
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sadao Mori | + 2585 word(s) | 2585 | 2022-01-04 08:44:58 | | | |
| 2 | Jessie Wu | Meta information modification | 2585 | 2022-01-13 06:54:54 | | | | |
| 3 | Jessie Wu | Meta information modification | 2585 | 2022-01-13 06:58:40 | | |
Video Upload Options
The genus Passiflora L. is widely cultivated in tropical and subtropical regions. The major species, Passiflora edulis Sims, is known as ‘passion fruit’ and is widely used in processed foods as well as eaten raw. P. edulis fruits are eaten for their pulp together with the seeds; however, the seeds are often discarded when used in processed foods. P. edulis seeds contain a variety of nutrients and functional components, and their industrial use is desirable from the perspective of waste reduction.
1. Introduction
The genus Passiflora L. is a highly diverse plant family with approximately 520 species distributed throughout the tropics of America, Asia, and Africa [1]. More than 90% of Passiflora species are distributed in the Americas; however, they are also widely distributed in India, China, Southeast Asia, Australia, the Pacific islands, and neighboring regions [2]. The Passiflora plant can be divided into pulp, peel, seeds, and bark, the constituents and health benefits of each have been investigated, particularly for P. edulis. The extract of the edible portion reportedly has protective effects against alcoholic liver disease [3]. Moreover, leaf extract has shown a variety of physiological functions, such as being anti-inflammatory [4]; providing intestine protection [5]; and having wound healing [6], antiplatelet [7], and antidepressant effects [8]. It has also been evaluated in animal studies for its safety when administered [9]. P. edulis peel is rich in dietary fiber and functional components, and various physiological effects of P. edulis peel extract have been reported, such as antihypotensive effects [10], hypoglycemic effects [11][12], and metabolic improvement [13][14]. Furthermore, P. edulis bark reportedly has anti-obesity properties [15].
2. Health Benefits of P. edulis Seed Components
2.1. Antioxidant Activity
2.2. Effect on Skin
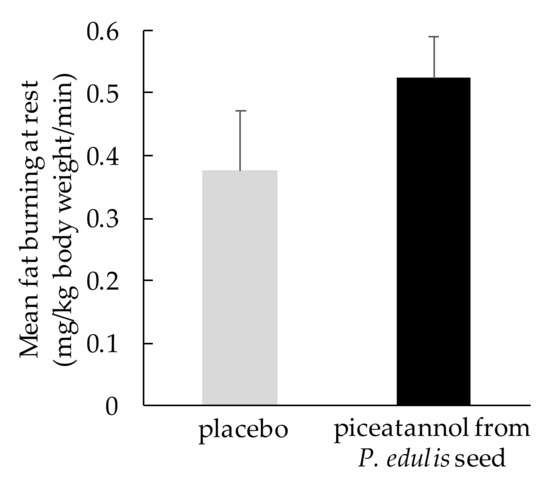
2.3. Effect on Fat Metabolism
2.4. Hypoglycemic Effect
2.5. Other Physiological Effects
References
- Ulmer, T.; MacDougal, J.M. Passiflora: Passionflowers of the World; Timber Press: Portland, OR, USA, 2004.
- Cerqueira-Silva, C.B.; Jesus, O.N.; Santos, E.S.; Corrêa, R.X.; Souza, A.P. Genetic breeding and diversity of the genus Passiflora: Progress and perspectives in molecular and genetic studies. Int. J. Mol. Sci. 2014, 15, 14122–14152.
- Zhang, Y.J.; Zhou, T.; Wang, F.; Zhou, Y.; Li, Y.; Zhang, J.J.; Zheng, J.; Xu, D.P.; Li, H.B. The Effects of Syzygium samarangense, Passiflora edulis and Solanum muricatum on Alcohol-Induced Liver Injury. Int. J. Mol. Sci. 2016, 17, 1616.
- Urrego, N.; Sepúlveda, P.; Aragón, M.; Ramos, F.A.; Costa, G.M.; Ospina, L.F.; Castellanos, L. Flavonoids and saponins from Passiflora edulis f. edulis leaves (purple passion fruit) and its potential anti-inflammatory activity. J. Pharm. Pharmacol. 2021, 73, 1530–1538.
