Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mohamed Koubaa | + 2758 word(s) | 2758 | 2022-01-05 07:07:47 | | | |
| 2 | Camila Xu | Meta information modification | 2758 | 2022-01-13 07:12:18 | | | | |
| 3 | Camila Xu | Meta information modification | 2758 | 2022-01-13 07:13:51 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Koubaa, M. 2-Phenylethanol. Encyclopedia. Available online: https://encyclopedia.pub/entry/18178 (accessed on 09 March 2026).
Koubaa M. 2-Phenylethanol. Encyclopedia. Available at: https://encyclopedia.pub/entry/18178. Accessed March 09, 2026.
Koubaa, Mohamed. "2-Phenylethanol" Encyclopedia, https://encyclopedia.pub/entry/18178 (accessed March 09, 2026).
Koubaa, M. (2022, January 13). 2-Phenylethanol. In Encyclopedia. https://encyclopedia.pub/entry/18178
Koubaa, Mohamed. "2-Phenylethanol." Encyclopedia. Web. 13 January, 2022.
Copy Citation
2-Phenylethanol (2-PE), also known as 2-phenethyl alcohol, is a higher aromatic alcohol characterized by one of the most popular and desired fragrances, which is the rosy scent.
2-phenylethanol
agro-industrial waste
biotechnology
microbial fermentation
1. Introduction
Over the years, fragrance usage became ubiquitous. A huge number of flavors and fragrances found their way into the daily life of almost all human beings. 2-Phenylethanol (2-PE), also known as 2-phenethyl alcohol, is a higher aromatic alcohol characterized by one of the most popular and desired fragrances, which is the rosy scent [1]. 2-PE has a wide range of applications in diverse types of products. It is commonly used in cosmetics including perfumes, in pharmaceuticals as a preservative and in various herbal products. Additionally, 2-PE is used in food and beverage industries as organoleptic enhancer of the final product [2][3]. For instance, this aroma is added to the composition of a variety of ice creams, candies, cookies, puddings, gelatins, cigarettes, and chewing gums [4]. This flavor has been approved by many worldwide organizations, including the Food and Drug Administration (FDA), Flavor and Extract Manufacturers’ Association (FEMA), the Joint Expert Committee on Food Additives (JECFA), and the Council of Europe (COE). They consider 2-PE as generally recognized as safe (GRAS, 2858), which gives it an added value [5]. Additionally, 2-PE exerts antimicrobial and antifungal properties. It can inhibit the growth of several Gram-positive and Gram-negative microorganisms, such as Escherichia coli, Staphylococcus aureus, Enterococcus faecium and many fungal species including Candida albicans, Candida dubliniensis, Saccharomyces cerevisiae, Kluyveromyces marxianus, and many other microorganisms [6]. These characteristics made this substance an appreciated additive in antiseptics, preservatives, cleaning, and personal care products [2]. It is noteworthy that 2-PE is also being used in aromatherapy. It was demonstrated that the odor of rose oil reduces plasma adrenaline concentration by 30% and human sympathetic activity by 40% [7]. In addition, 2-PE is used as a precursor for the synthesis of other valuable chemicals such as 2-phenethyl acetate (2-PEA), which is a volatile ester having also a rose-like odor [8].
The world production of 2-PE is approximately 10,000 tons per year, most of which is obtained by chemical synthesis, with a price between 3.5 and 5 US$/kg [9][10]. However, natural 2-PE, extracted from the essential oil of some flowers, is much more expensive compared to the chemically synthesized 2-PE with an estimated price of about 1000 US$/kg [11]. Martinez-Avila et al. estimated that the bioproduction of 2-PE via a microbial route would actually cost around 220 US$/kg [12].
Three different paths are used in the industry to chemically synthesize 2-PE. First, the Friedel-Crafts reaction of ethylene oxide with benzene in the presence of aluminum chloride. Second, the hydrogenation of styrene oxide with a small amount of sodium hydroxide and Raney nickel as a catalyst. Third, the oxidation of propylene with 2-phenylethyl hydroperoxide [13]. These methods involve the use of toxic chemicals (e.g., benzene, styrene oxide), which are carcinogenic and hazardous to the health and environment. Furthermore, chemical synthesis needs high temperatures, high pressure, and strong alkali or acid conditions. It is also associated with the production of unwanted byproducts. All of this, affects the quality of the final product, making its purification difficult [14][15].
