutans in male and female ICR mice. Int. J. Pharm Pharm Sci 2018, 10, 25.
- Aliyu, A.; Shaari, M.R.; Ahmad Sayuti, N.S.; Reduan, M.F.H.; Sithambaram, S.; Noordin, M.M.; Shaari, K.; Hamzah, H. Subacute oral administration of Clinacanthus nutans ethanolic leaf extract induced liver and kidney toxicities in ICR mice. Molecules 2020, 25, 2631.
- P’ng, X.W.; Akowuah, G.A.; Chin, J.H. Evaluation of the sub–acute oral toxic effect of methanol extract of Clinacanthus nutans leaves in rats. J. Acute Dis. 2013, 2, 29–32.
More
Information
Subjects:
Urology & Nephrology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.5K
Revisions:
2 times
(View History)
Update Date:
13 Jan 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