- Do Carmo, M.C.L.; Martins, I.M.; Magalhães, A.E.R.; Júnior, M.R.M.; Macedo, J.A. Passion fruit (Passiflora edulis) leaf aqueous extract ameliorates intestinal epithelial barrier dysfunction and reverts inflammatory parameters in Caco-2 cells monolayer. Food Res. Int. 2020, 133, 109162.
- Soares, R.D.F.; Campos, M.G.N.; Ribeiro, G.P.; Salles, B.C.C.; Cardoso, N.S.; Ribeiro, J.R.; Souza, R.M.; Leme, K.C.; Soares, C.B.; de Oliveira, C.M.; et al. Development of a chitosan hydrogel containing flavonoids extracted from Passiflora edulis leaves and the evaluation of its antioxidant and wound healing properties for the treatment of skin lesions in diabetic mice. J. Biomed. Mater. Res. A 2020, 108, 654–662.
- Salles, B.C.C.; da Silva, M.A.; Taniguthi, L.; Ferreira, J.N.; da Rocha, C.Q.; Vilegas, W.; Dias, P.H.; Pennacchi, P.C.; Duarte, S.; Rodrigues, M.R.; et al. Passiflora edulis Leaf Extract: Evidence of Antidiabetic and Antiplatelet Effects in Rats. Biol. Pharm. Bull. 2020, 43, 169–174.
- Alves, J.S.F.; Silva, A.; da Silva, R.M.; Tiago, P.R.F.; de Carvalho, T.G.; de Araújo Júnior, R.F.; de Azevedo, E.P.; Lopes, N.P.; Ferreira, L.S.; Gavioli, E.C.; et al. In Vivo Antidepressant Effect of Passiflora edulis f. flavicarpa into Cationic Nanoparticles: Improving Bioactivity and Safety. Pharmaceutics 2020, 12, 383.
- Devaki, K.; Beulah, U.; Akila, G.; Gopalakrishnan, V.K. Effect of Aqueous Extract of Passiflora edulis on Biochemical and Hematological Parameters of Wistar Albino Rats. Toxicol. Int. 2012, 19, 63–67.
- Cabral, B.; Gonçalves, T.A.F.; Abreu, L.S.; Andrade, A.W.L.; de Azevedo, F.; de Castro, F.D.; Tavares, J.F.; Guerra, G.C.B.; de Rezende, A.A.; de Medeiros, I.A.; et al. Cardiovascular Effects Induced by Fruit Peels from Passiflora edulis in Hypertensive Rats and Fingerprint Analysis by HPLC-ESI-MSn spectrometry. Planta Med. 2021.
- Guan, Y.; Sun, H.; Chen, H.; Li, P.; Shan, Y.; Li, X. Physicochemical characterization and the hypoglycemia effects of polysaccharide isolated from Passiflora edulis Sims peel. Food Funct. 2021, 12, 4221–4230.
- Goss, M.J.; Nunes, M.L.O.; Machado, I.D.; Merlin, L.; Macedo, N.B.; Silva, A.M.O.; Bresolin, T.M.B.; Santin, J.R. Peel flour of Passiflora edulis Var. Flavicarpa supplementation prevents the insulin resistance and hepatic steatosis induced by low-fructose-diet in young rats. Biomed. Pharmacother. 2018, 102, 848–854.
- Vuolo, M.M.; Lima, G.C.; Batista, Â.G.; Carazin, C.B.B.; Cintra, D.E.; Prado, M.A.; Júnior, M.R.M. Passion fruit peel intake decreases inflammatory response and reverts lipid peroxidation and adiposity in diet-induced obese rats. Nutr. Res. 2020, 76, 106–117.
- De Faveri, A.; De Faveri, R.; Broering, M.F.; Bousfield, I.T.; Goss, M.J.; Muller, S.P.; Pereira, R.O.; de Oliveira, E.S.A.M.; Machado, I.D.; Quintão, N.L.M.; et al. Effects of passion fruit peel flour (Passiflora edulis f. flavicarpa O. Deg.) in cafeteria diet-induced metabolic disorders. J. Ethnopharmacol. 2020, 250, 112482.