On the other hand, natural 2-PE can be extracted from the essential oils of various flowers, including jasmine, hyacinth, lilies, and daffodils. However, the concentration of 2-PE in these flowers is too low, except for rose oil which can contain up to 60% 2-PE [11]. For instance, the dominant aroma compound produced by Rosa damascena, also known as damask roses, is 2-PE [16]. Because of the low concentration of 2-PE in almost all types, multiple separation steps are required [12]. As a consequence of the aforementioned rarity of natural 2-PE in flowers, requiring complicated and costly downstream processing, the large market demand cannot be satisfied [14].
The uses of 2-PE in cosmetic products and in different foods and pharmaceuticals drove increased demand for natural methods of production of this aroma [17], thus boosting research to find alternative methods for the production of natural 2-PE through biotechnological approaches [18]. The most promising method is through microbial fermentation, mainly the one based on the synthesis of 2-PE using yeasts [19]. Yeasts have the ability to synthesize 2-PE using L-phenylalanine (L-Phe) or glucose as substrates using the Ehrlich pathway and the shikimate pathway, respectively [4][20]. In fact, biotechnological production of flavors and fragrances is becoming more attractive, since it produces a final product classified as natural by the US Food and Drug Administration and by the European legislation if the substrates used in the process are of natural origin [21]. In addition to the formation of a “natural” product, the production cycle of microbial fermentation is short and environmentally friendly, decreasing the environmental pollution caused by the chemical synthesis of 2-PE [22][23]. However, it is noteworthy that microbial fermentation techniques need highly productive yeasts and cheap feedstocks in order to be economically attractive and compete with the chemical synthesis routes [24]. Agro-industrial waste and by-products having high nutritional value offer suitable environments for the growth of microorganisms. Through fermentation, microorganisms have the ability to use them as potential raw materials for the production of value-added products [25]. This is known as “biorefinery”, whereby the waste and by-products of an industry can serve as the raw material for another [26].
2. Yeasts Producing 2-PE
Yeasts are microorganisms with large synthetic abilities. Through enzyme-catalyzed reactions, they can convert simple carbohydrates and nitrogen sources into different complex molecules, particularly various flavor compounds [27]. In fact, numerous yeast species have the ability to produce 2-PE [9], and this production is strain-specific, which means that a significant difference in 2-PE production level may be seen between different strains of one species [28]. It is noteworthy that recently numerous metabolically engineered yeast strains have been constructed in order to significantly enhance 2-PE yield [29].
Saccharomyces cerevisiae, a significant eukaryotic model organism, is a well-known promising microorganism for its ability to produce 2-PE [30]. Many studies have proved that other so-called non-conventional yeasts are also able to produce 2-PE and some of them show a higher capacity for aroma metabolite production compared to S. cerevisiae [31]. Kluyveromyces marxianus [19], Kluyveromyces lactis, Pichia fermentans, Pichia anomala, Pichia membranaefaciens, Candida utilis [32], Phellinus ignarius, Ischnoderma benzoinum, Geotrichum penicillatum, Aspergillus niger [33], Meyerozyma caribbica, Meyerozyma guilliermondii, Metschnikowia chrysoperlae, Clavispora lusitaniae, Candida tropicalis [24], Yarrowia lipolytica, Zygosaccharomyces rouxii, Pichia kudriavzevii [2], and Pichia pastoris have all been previously reported to produce 2-PE, but to different extents [34].
Later in this entry, the ability of conventional and non-conventional yeasts to produce 2-PE on synthetic media and on agro-industrial waste and by-products will be discussed, along with the factors and fermentation conditions affecting the concentration of 2-PE produced by each species.
3. Biochemical Pathways for 2-PE Production
Yeast cells are capable of producing 2-PE via normal metabolism. 2-PE can be synthesized through two independent routes in yeast cells, either de novo via the shikimate pathway or by the bioconversion of L-Phe via the Ehrlich pathway (Figure 1) [35].
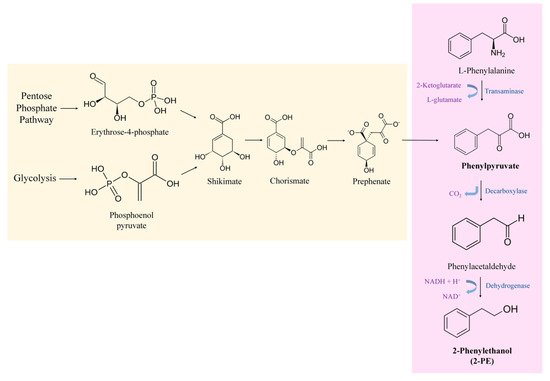
Figure 1. Metabolic pathways involved in the production of 2-PE in yeasts: Shikimate pathway for de novo synthesis of 2-PE and Ehrlich pathway for 2-PE production from L-Phe.
