Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jin Wang | + 1355 word(s) | 1355 | 2022-01-06 07:30:58 | | | |
| 2 | Yvaine Wei | Meta information modification | 1355 | 2022-01-12 04:35:11 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wang, J. Effects of Microwaves, Ultrasonication, and Thermosonication on Milk. Encyclopedia. Available online: https://encyclopedia.pub/entry/18103 (accessed on 07 February 2026).
Wang J. Effects of Microwaves, Ultrasonication, and Thermosonication on Milk. Encyclopedia. Available at: https://encyclopedia.pub/entry/18103. Accessed February 07, 2026.
Wang, Jin. "Effects of Microwaves, Ultrasonication, and Thermosonication on Milk" Encyclopedia, https://encyclopedia.pub/entry/18103 (accessed February 07, 2026).
Wang, J. (2022, January 12). Effects of Microwaves, Ultrasonication, and Thermosonication on Milk. In Encyclopedia. https://encyclopedia.pub/entry/18103
Wang, Jin. "Effects of Microwaves, Ultrasonication, and Thermosonication on Milk." Encyclopedia. Web. 12 January, 2022.
Copy Citation
Cow’s milk is considered an excellent protein source. However, the digestibility of milk proteins needs to be improved. The milk protein content was reduced as the microwave processing time and the temperature increased. The final milk protein available in the sample was lower when microwave processing was conducted at 75 °C and 90 °C compared to 60 °C, whereas the ultrasound treatment significantly improved the protein content, and no particular trend was observed for the thermosonicated samples. Thus, ultrasound processing shows a potential application in improving the protein quality of cow’s milk.
cow’s milk
protein secondary structure
protein digestibility
1. Introduction
Cow’s milk is considered an excellent food source for human body growth due its high content of minerals (calcium and phosphorous) and protein. Cow’s milk can provide all essential amino acids including a high level of lysine, which can help in synthesizing important proteins important for human health [1][2]. Milk proteins perform several functions such as immune system stimulation, shielding the human body against different types of bacteria, viruses, and fungi, and gut development [3][4]. However, the protein quality of cow’s milk still nees to be improved through various food-processing techniques due to these proteins’ low digestibility and their allergenicity, which could lead to gastrointestinal discomfort, respiratory failure, as well as anaphylactic shock [5].
Thermal processing of milk aids in the extension of its shelf life and in the reduction of microbial activity [6]. However, thermal process is known to induce some structural changes in milk, such as protein denaturation. It can further cause the permanent unfolding of protein and even might expose hydrophobic groups and reduce disulphide bridges [7][8]. A study reported that a microwave treatment caused a decrease in the content of lactose, fat, and protein in cow’s milk, whereas milk’s average density was increased [9]. There was a decrease in α-helixes and β-sheets when milk was treated with microwaves above 50 °C [10]. In recent years, non-thermal processing has received high attention because it can retain the original characteristics, freshness, and nutritional value compared to thermal treatment [6]. Studies reported that ultrasounds had a minor effect on the secondary structure and hydrophobicity of a whey protein concentrate [11]. In sodium caseinate (biochemical name of casein protein), study also concluded that during ultrasound treatment, there were no major structural deviations, but a slight deviation was observed for lactoferrin [12]. Moreover, during ultrasonication, there was minimal loss in flavor, and this processing technique exhibited higher consistency in terms of homogenization and viscosity compared to other non-thermal techniques [13]. In addition, ultrasound processing has a lower operating cost and an effective power output [14][15].
2. FTIR Analysis
FTIR spectroscopy was used to investigate the structural changes in protein due to the processing techniques. Among all regions, the Amide I band ranging from 1700 to 1600 cm−1 was chosen for the study, as it is considered a sensitive region for the study of conformational changes occurring in a protein secondary structure. The Amide I band consist of C=O stretching vibrations (almost 80%) in addition to C–H stretching modes and in-plane N–H bending. The C=O stretching vibrations are the result of changes in the secondary structure of the protein as well as of inter- or intramolecular effects. The hydrogen bonding pattern and geometry of a molecule are also sometimes responsible for these vibrations [16][17]. The Amide I band consists of various secondary structural components such as β sheets, random coils, α helices, and β turns [8].