- Panelli, M.F.; Pierine, D.T.; de Souza, S.L.B.; Ferron, A.J.T.; Garcia, J.L.; Santos, K.C.D.; Belin, M.A.F.; Lima, G.P.P.; Borguini, M.G.; Minatel, I.O.; et al. Bark of Passiflora edulis Treatment Stimulates Antioxidant Capacity, and Reduces Dyslipidemia and Body Fat in db/db Mice. Antioxidants 2018, 7, 120.
- De Santana, F.C.; de Oliveira Torres, L.R.; Shinagawa, F.B.; de Oliveira, E.S.A.M.; Yoshime, L.T.; de Melo, I.L.P.; Marcellini, P.S.; Mancini-Filho, J. Optimization of the antioxidant polyphenolic compounds extraction of yellow passion fruit seeds (Passiflora edulis Sims) by response surface methodology. J. Food Sci. Technol. 2017, 54, 3552–3561.
- Lourith, N.; Kanlayavattanakul, M. Antioxidant activities and phenolics of Passiflora edulis seed recovered from juice production residue. J. Oleo Sci. 2013, 62, 235–240.
- Yepes, A.; Ochoa-Bautista, D.; Murillo-Arango, W.; Quintero-Saumeth, J.; Bravo, K.; Osorio, E. Purple passion fruit seeds (Passiflora edulis f. edulis Sims) as a promising source of skin anti-aging agents: Enzymatic, antioxidant and multi-level computational studies. Arab. J. Chem. 2021, 14, 102905.
- Loizzo, M.R.; Lucci, P.; Núñez, O.; Tundis, R.; Balzano, M.; Frega, N.G.; Conte, L.; Moret, S.; Filatova, D.; Moyano, E.; et al. Native Colombian Fruits and Their by-Products: Phenolic Profile, Antioxidant Activity and Hypoglycaemic Potential. Foods 2019, 8, 89.
- Sano, S.; Sugiyama, K.; Ito, T.; Katano, Y.; Ishihata, A. Identification of the strong vasorelaxing substance scirpusin B, a dimer of piceatannol, from passion fruit (Passiflora edulis) seeds. J. Agric. Food Chem. 2011, 59, 6209–6213.
- Wen, H.; Fu, Z.; Wei, Y.; Zhang, X.; Ma, L.; Gu, L.; Li, J. Antioxidant Activity and Neuroprotective Activity of Stilbenoids in Rat Primary Cortex Neurons via the PI3K/Akt Signalling Pathway. Molecules 2018, 23, 2328.
- Kandandapani, S.; Balaraman, A.K.; Ahamed, H.N. Extracts of passion fruit peel and seed of Passiflora edulis (Passifloraceae) attenuate oxidative stress in diabetic rats. Chin. J. Nat. Med. 2015, 13, 680–686.
- Taborda, J.A.V.; Arango, W.M.; Méndez Arteaga, J.J.; Guerra Almonacid, C.M. Encapsulation of bioactive compounds from byproducts of two species of passionflowers: Evaluation of the physicochemical properties and controlled release in a gastrointestinal model. Heliyon 2021, 7, e07627.
- Yepes, D.F.M.; Arango, W.M.; Rodríguez, Á.A.J.; Arteaga, J.J.M.; Porras, Á.E.A. Encapsulation of phenols of gulupa seed extract using acylated rice starch: Effect on the release and antioxidant activity. J. Funct. Foods 2021, 87, 104788.
- Rotta, E.M.; Giroux, H.J.; Lamothe, S.; Bélanger, D.; Sabik, H.; Visentainer, J.V.; Britten, M. Use of passion fruit seed extract (Passiflora edulis Sims) to prevent lipid oxidation in dairy beverages during storage and simulated digestion. LWT 2020, 123, 109088.
- De Santana, F.C.; Shinagawa, F.B.; Araujo Eda, S.; Costa, A.M.; Mancini-Filho, J. Chemical Composition and Antioxidant Capacity of Brazilian Passiflora Seed Oils. J. Food Sci. 2015, 80, C2647–C2654.
- Ferreira, B.S.; de Almeida, C.G.; Faza, L.P.; de Almeida, A.; Diniz, C.G.; da Silva, V.L.; Grazul, R.M.; Le Hyaric, M. Comparative properties of Amazonian oils obtained by different extraction methods. Molecules 2011, 16, 5875–5885.