The Ehrlich pathway is considered the most efficient biotechnological approach and thus the more industrially attractive option. Most advances in biotechnology have focused on the development of this process [18]. In this mechanism, the yeast cells use the aromatic amino acid, L-phenylalanine, as a sole nitrogen source in order to produce 2-PE. It involves three steps, where the first one consists of converting L-Phe into phenylpyruvate. In the model yeast S. cerevisiae, this transamination reaction is catalyzed by two amino acid transaminase isoenzymes Aro8p and Aro9p. Second, phenylpyruvate is decarboxylated to phenylacetaldehyde. The second step of the Ehrlich pathway is catalyzed by the phenylpyruvate decarboxylase enzyme, Aro10p, and the three pyruvate decarboxylase isoenzymes Pdc1p, Pdc5p and Pdc6p. Finally, the third step consists of reducing the phenylacetaldehyde to 2-PE by alcohol dehydrogenases (Adh1p, Adh2p, Adh3p, Adh4p, Adh5p) and formaldehyde dehydrogenase Sfa1p [30][35]. This natural process is significantly improved when the amino acid L-Phe is available in the media, since high concentrations of L-Phe are needed to shift cell metabolism to the Ehrlich pathway [36].
On the other hand, yeasts can produce 2-PE from intermediate molecules of their metabolism (e.g., phosphoenolpyruvate (PEP) and erythrose-4-phosphate (E4P)). This is known by the de novo synthesis of 2-PE which takes place through the shikimate pathway. In this pathway, simple sugars are transformed into 2-PE [37]. PEP and E4P resulting from glycolysis and pentose phosphate pathway, respectively, are catalyzed to synthesize 3-deoxy-D-arabinoheptulosonate-7-phosphate (DAHP). Then, through a series of intermediates including shikimate, chorismate, prephenate, etc., phenylpyruvate is produced (Figure 1). Phenylpyruvate, the product of the shikimate pathway, next enters the Ehrlich pathway. Hence, phenylpyruvate is then decarboxylated to phenylacetaldehyde and dehydrogenated to 2-PE [38]. It is noteworthy that the shikimate pathway involves many steps with branched metabolic pathways and a variety of feedback inhibitions. Moreover, the glycolysis and the pentose phosphate pathways are mainly utilized for cell growth rather than 2-PE production. This will limit the transformation of simple sugars to 2-PE, hence the final concentration of 2-PE obtained is very low [35].
4. Factors Affecting the Rate of 2-PE Production in Yeasts
The extent to which yeasts are capable of producing 2-PE does not only depend on the species itself, but also on the media composition and the fermentation conditions [4].
If amino acids, particularly L-Phe, are the sole nitrogen source in the medium, the Ehrlich pathway predominates over de novo synthesis, which usually dominates at low amino acid concentrations [39]. However, in the presence of more assimilable nitrogen sources, L-Phe will be metabolized through the cinnamate pathway, limiting the 2-PE formation. Therefore, high concentrations of 2-PE can be achieved by supplying L-Phe as a sole nitrogen source [40]. It is important to note that the usage of L-Phe as a substrate for 2-PE production is costly [12]. Therefore, metabolic engineering is being used to boost the aromatic amino acids pathway (AAA) for providing L-Phe for the bioconversion and also overexpression of the key enzymes of the Ehrlich pathway [41].
On the other hand, fermentation conditions, particularly the availability of nutrients, nitrogen sources, and sugars supplied in the culture media, affect the metabolic activity of the microorganisms [42]. Additionally, temperature, pH, oxygen availability, and airflow rate greatly affect 2-PE production by altering the microbial growth, the microbial community structures, or the key enzyme activities [15].