Figure 1a shows the percentage peak areas obtained for microwave-processed samples at 60 °C, 75 °C, and 90 °C for 1, 3, and 5 min, respectively, within the Amide I region. Figure 1b,c shows the peak areas obtained for milk processed with ultrasonication and thermosonication for 1, 3, 5, 7, and 9 min, respectively.
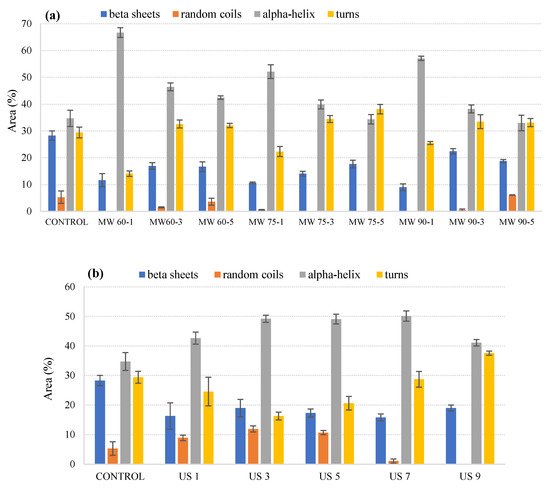
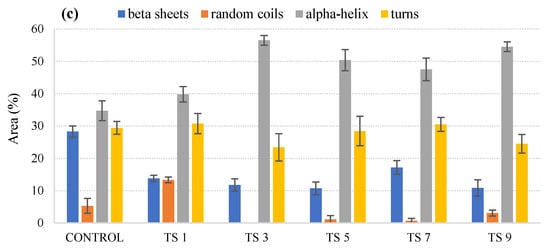
Figure 1. Variations in relative areas of the bands fitted to the normalized FTIR spectra of the Amide I region (1700–1600 cm–1) of microwave-processed (a), ultrasound-processed (b), and thermosonication-processed (c) cow milk. Note: the error bar means standard error of the mean.
The results for thermal treatment showed a decrease in the α helix structure for all temperature–time combinations and, due to the relocation of the α helix structure, there was an increase in β-sheets and turns. These trends were also reported in previous studies [18][19] and suggested that the reduction in α helices exposed the free thiol group, Cys-121; this group causes aggregation of whey protein, which in turn increases the viscosity of milk. The increase in turns can contribute to the increased involvement of casein in forming aggregates, whereas the increase in β-sheets can be due to interactions between proteins and lipids, which tend to change the physical state of milk fat when stored at high temperatures [20][21]. Therefore, further research needs to be focused on the effect of ultrasound and thermosonication on protein structure and allergenicity, as deviations in protein structural affect functionality and hence allergenicity. Moreover, more stable α helix structures were found in ultrasonicated and thermo-sonicated samples, suggesting that non-thermal processing techniques do not affect the stability of proteins’ secondary structure.
3. Effect of Processing on Protein Digestibility
In vitro digestion was carried out with pepsin and pancreatic enzymes in microwave-treated, ultrasound-treated, and thermosonicated samples. Figure 2a–c represents the soluble protein content of initial, intermediary, i.e., after pepsin digestion, and final, i.e., after pancreatic digestion, samples treated with microwaves, ultrasound waves, and a combination of thermal treatment and ultrasound waves. In Figure 2a, the initial soluble protein content in microwave-treated samples at 60 °C for 1, 3, and 5 min was significantly higher compared to the initial protein content at 75 °C and 90 °C. This could be because at higher temperatures, protein denatures, leading to the release of hydrophobic cores that expose hydrophobic residues to the surroundings. Furthermore, no trend was observed in protein content determined after pepsin digestion, but protein content was reduced after pancreatic digestion at 60 °C and 90 °C (except for some variations) and increased at 75 °C.