- Matsui, Y.; Sugiyama, K.; Kamei, M.; Takahashi, T.; Suzuki, T.; Katagata, Y.; Ito, T. Extract of passion fruit (Passiflora edulis) seed containing high amounts of piceatannol inhibits melanogenesis and promotes collagen synthesis. J. Agric. Food Chem. 2010, 58, 11112–11118.
- Hartanto, S.; Lister, I.N.E.; Fachrial, E. A Comparative Study of Peel and Seed Extract of Passion Fruit (Passiflora edulis) as Anti Collagenase. Am. Sci. Res. J. Eng. Technol. Sci. 2019, 54, 42–48.
- Vera, K.; Raif, A.; Ikhtiari, R. Antioxidant and Anti-elastase Activity of Seed and Peel Extract of P. edulis. Am. Sci. Res. J. Eng. Technol. Sci. 2019, 53, 43–48.
- Chung, J.H.; Seo, J.Y.; Choi, H.R.; Lee, M.K.; Youn, C.S.; Rhie, G.; Cho, K.H.; Kim, K.H.; Park, K.C.; Eun, H.C. Modulation of skin collagen metabolism in aged and photoaged human skin in vivo. J. Investig. Dermatol. 2001, 117, 1218–1224.
- Varani, J.; Dame, M.K.; Rittie, L.; Fligiel, S.E.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Decreased collagen production in chronologically aged skin: Roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am. J. Pathol. 2006, 168, 1861–1868.
- Kang, S.; Fisher, G.J.; Voorhees, J.J. Photoaging and topical tretinoin: Therapy, pathogenesis, and prevention. Arch. Dermatol. 1997, 133, 1280–1284.
- Kim, S.; Kim, Y.; Lee, Y.; Chung, J.H. Ceramide accelerates ultraviolet-induced MMP-1 expression through JAK1/STAT-1 pathway in cultured human dermal fibroblasts. J. Lipid Res. 2008, 49, 2571–2581.
- Dong, K.K.; Damaghi, N.; Picart, S.D.; Markova, N.G.; Obayashi, K.; Okano, Y.; Masaki, H.; Grether-Beck, S.; Krutmann, J.; Smiles, K.A.; et al. UV-induced DNA damage initiates release of MMP-1 in human skin. Exp. Dermatol. 2008, 17, 1037–1044.
- Kang, S.; Chung, J.H.; Lee, J.H.; Fisher, G.J.; Wan, Y.S.; Duell, E.A.; Voorhees, J.J. Topical N-acetyl cysteine and genistein prevent ultraviolet-light-induced signaling that leads to photoaging in human skin in vivo. J. Investig. Dermatol. 2003, 120, 835–841.
- Maruki-Uchida, H.; Kurita, I.; Sugiyama, K.; Sai, M.; Maeda, K.; Ito, T. The protective effects of piceatannol from passion fruit (Passiflora edulis) seeds in UVB-irradiated keratinocytes. Biol. Pharm. Bull. 2013, 36, 845–849.
- Yamamoto, T.; Setoguchi, Y.; Mori, S.; Morita, M.; Yano, S.; Maeda, K. Effects of oral intake of piceatannol on skin moisture—A randomized, double-blind, placebo-controlled parallel-group, comparison study. Jpn. Pharmacol. Ther. 2018, 46, 1191–1199.
- Yokozawa, T.; Kim, Y.J. Piceatannol inhibits melanogenesis by its antioxidative actions. Biol. Pharm. Bull. 2007, 30, 2007–2011.
- Krambeck, K.; Oliveira, A.; Santos, D.; Pintado, M.M.; Baptista Silva, J.; Sousa Lobo, J.M.; Amaral, M.H. Identification and Quantification of Stilbenes (Piceatannol and Resveratrol) in Passiflora edulis By-Products. Pharmaceuticals 2020, 13, 73.
- Krambeck, K.; Silva, V.; Silva, R.; Fernandes, C.; Cagide, F.; Borges, F.; Santos, D.; Otero-Espinar, F.; Lobo, J.M.S.; Amaral, M.H. Design and characterization of Nanostructured lipid carriers (NLC) and Nanostructured lipid carrier-based hydrogels containing Passiflora edulis seeds oil. Int. J. Pharm. 2021, 600, 120444.