5. 2-PE Production via Fermentation of Yeasts on Synthetic Media
Various yeast species were capable of producing different amounts of 2-PE during growth on synthetic media. A study done by Chreptowicz et al. showed that S. cerevisiae JM2014 strain, a non-genetically modified strain that was isolated from a fermented milk drink, was able to produce 3.6 g/L of 2-PE after 72 h incubation at 30 °C. The batch culture was done in a 6.2 L bioreactor containing 4 L of medium 8 and 5 g/L L-Phe. Medium 8 is specific for L-Phe biotransformation to 2-PE and its major constituents are 15 g/L glucose, 8 g/L sucrose, and 5 g/L L-Phe [43]. Another study done by de Lima et al. reported that the wild-type (WT) K. marxianus CCT 7735 strain was able to produce 3.44 g/L of 2-PE under optimized conditions. The batch culture was done in a 125 mL Erlenmeyer flask containing 25 mL synthetic medium (3 g/L glucose and 4 g/L L-Phe) at 30 °C, with a stirring rate of 200 rpm for 72 h [44]. Additionally, the WT strain C. glycerinogenes WL2002-5 was able to produce 5 g/L of 2-PE under optimized conditions. Batch fermentation was done in a 5 L bioreactor containing 3 L of synthetic medium supplemented with 90 g/L glucose and 7 g/L L-Phe for 50 h at 30 °C and 500 rpm [45]. Furthermore, the WT Clavispora lusitaniae WUT17 was able to produce 2.04 g/L of 2-PE in medium 8. The experiment was done in batch cultures incubated at 30 °C and 240 rpm for 72 h [46]. WT Pichia kudriavzevii YF1702 was reported to produce 5.09 g/L of 2-PE under its optimal fermentation conditions. Fermentation was done in 250 mL flasks containing 25.5 mL cultivation media (50 g/L glucose and 10.7 g/L L-Phe) for 56 h at 26 °C and 210 rpm shaking [47]. Z. rouxii M2013310 strain was able to produce 3.58 g/L 2-PE after 72 h fermentation in M3 culture medium containing 30 g/L glucose, 8 g/L sucrose, and 9 g/L L-Phe [48].
Additionally, a S. cerevisiae CWY132 mutant strain was able to produce 3.76 g/L 2-PE under optimized conditions. Fermentation was done in a batch process with a medium containing 34.16 g/L glucose and 5 g/L L-Phe [49]. Another study reported that S. cerevisiae PFP-26, a mutant strain resistant to the phenylalanine analog p-fluoro-d,l-phenylalanine (PFP) and isolated from the parent yeast S. cerevisiae Kyokai No.9 was able to produce 0.973 g/L. S. cerevisiae PFP-26 cells were shaken in MM5 medium at 30 °C for 48 h. MM5 is a minimal medium containing 5% glucose [50].
On the other hand, several genetic engineering strategies were developed in order to improve 2-PE production in various yeast strains. A study done by Kim et al. reported that a genetically engineered S. cerevisiae strain, JHY315 strain, was able to produce 4.8 g/L of 2-PE. JHY315 is an aldehyde dehygrogenase 3 (ALD3) deletion strain overexpressing ARO9 and ARO10 genes by episomal overexpression and overexpressing the transcription factor Aro80 in order to induce the endogenous genes. This strain was grown on a synthetic media composed mainly of 20 g/L glucose and 10 g/L L-Phe amino acid. The batch culture was done in a 250 mL shake flask and cultivated at 30 °C with shaking at 250 rpm [51]. Another study done by Kim et al. showed that the genetically modified K. marxianus BY25569 strain, overexpressing a feedback resistant (fbr) DAHP synthase, known as AroGfbr, from Klebsiella pneumoniae and hence improving the production of DAHP from glucose, can produce 1.3 g/L of 2-PE without the presence of L-Phe. The inoculum was cultivated in 5 mL of defined synthetic medium, containing 20 g/L glucose [52]. Additionally, a novel promising 2-PE producer is Y. lipolytica. At 95 h after the addition of the L-Phe, the genetically engineered Y. lipolytica NCYC3825 strain produced 1.98 g/L of 2-PE. Bioconversion of L-Phe to 2-PE was done in shake flasks containing the cultivation media, containing mainly 40 g glucose, and supplemented with 7 g/L L-Phe after 73 h of cultivation [9][53]. Finally, a P. pastoris SK004 strain overexpressing ARO10, ARO8, the aldehyde reductase ADH6 gene, aroGfbr, and chorismate mutase-prephenate dehydratase (pheAfbr) which is a feedback resistance mutant gene. aroGfbr and pheAfbr were previously reported, in the bacterium E. coli, to reduce the feedback inhibition of l-Phe biosynthesis by the accumulated phenylpyruvate. After 36 h incubation at 30 °C, in a rotary shaker at 200 rpm, this strain was able to produce 1.169 g/L 2-PE [34].
