Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Angelika Graiff | + 2331 word(s) | 2331 | 2021-12-16 05:19:26 | | | |
| 2 | Vivi Li | Meta information modification | 2331 | 2022-01-12 03:02:00 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Graiff, A. Baltic Sea Keystone Macroalgae (Fucus vesiculosus, Phaeophyceae). Encyclopedia. Available online: https://encyclopedia.pub/entry/18068 (accessed on 07 February 2026).
Graiff A. Baltic Sea Keystone Macroalgae (Fucus vesiculosus, Phaeophyceae). Encyclopedia. Available at: https://encyclopedia.pub/entry/18068. Accessed February 07, 2026.
Graiff, Angelika. "Baltic Sea Keystone Macroalgae (Fucus vesiculosus, Phaeophyceae)" Encyclopedia, https://encyclopedia.pub/entry/18068 (accessed February 07, 2026).
Graiff, A. (2022, January 11). Baltic Sea Keystone Macroalgae (Fucus vesiculosus, Phaeophyceae). In Encyclopedia. https://encyclopedia.pub/entry/18068
Graiff, Angelika. "Baltic Sea Keystone Macroalgae (Fucus vesiculosus, Phaeophyceae)." Encyclopedia. Web. 11 January, 2022.
Copy Citation
The brown seaweed Fucus vesiculosus is the dominant and the most ecologically crucial primary producer and habitat founder in the Baltic Sea. In the shallow coastal zone, F. vesiculosus is particularly exposed to strongly and rapidly changing environmental conditions due to global change.
antioxidative potential
bladder wrack
photosynthetic performance
lipid peroxidation
mesocosm
multifactorial change
seasonal pattern
superoxide dismutase
1. Introduction
Marine seaweeds that inhabit the shallow coastal area of temperate shorelines frequently experience an environment characterized by strong daily and seasonal variations in abiotic drivers, such as high and low irradiances, rapid temperature changes, and pH fluctuations. Due to anthropogenic global change, the continuing warming and its amplification of seasonal fluctuations generates stressful conditions in shallow waters, which seems to be a severe challenge for seaweeds [1][2][3]. The response of seaweeds to unfavorable environmental conditions is mediated through various physiological and biochemical mechanisms, of which the excessive formation and accumulation of reactive oxygen species (ROS) plays a central role by imposing oxidative stress on the cells (reviewed by [4][5][6]).
Perennial seaweeds in temperate latitudes can photosynthesize over broad temperature ranges, as they remain photosynthetically active throughout the year. Various metabolic processes in seaweeds induce ROS formation, particularly photosynthesis. Plants and seaweeds accumulate ROS as a concomitant process of the electron transport systems during photosynthesis and respiration, even under normal metabolic conditions [7]. For instance, the main source of ROS in plant and seaweed tissues is the photosynthetic electron transport system that generates singlet oxygen (1O2) and superoxide radicals (·O2−) [8][9]. Additionally, in a sequential reduction of molecular oxygen (O2), hydrogen peroxide (H2O2) as well as hydroxyl radical (·OH) are produced. Various environmental factors, e.g., high or low temperature, rapid temperature changes, nutrient (also carbon) deficiency, high irradiance, and ultraviolet radiation (UVR) stimulate, as a general stress response, the gradual and continued production of ROS. Under stressful environmental conditions, photosynthesis is impaired and surplus energy leads to ROS production [10][11][12][13]. Oxidative stress is recognized as a physiological condition established when ROS formation surpasses the antioxidant defensive systems of organisms, leading to oxidative impairment in lipids, proteins, and DNA [7][14][15]. For instance, a direct indicator of oxidative stress and damage is the level of malondialdehyde (MDA) as a biochemical marker for lipid peroxidation, which is the result of the decomposition of polyunsaturated fatty acids in cell membranes [16]. Thus, MDA often accumulates under oxidative stress, and it has widely been used to distinguish between stressed and unstressed seaweeds (e.g., [13][17][18]).