- Krambeck, K.; Santos, D.; Otero-Espinar, F.; Sousa Lobo, J.M.; Amaral, M.H. Lipid nanocarriers containing Passiflora edulis seeds oil intended for skin application. Colloids Surf. B Biointerfaces 2020, 193, 111057.
- Dewi, N.K.; Putra, I.B.; Jusuf, N.K. Passion fruit purple variant (Passiflora edulis Sims var. edulis) seeds extract 10% cream in acne vulgaris treatment: An open-label pilot study. Int. J. Dermatol. 2020, 59, 1506–1512.
- Jusuf, N.K.; Putra, I.B.; Dewi, N.K. Antibacterial Activity of Passion Fruit Purple Variant (Passiflora edulis Sims var. edulis) Seeds Extract Against Propionibacterium acnes. Clin. Cosmet. Investig. Dermatol. 2020, 13, 99–104.
- Aryunisari, C.G.; Putra, I.B.; Jusuf, N.K. Effect of Purple Passion Fruit Extract Cream (Passiflora edulis Sims var. Edulis) 6% against Striae Distensae. Open Access Maced. J. Med. Sci. 2021, 9, 720–725.
- Ishihata, A.; Maruki-Uchida, H.; Gotoh, N.; Kanno, S.; Aso, Y.; Togashi, S.; Sai, M.; Ito, T.; Katano, Y. Vascular- and hepato-protective effects of passion fruit seed extract containing piceatannol in chronic high-fat diet-fed rats. Food Funct. 2016, 7, 4075–4081.
- Fujiwara, Y.; Shiokoshi, M.; Kawawa, R.; Ishikawa, T.; Ichi, I.; Mori, S.; Morita, M. Abstracts of the Asian Congress of Nutrition 2019. Ann. Nutr. Metab. 2019, 75, 1–424.
- Tung, Y.C.; Lin, Y.H.; Chen, H.J.; Chou, S.C.; Cheng, A.C.; Kalyanam, N.; Ho, C.T.; Pan, M.H. Piceatannol Exerts Anti-Obesity Effects in C57BL/6 Mice through Modulating Adipogenic Proteins and Gut Microbiota. Molecules 2016, 21, 1419.
- Adrianus, D.T.; Kawakami, S.; Mori, S.; Morita, M.; Yano, S. Effects of Oral Intake of Piceatannol on Fat Burning―A Randomized, Double-blind, Placebo-controlled Crossover Comparison Study. Jpn. Pharmacol. Ther. 2020, 48, 1235–1240.
- Matsui, N.; Uchida-Maruki, H.; Yamamoto, T.; Ito, R.; Ebisihara, S.; Morita, M. Effects of Oral Intake of Piceatannol on Fat Burning During Moderate-Intensity Exercise—A Randomized, Double-blind, Placebo-controlled Crossover Comparison Study. Jpn. Pharmacol. Ther. 2021, 49, 731–738.
- Kawakami, S.; Kinoshita, Y.; Maruki-Uchida, H.; Yanae, K.; Sai, M.; Ito, T. Piceatannol and its metabolite, isorhapontigenin, induce SIRT1 expression in THP-1 human monocytic cell line. Nutrients 2014, 6, 4794–4804.
- Lee, H.J.; Kang, M.G.; Cha, H.Y.; Kim, Y.M.; Lim, Y.; Yang, S.J. Effects of Piceatannol and Resveratrol on Sirtuins and Hepatic Inflammation in High-Fat Diet-Fed Mice. J. Med. Food 2019, 22, 833–840.
- Purushotham, A.; Schug, T.T.; Xu, Q.; Surapureddi, S.; Guo, X.; Li, X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009, 9, 327–338.
- Yang, J.S.; Tongson, J.; Kim, K.H.; Park, Y. Piceatannol attenuates fat accumulation and oxidative stress in steatosis-induced HepG2 cells. Curr. Res. Food. Sci. 2020, 3, 92–99.