Subsequently, various yeasts were able to produce 2-PE to different extents, depending on the characteristics and type of the species and strain used and the nutrients present in the synthetic media. Based on the studies mentioned above, it can be noted that the different yeasts growing on multiple synthetic media were able to produce between 1.17 and 5.08 g/L 2-PE. An important factor being the amount of L-Phe. Increasing the supply of L-Phe leads to a higher production of 2-PE. It is noteworthy that the key parameter of 2-PE production is to have strains capable of producing 2-PE through their normal metabolism, however, L-Phe supplementation to the medium allowed higher production of 2-PE [40].
Additionally, conventional culture media are expensive due to their constituents particularly some amino acids and the gelling agents, such as L-Phe and agar, respectively. Therefore, these media are not cost-effective on a large scale, and industries have started to search for and use various alternative cheap carbon sources (Table 1) [54].
Table 1. Advantages and disadvantages of using synthetic media versus agro-industrial waste and by-products for 2-PE production [25][26][54][55].
| Synthetic Culture Media | Agro-Industrial Waste and By-Products | |
|---|---|---|
| Advantages |
|
|
| Disadvantages |
|
|
References
- Chantasuban, T.; Santomauro, F.; Gore-Lloyd, D.; Parsons, S.; Henk, D.; Scott, R.J.; Chuck, C. Elevated production of the aromatic fragrance molecule, 2-phenylethanol, using Metschnikowia pulcherrima through both de novo and ex novo conversion in batch and continuous modes. J. Chem. Technol. Biotechnol. 2018, 93, 2118–2130.
- Martínez-Avila, O.; Sánchez, A.; Font, X.; Barrena, R. Bioprocesses for 2-phenylethanol and 2-phenylethyl acetate production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2018, 102, 9991–10004.
- Guo, D.; Zhang, L.; Kong, S.; Liu, Z.; Li, X.; Pan, H. Metabolic Engineering of Escherichia coli for Production of 2-Phenylethanol and 2-Phenylethyl Acetate from Glucose. J. Agric. Food Chem. 2018, 66, 5886–5891.
- Garavaglia, J.; Flôres, S.H.; Pizzolato, T.M.; Peralba, M.D.C.; Ayub, M.A.Z. Bioconversion of l-phenylalanine into 2-phenylethanol by Kluyveromyces marxianus in grape must cultures. World J. Microbiol. Biotechnol. 2007, 23, 1273–1279.
- Scognamiglio, J.; Jones, L.; Letizia, C.S.; Api, A.M. Fragrance material review on phenylethyl alcohol. Food Chem. Toxicol. 2012, 50, S224–S239.
- Majdabadi, N.; Falahati, M.; Heidarie-Kohan, F.; Farahyar, S.; Rahimi-Moghaddam, P.; Ashrafi-Khozani, M.; Razavi, T.; Mohammadnejad, S. Effect of 2-Phenylethanol as Antifungal Agent and Common Antifungals (Amphotericin B, Fluconazole, and Itraconazole) on Candida Species Isolated from Chronic and Recurrent Cases of Candidal Vulvovaginitis. Assay Drug Dev. Technol. 2018, 16, 141–149.
- Ueno, H.; Shimada, A.; Suemitsu, S.; Murakami, S.; Kitamura, N.; Wani, K.; Matsumoto, Y.; Okamoto, M.; Ishihara, T. Anti-depressive-like effect of 2-phenylethanol inhalation in mice. Biomed. Pharmacother. 2019, 111, 1499–1506.
- Kuo, C.H.; Chiang, S.H.; Ju, H.Y.; Chen, Y.M.; Liao, M.Y.; Liu, Y.C.; Shieh, C.J. Enzymatic synthesis of rose aromatic ester (2-phenylethyl acetate) by lipase. J. Sci. Food Agric. 2012, 92, 2141–2147.
- Celińska, E.; Kubiak, P.; Białas, W.; Dziadas, M.; Grajek, W. Yarrowia lipolytica: The novel and promising 2-phenylethanol producer. J. Ind. Microbiol. Biotechnol. 2013, 40, 389–392.