H2O2 is omnipresent in the oceans worldwide, with concentrations fluctuating temporally and spatially [19][20][21]. Seawater H2O2 concentrations between 10 and 300 nM are reported from different water masses and habitats [22][23][24]. In shallow and calm coastal areas, seaweeds may also experience direct oxidative stress where H2O2 is formed by the photoactivation of dissolved organic material (DOM) due to ultraviolet radiation (UVR) and the release of excited electrons, initiating the reduction of molecular oxygen. The formation of H2O2 is mainly observed in surface waters or flat water zones characterized by high concentrations of DOM and oxygen [19][25][26][27][28]. In surface waters and/or intertidal pools, H2O2 can reach even micromolar concentrations (<2 µM; [29][30]). H2O2 undergoes relatively few reactions with biologically important molecules, but it passes quickly through membranes by diffusion; thus, it is most likely the intracellular preliminary stage for more reactive oxidants (reviewed by Winterbourn [31]). However, H2O2 directly inhibits photosynthesis by affecting several photosynthetically important enzymes such as RuBisCO [32][33][34].
Seaweeds, however, are equipped with various defense mechanisms that effectively remove ROS. Common antioxidative systems in seaweeds consist of several enzymes, e.g., superoxide dismutase (SOD), ascorbate peroxidase (APX), and catalase (CAT), as well as the antioxidants ascorbic acid, glutathione, β-carotene, and α-tocopherol [11][35][36]. The antioxidative enzymes transform distinct ROS to less toxic compounds. For example, SOD scavenges and produces ROS at the same time because it catalyzes the conversion of ·O2− to H2O2 and oxygen [7]. Then, H2O2 is further deprotonated by CAT or APX. SOD is known as “the first line of defense” against ROS and plays a central role in the antioxidative system, as it is a powerful scavenger of ·O2−, which otherwise initiates a ROS cascade [6][37]. Furthermore, seaweeds are also able to detoxify H2O2 by excreting H2O2 to reduce intracellular concentrations [38][39]. In recent years, several studies have investigated the underlying molecular processes of ROS acclimation. These include the regulation of gene expression, for example the induction of various ROS scavenging enzymes (e.g., genes encoding for SOD, APX, CAT), heat shock proteins (HSPs), early light-inducible proteins (ELIPs), and the general adjustment of the primary metabolism towards abiotic factors (i.e., high light, high temperature, rapid changes in salinity) in seaweeds [40][41][42][43][44][45]. An overall trend was revealed by Collén et al. [43], who concluded that abiotic stress initiated an increased expression of “stress key genes” (e.g., HSP and ELIP genes), which appear together with decreased expression of energy protein synthesis-related genes.
Along the rocky and stony shores of the Baltic Sea, the brown seaweed Fucus vesiculosus L. forms biomass-rich belts and thereby founds the basis of a productive and structurally complex community [46][47]. F. vesiculosus is a keystone species in shallow Baltic coastal habitats, which are characterized by fluctuating environmental conditions, particularly by annual and seasonal variations in pH (7.4–8.5) and temperature (<0 to 20/25 °C), which are tolerated by perennial F. vesiculosus [48][49]. The formation and structure of the F. vesiculosus ecosystem has been attributed to different abiotic factors and is maintained by fine-tuned biotic interactions [49][50][51][52]. For example, F. vesiculosus populations seem to severely suffer from environmental changes over the past decades, as reflected by their decline in abundance and depth penetration along Baltic shores [53][54].
Baltic Sea surface temperature has warmed rapidly during recent decades and is predicted to increase by 3–6 °C until 2100 [55]. Additionally, to this continuous warming trend, short-term extreme warming events known as “marine heat-waves” (sensu Hobday et al. [56]) became also more frequent in this region [57]. The simultaneous rise in pCO2 and the accompanying acidification of the brackish Baltic Sea is challenging to forecast [58], but model simulations for the Baltic Proper projected a gradually decreasing mean surface pH until the end of this century [59].
Thus, co-occurring changes of environmental variables such as warming and acidification of the Baltic Sea may individually or interactively impact the antioxidative properties of F. vesiculosus. Their effect on F. vesiculosus may differ seasonally, depending on, for example, growth periods. The present study investigates single and joint effects of increased seawater temperature (Δ + 5 °C) and pCO2 (1100 ppm) as predicted for shallow shores until the end of this century in the Baltic Sea [58][60][61] on adult F. vesiculosus in all four seasons. To simulate these specific global change scenarios, benthic mesocosms (Kiel Outdoor Benthocosms (KOBs)) were used. Temperature and pCO2 elevation were added on top of the natural fluctuations and variabilities of all abiotic factors present in the KOBs [62]. We hypothesized that higher inorganic carbon availability under elevated pCO2 may decrease, but higher temperatures may enhance oxidative stress for F. vesiculosus. Therefore, the antioxidative properties (SOD activity and lipid peroxidation), as well as the sensitivity of F. vesiculosus photosynthetic performance (i.e., effective quantum yield) to artificial oxidative stress resulting from exposure to H2O2 under these global change scenarios, were examined for the first time.