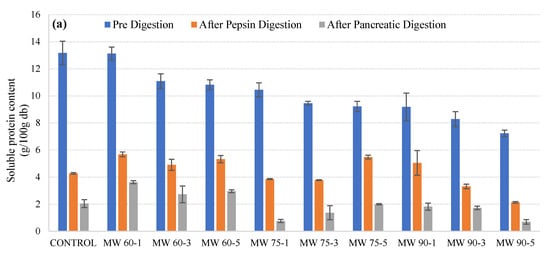
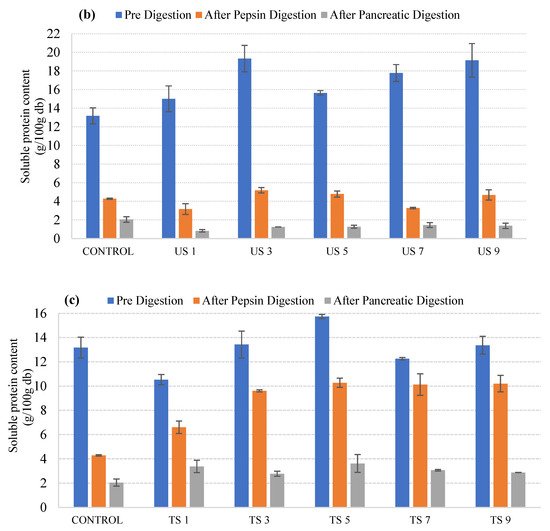
Figure 2. Protein content changes of microwave-processed (a), ultrasound-processed (b), and thermosonication-processed (c) cow milk under different conditions (pre-digestion, after pepsin digestion, and after pancreatic digestion).
In Figure 2b, it can be observed that the initial soluble protein content was higher when the samples were treated with ultrasound compared to untreated samples. The increase in the soluble protein content could result from cavitation effects which lead to the transformation between soluble and insoluble proteins during ultrasound processing. The protein content after pepsin digestion showed an increase when the treatment time was increased from 1 min to 3 min, after which there was a slight decrease when the treatment time was raised to 5 min. The protein content after pepsin digestion again showed a raise as the treatment time increased to 7 min and 9 min. The final protein content after pancreatic protein digestion showed a different trend. The protein content values gradually increased from 1 min to 9 min, with the higher peak observed at 7 min. Figure 2c shows the pre-digestion, after-pepsin-digestion, and after-pancreatic-digestion protein content of the thermosonicated samples. It can be observed that the initial protein content increased after treatment for 1 min to 5 min, after which it showed a decrease at 7 min and a gradual increase at 9 min. The protein content after pepsin digestion showed an increasing trend, with slight variations when the treatment time was increased from 1 to 9 min. Furthermore, no particular trend was observed in protein content after pancreatic digestion for all treated samples.
4. Conclusions
Secondary structural changes in milk proteins due to microwave processing, ultrasonication, and thermosonication were evaluated using Fourier Transform Infrared spectroscopy (FTIR). As the applied temperature and time were raised in microwave processing, rearrangement in α-helices occurred, and as a result there was an increase in turns and β-sheets in the proteins. The highest protein digestibility was reported when the milk was treated at 75 °C for 1 min. In the case of ultrasonication, no major changes in secondary structures were observed, except when milk was treated for 9 min, which showed a small rearrangement of α-helix structures. However, no significant changes were observed in the in vitro protein digestibility after ultrasound processing. Very few studies have been conducted on the effect of combined thermal treatment and ultrasound waves, but it is clear that the thermosonicated samples showed poor digestibility, ranging from 68.76 to 78.81%, compared to the microwave samples (72.56–93.4%) and the ultrasound samples (90.20–94.41%), which exhibited higher digestibility.
References
- Alkanhal, H.A.; Al-Othman, A.A.; Hewedi, F.M. Changes in protein nutritional quality in fresh and recombined ultra high temperature treated milk during storage. Int. J. Food Sci. Nutr. 2001, 52, 509–514.
- Bannon, G.; Fu, T.-J.; Kimber, I.; Hinton, D.M. Protein digestibility and relevance to allergenicity. Environ. Health Perspect. 2003, 111, 1122–1124.
- Bu, G.; Luo, Y.; Chen, F.; Liu, K.; Zhu, T. Milk processing as a tool to reduce cow’s milk allergenicity: A mini-review. Dairy Sci. Technol. 2013, 93, 211–223.