- Quintelas, C.; Braga, A.; Mesquita, D.P.; Amaral, A.L.; Ferreira, E.C.; Belo, I. NIR spectroscopy applied to the determination of 2-phenylethanol and l-phenylalanine concentrations in culture medium of Yarrowia lipolytica. J. Chem. Technol. Biotechnol. 2019, 94, 812–818.
- Oliveira, S.M.M.; Gomes, S.D.; Sene, L.; Christ, D.; Piechontcoski, J. Production of natural aroma by yeast in wastewater of cassava starch industry. Eng. Agric. 2015, 35, 721–732.
- Martínez-Avila, O.; Sánchez, A.; Font, X.; Barrena, R. Fed-Batch and Sequential-Batch Approaches to Enhance the Bioproduction of 2-Phenylethanol and 2-Phenethyl Acetate in Solid-State Fermentation Residue-Based Systems. J. Agric. Food Chem. 2019, 67, 3389–3399.
- Conde-Báez, L.; López-Molina, A.; Gómez-Aldapa, C.; Pineda-Muñoz, C.; Conde-Mejía, C. Economic projection of 2-phenylethanol production from whey. Food Bioprod. Process. 2019, 115, 10–16.
- Lukito, B.R.; Wu, S.; Saw, H.J.J.; Li, Z. One-Pot Production of Natural 2-Phenylethanol from l-phenylalanine via Cascade Biotransformations. ChemCatChem 2019, 11, 831–840.
- Mu, L.; Hu, X.; Liu, X.; Zhao, Y.; Xu, Y. Production of 2-phenylethanol by microbial mixed cultures allows resource recovery of cane molasses wastewater. Fresenius Environ. Bull. 2014, 23, 1356–1365.
- Sakai, M.; Hirata, H.; Sayama, H.; Sekiguchi, K.; Itano, H.; Asai, T.; Dohra, H.; Hara, M.; Watanabe, N. Production of 2-phenylethanol in roses as the dominant floral scent compound from l-phenylalanine by two key enzymes, a PLP-dependent decarboxylase and a phenylacetaldehyde reductase. Biosci. Biotechnol. Biochem. 2007, 10, 2408–2419.
- Cordente, A.G.; Solomon, M.; Schulkin, A.; Leigh Francis, I.; Barker, A.; Borneman, A.R.; Curtin, C.D. Novel wine yeast with ARO4 and TYR1 mutations that overproduce ‘floral’ aroma compounds 2-phenylethanol and 2-phenylethyl acetate. Appl. Microbiol. Biotechnol. 2018, 102, 5977–5988.
- Etschmann, M.M.W.; Schrader, J. An aqueous-organic two-phase bioprocess for efficient production of the natural aroma chemicals 2-phenylethanol and 2-phenylethylacetate with yeast. Appl. Microbiol. Biotechnol. 2006, 71, 440–443.
- Mierzejewska, J.; Dąbkowska, K.; Chreptowicz, K.; Sokołowska, A. Hydrolyzed corn stover as a promising feedstock for 2-phenylethanol production by nonconventional yeast. J. Chem. Technol. Biotechnol. 2019, 94, 777–784.
- Dai, J.; Xia, H.; Yang, C.; Chen, X. Sensing, Uptake and Catabolism of l-phenylalanine During 2-Phenylethanol Biosynthesis via the Ehrlich Pathway in Saccharomyces cerevisiae. Front. Microbiol. 2021, 12, 12.
- Hua, D.; Xu, P. Recent advances in biotechnological production of 2-phenylethanol. Biotechnol. Adv. 2011, 29, 654–660.
- Wang, H.; Dong, Q.; Guan, A.; Meng, C.; Shi, X.; Guo, Y. Synergistic inhibition effect of 2-phenylethanol and ethanol on bioproduction of natural 2-phenylethanol by Saccharomyces cerevisiae and process enhancement. J. Biosci. Bioeng. 2011, 112, 26–31.
- Conde-Báez, L.; Castro-Rosas, J.; Villagómez-Ibarra, J.R.; Páez-Lerma, J.B.; Gómez-Aldapa, C. Evaluation of Waste of the Cheese Industry for the Production of Aroma of Roses (Phenylethyl Alcohol). Waste Biomass Valorization 2017, 8, 1343–1350.
- Chreptowicz, K.; Sternicka, M.K.; Kowalska, P.D.; Mierzejewska, J. Screening of yeasts for the production of 2-phenylethanol (rose aroma) in organic waste-based media. Lett. Appl. Microbiol. 2018, 66, 153–160.