- Carpéné, C.; Pejenaute, H.; Del Moral, R.; Boulet, N.; Hijona, E.; Andrade, F.; Villanueva-Millán, M.J.; Aguirre, L.; Arbones-Mainar, J.M. The Dietary Antioxidant Piceatannol Inhibits Adipogenesis of Human Adipose Mesenchymal Stem Cells and Limits Glucose Transport and Lipogenic Activities in Adipocytes. Int. J. Mol. Sci. 2018, 19, 2081.
- Takam, P.N.; Djikeng, F.T.; Kuate, D.; Kengne, A.P.N.; Tsafack, H.D.; Makamwé, I.; Oben, J.E. Passiflora edulis seed oil from west Cameroon: Chemical characterization and assessment of its hypolipidemic effect in high-fat diet-induced rats. Food Sci. Nutr. 2019, 7, 3751–3758.
- Uchida-Maruki, H.; Inagaki, H.; Ito, R.; Kurita, I.; Sai, M.; Ito, T. Piceatannol lowers the blood glucose level in diabetic mice. Biol. Pharm. Bull. 2015, 38, 629–633.
- Oritani, Y.; Okitsu, T.; Nishimura, E.; Sai, M.; Ito, T.; Takeuchi, S. Enhanced glucose tolerance by intravascularly administered piceatannol in freely moving healthy rats. Biochem. Biophys. Res. Commun. 2016, 470, 753–758.
- Pan, Z.H.; Ning, D.S.; Fu, Y.X.; Li, D.P.; Zou, Z.Q.; Xie, Y.C.; Yu, L.L.; Li, L.C. Preparative Isolation of Piceatannol Derivatives from Passion Fruit (Passiflora edulis) Seeds by High-Speed Countercurrent Chromatography Combined with High-Performance Liquid Chromatography and Screening for α-Glucosidase Inhibitory Activities. J. Agric. Food Chem. 2020, 68, 1555–1562.
- Kitada, M.; Ogura, Y.; Maruki-Uchida, H.; Sai, M.; Suzuki, T.; Kanasaki, K.; Hara, Y.; Seto, H.; Kuroshima, Y.; Monno, I.; et al. The Effect of Piceatannol from Passion Fruit (Passiflora edulis) Seeds on Metabolic Health in Humans. Nutrients 2017, 9, 1142.
- Jiménez, Á.A.R.; Méndez, J.J.A.; Murillo, W.A.; Guerrero, M.F.P. Vasodilator effect of ethanolic extracts of Passiflora vitifolia and Passiflora edulis f. edulis seeds. J. Appl. Pharm. Sci. 2021, 11, 61–69.
- Yamamoto, T.; Sato, A.; Takai, Y.; Yoshimori, A.; Umehara, M.; Ogino, Y.; Inada, M.; Shimada, N.; Nishida, A.; Ichida, R.; et al. Effect of piceatannol-rich passion fruit seed extract on human glyoxalase I-mediated cancer cell growth. Biochem. Biophys. Rep. 2019, 20, 100684.
- Mota, N.; Kviecinski, M.R.; Zeferino, R.C.; de Oliveira, D.A.; Bretanha, L.C.; Ferreira, S.R.S.; Micke, G.A.; Filho, D.W.; Pedrosa, R.C.; Ourique, F. In vivo antitumor activity of by-products of Passiflora edulis f. flavicarpa Deg. Rich in medium and long chain fatty acids evaluated through oxidative stress markers, cell cycle arrest and apoptosis induction. Food Chem. Toxicol. 2018, 118, 557–565.
- Kido, L.A.; Hahm, E.R.; Kim, S.H.; Baseggio, A.M.; Cagnon, V.H.A.; Singh, S.V.; Maróstica, M.R., Jr. Prevention of Prostate Cancer in Transgenic Adenocarcinoma of the Mouse Prostate Mice by Yellow Passion Fruit Extract and Antiproliferative Effects of Its Bioactive Compound Piceatannol. J. Cancer. Prev. 2020, 25, 87–99.
- Banik, K.; Ranaware, A.M.; Harsha, C.; Nitesh, T.; Girisa, S.; Deshpande, V.; Fan, L.; Nalawade, S.P.; Sethi, G.; Kunnumakkara, A.B. Piceatannol: A natural stilbene for the prevention and treatment of cancer. Pharmacol Res. 2020, 153, 104635.