- Chandrapala, J.; Zisu, B.; Palmer, M.; Kentish, S.; Ashokkumar, M. Effects of ultrasound on the thermal and structural characteristics of proteins in reconstituted whey protein concentrate. Ultrason. Sonochemistry 2011, 18, 951–957.
- Chouliara, E.; Georgogianni, K.; Kanellopoulou, N.; Kontominas, M. Effect of ultrasonication on microbiological, chemical and sensory properties of raw, thermized and pasteurized milk. Int. Dairy J. 2010, 20, 307–313.
- D’amico, D.J.; Silk, T.M.; Wu, J.; Guo, M. Inactivation of microorganisms in milk and apple cider treated with ultrasound. J. Food Prot. 2006, 69, 556–563.
- De Figueiredo Furtado, G.; Mantovani, R.A.; Consoli, L.; Hubinger, M.D.; da Cunha, R.L. Structural and emulsifying properties of sodium caseinate and lactoferrin influenced by ultrasound process. Food Hydrocoll. 2017, 63, 178–188.
- Friedman, M. Chemistry, nutrition, and microbiology of D-amino acids. J. Agric. Food Chem. 1999, 47, 3457–3479.
- Gomaa, A.I.; Nsonzi, F.; Sedman, J.; Ismail, A.A. Enhanced Unfolding of Bovine β-Lactoglobulin Structure Using Microwave Treatment: A Multi-Spectroscopic Study. Food Biophys. 2016, 11, 370–379.
- Grewal, M.K.; Chandrapala, J.; Donkor, O.; Apostolopoulos, V.; Stojanovska, L.; Vasiljevic, T. Fourier transform infrared spectroscopy analysis of physicochemical changes in UHT milk during accelerated storage. Int. Dairy J. 2017, 66, 99–107.
- Hejazi, S.N.; Orsat, V. Malting process optimization for protein digestibility enhancement in finger millet grain. J. Food Sci. Technol. 2016, 53, 1929–1938.
- Iuliana, C.; Rodica, C.; Sorina, R.; Oana, M. Impact of microwaves on the physico-chemical characteristics of cow milk. Rom. Rep. Phys. 2015, 67, 423–430.
- Kakade, M.; Evans, R.J. Chemical and enzymatic determinations of available lysine in raw and heated navy beans (Phaseolus vulgaris). Can. J. Biochem. 1966, 44, 648–650.
- Lacroix, M.; Léonil, J.; Bos, C.; Henry, G.; Airinei, G.; Fauquant, J.; Tomé, D.; Gaudichon, C. Heat markers and quality indexes of industrially heat-treated milk protein measured in rats. J. Agric. Food Chem. 2006, 54, 1508–1517.
- Lönnerdal, B. Nutritional and physiologic significance of human milk proteins. Am. J. Clin. Nutr. 2003, 77, 1537S–1543S.
- Patel, H.A.; Singh, H.; Anema, S.G.; Creamer, L.K. Effects of heat and high hydrostatic pressure treatments on disulfide bonding interchanges among the proteins in skim milk. J. Agric. Food Chem. 2006, 54, 3409–3420.
- Peram, M.R.; Loveday, S.M.; Ye, A.; Singh, H. In vitro gastric digestion of heat-induced aggregates of β-lactoglobulin. J. Dairy Sci. 2013, 96, 63–74.
- Raikos, V. Effect of heat treatment on milk protein functionality at emulsion interfaces. A review. Food Hydrocoll. 2010, 24, 259–265.
- Raikos, V.; Dassios, T. Health-promoting properties of bioactive peptides derived from milk proteins in infant food: A review. Dairy Sci. Technol. 2014, 94, 91–101.
- Qi, X.L.; Carl, H.; Mcnulty, D.; Clarke, D.T.; Brownlow, S.; Jones, G.R. Effect of temperature on the secondary structure of β-lactoglobulin at pH 6.7, as determined by CD and IR spectroscopy: A test of the molten globule hypothesis. Biochem. J. 1997, 324, 341–346.
- Rérat, A.; Calmes, R.; Vaissade, P.; Finot, P.-A. Nutritional and metabolic consequences of the early Maillard reaction of heat treated milk in the pig. Eur. J. Nutr. 2002, 41, 1–11.
More
Information
Subjects:
Food Science & Technology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
13 Jan 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