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid state fermentation: A review. Bioresour. Bioprocess. 2018, 5, 1.
- Ravindran, R.; Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. A review on bioconversion of agro-industrial wastes to industrially important enzymes. Bioengineering 2018, 5, 93.
- Carlquist, M.; Gibson, B.; Karagul Yuceer, Y.; Paraskevopoulou, A.; Sandell, M.; Angelov, A.I.; Gotcheva, V.; Angelov, A.D.; Etschmann, M.; de Billerbeck, G.M.; et al. Process engineering for bioflavour production with metabolically active yeasts—A mini-review. Yeast 2015, 32, 123–143.
- Eshkol, N.; Sendovski, M.; Bahalul, M.; Katz-Ezov, T.; Kashi, Y.; Fishman, A. Production of 2-phenylethanol from l-phenylalanine by a stress tolerant Saccharomyces cerevisiae strain. J. Appl. Microbiol. 2009, 106, 534–542.
- Zhu, L.; Xu, S.; Li, Y.; Shi, G. Improvement of 2-phenylethanol production in Saccharomyces cerevisiae by evolutionary and rational metabolic engineering. PLoS ONE 2021, 16, e0258180.
- Wang, Z.; Jiang, M.; Guo, X.; Liu, Z.; He, X. Reconstruction of metabolic module with improved promoter strength increases the productivity of 2-phenylethanol in Saccharomyces cerevisiae. Microb. Cell Fact. 2018, 17, 1–14.
- de Jesús Rodríguez-Romero, J.; Aceves-Lara, C.A.; Silva, C.F.; Gschaedler, A.; Amaya-Delgado, L.; Arrizon, J. 2-Phenylethanol and 2-phenylethylacetate production by nonconventional yeasts using tequila vinasses as a substrate. Biotechnol. Rep. 2020, 25, e00420.
- Mameeva, O.G.; Ostapchuk, A.M.; Podgorsky, V.S. The 2-Phenylethanol and Ethanol Production by Yeast Saccharomyces cerevisiae. Microbiol. Biotechnol. 2010, 1, 14–22.
- Wang, Q.; Song, Y.; Jin, Y.; Liu, H.; Zhang, H.; Sun, Y.; Liu, G. Biosynthesis of 2-phenylethanol using tobacco waste as feedstock. Biocatal. Biotransform. 2013, 31, 292–298.
- Kong, S.; Pan, H.; Liu, X.; Li, X.; Guo, D. De novo biosynthesis of 2-phenylethanol in engineered Pichia pastoris. Enzyme Microb. Technol. 2020, 133, 109459.
- Wang, Z.; Bai, X.; Guo, X.; He, X. Regulation of crucial enzymes and transcription factors on 2-phenylethanol biosynthesis via Ehrlich pathway in Saccharomyces cerevisiae. J. Ind. Microbiol. Biotechnol. 2017, 44, 129–139.
- Sendovski, M.O.R.; Nir, N.; Fishman, A. Bioproduction of 2-Phenylethanol in a Biphasic Ionic liquid Aqueous system. J. Agric. Food Chem. 2010, 58, 2260–2265.
- Koma, D.; Yamanaka, H.; Moriyoshi, K.; Ohmoto, T.; Sakai, K. Production of aromatic compounds by metabolically engineered Escherichia coli with an expanded shikimate pathway. Appl. Environ. Microbiol. 2012, 78, 6203–6216.
- Liu, J.; Jiang, J.; Bai, Y.; Fan, T.P.; Zhao, Y.; Zheng, X.; Cai, Y. Mimicking a New 2-Phenylethanol Production Pathway from Proteus mirabilis JN458 in Escherichia coli. J. Agric. Food Chem. 2018, 66, 3498–3504.
- Etschmann, M.M.W.; Sell, D.; Schrader, J. Screening of yeasts for the production of the aroma compound 2-phenylethanol in a molasses-based medium. Biotechnol. Lett. 2003, 25, 531–536.
- Etschmann, M.; Bluemke, W.; Sell, D.; Schrader, J. Biotechnological production of 2-phenylethanol. Appl. Microbiol. Biotechnol. 2002, 59, 1–8.
- Tzin, V.; Galili, G. The Biosynthetic Pathways for Shikimate and Aromatic Amino Acids in Arabidopsis thaliana. In The Arabidopsis Book; American Society of Plant Biologists: Rockville, MD, USA, 2010; Volume 8, p. e0132.
- Seguinot, P.; Ortiz-Julien, A.; Camarasa, C. Impact of Nutrient Availability on the Fermentation and Production of Aroma Compounds Under Sequential Inoculation with M. pulcherrima and S. cerevisiae. Front. Microbiol. 2020, 11, 305.
- Chreptowicz, K.; Wielechowska, M.; Główczyk-Zubek, J.; Rybak, E.; Mierzejewska, J. Production of natural 2-phenylethanol: From biotransformation to purified product. Food Bioprod. Process. 2016, 100, 275–281.
- de Lima, L.A.; Diniz, R.H.S.; de Queiroz, M.V.; Fietto, L.G.; da Silveira, W.B. Screening of Yeasts Isolated from Brazilian Environments for the 2-Phenylethanol (2-PE) Production. Biotechnol. Bioprocess Eng. 2018, 23, 326–332.
- Lu, X.; Wang, Y.; Zong, H.; Ji, H.; Zhuge, B.; Dong, Z. Bioconversion of l-phenylalanine to 2-phenylethanol by the novel stress-tolerant yeast Candida glycerinogenes WL2002-5. Bioengineered 2016, 7, 418–423.
- Adame-Soto, P.J.; Aréchiga-Carvajal, E.T.; López, M.G.; González-Herrera, S.M.; Moreno-Jiménez, M.R.; Urtiz-Estrada, N.; Rutiaga-Quiñones, O.M. Potential production of 2-phenylethanol and 2-phenylethylacetate by non-Saccharomyces yeasts from Agave durangensis. Ann. Microbiol. 2019, 69, 989–1000.
- Fan, G.; Cheng, L.; Fu, Z.; Sun, B.; Teng, C.; Jiang, X.; Li, X. Screening of yeasts isolated from Baijiu environments for 2-phenylethanol production and optimization of production conditions. 3 Biotech 2020, 10, 1–17.
- Dai, J.; Li, K.; Song, N.; Yao, W.; Xia, H.; Yang, Q.; Zhang, X.; Li, X.; Wang, Z.; Yao, L.; et al. Zygosaccharomyces rouxii, an Aromatic Yeast Isolated from Chili Sauce, Is Able to Biosynthesize 2-Phenylethanol via the Shikimate or Ehrlich Pathways. Front. Microbiol. 2020, 11, 597454.
- Cui, Z.; Yang, X.; Shen, Q.; Wang, K.; Zhu, T. Optimisation of biotransformation conditions for production of 2-phenylethanol by a Saccharomyces cerevisiae CWY132 mutant. Nat. Prod. Res. 2011, 25, 754–759.
- Fukuda, K.; Watanabe, M.; Asano, K. Altered Regulation of Aromatic Amino Acid Biosynthesis in β-Phenylethyl-alcohol-overproducing Mutants of Sake Yeast Saccharomyces cerevisiae. Agric. Biol. Chem. 1990, 54, 3151–3156.
- Kim, B.; Cho, B.R.; Hahn, J.S. Metabolic engineering of Saccharomyces cerevisiae for the production of 2-phenylethanol via Ehrlich pathway. Biotechnol. Bioeng. 2014, 111, 115–124.
- Kim, T.Y.; Lee, S.W.; Oh, M.K. Biosynthesis of 2-phenylethanol from glucose with genetically engineered Kluyveromyces marxianus. Enzyme Microb. Technol. 2014, 61–62, 44–47.
- Miller, K.K.; Alper, H.S. Yarrowia lipolytica: More than an oleaginous workhorse. Appl. Microbiol. Biotechnol. 2019, 103, 9251–9262.
- Basu, S.; Bose, C.; Ojha, N.; Das, N.; Das, J.; Pal, M.; Khurana, S. Evolution of bacterial and fungal growth media. Bioinformation 2015, 11, 182–184.
- Santos, F.P.; de Magalhães, D.C.M.M.; Nascimento, J.d.S.; Ramos, G.L.d.P.A. Use of products of vegetable origin and waste from hortofruticulture for alternative culture media. Food Sci. Technol. 2021.
More
Information
Subjects:
Biotechnology & Applied Microbiology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.4K
Revisions:
3 times
(View History)
Update Date:
13 Jan 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