2. Antioxidative Potential
Photosynthetic performance measured as effective quantum yield of Fucus vesiculosus under short-term H2O2 stress was regarded as an indicator for its antioxidative potential. In summer, at increased water temperatures (>26 °C), F. vesiculosus deceased, but during the other seasons and treatments, F. vesiculosus tolerated up to 5 mM H2O2, resulting in high effective quantum yields. The effective quantum yield of F. vesiculosus was significantly reduced, but it was still above 50% of the control in 10 mM H2O2 (Figure 1). A 50–70% reduction in effective quantum yield of F. vesiculosus at 20 mM was observed under all treatments in spring, summer, and autumn. The reduction of the quantum yield was stronger in spring and autumn compared to summer and winter. In winter, especially, the quantum yield was only reduced by 30–40% under all treatments in 20 mM H2O2 (Figure 1).
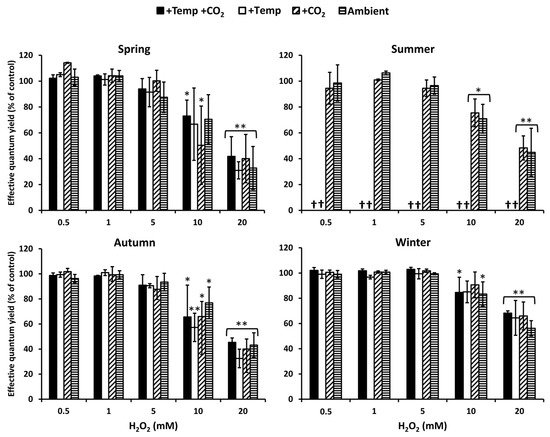
Figure 1. The effect of ascending H2O2 concentrations on effective quantum yield of Fucus vesiculosus (expressed as % of the control) grown for three months under various temperature and pCO2 conditions during different seasons. Seasons: spring: 4 April–19 June 2013; summer: 4 July–17 September 2013; autumn: 10 October–18 December 2013; winter: 16 January–01 April 2014. Temperature and pCO2 conditions: +Temp +CO2: elevated temperature Δ + 5°C with elevated pCO2; +Temp: elevated temperature Δ + 5 °C with in situ pCO2; +CO2: in situ Kiel Fjord temperature with elevated pCO2; Ambient: in situ Kiel Fjord temperature and pCO2. Values are means ± SD (standard deviation), n = 3. Effective quantum yield values of the controls were between 0.57 and 0.74 for all F. vesiculosus apices. *, **: Significant differences in comparison to the paired control value at p < 0.05 and p < 0.001, respectively (Tukey-HSD). Cross (†) marks death of F. vesiculosus in summer under simulated ocean warming.
3. Lipid Peroxidation
Lipid peroxidation was examined by measuring the concentration of the biochemical marker malondialdehyde (MDA). MDA levels of all initial F. vesiculosus samples from its native habitat varied slightly but significantly over the course of one year (p < 0.05, Tukey-HSD), with higher levels in April and January compared to July and October (Figure 2). During spring, MDA content significantly increased by 20–40% among the measurement dates and under elevated temperatures (Figure 2 and Table 1). During summer, a natural heat-wave increased the Kiel Fjord water temperature dramatically [62]. Thus, the water temperature under simulated ocean warming achieved maximum levels (peak temperatures: 27–30 °C for 30 days) that surpassed the thermal tolerance of F. vesiculosus (>26 °C, [63]). Therefore, simulated ocean warming in combination with a summer heatwave led to a drastic dieback of the F. vesiculosus individuals and resulted in significant differences between the measurement dates (Figure 2 and Table 1). MDA content of F. vesiculosus at the end of the summer experiment was neither increased nor decreased under elevated pCO2 (Figure 2). Levels of MDA increased significantly in F. vesiculosus until the end of the autumn experiment under simulated ocean warming by 40% (Figure 2 and Table 1). In winter, the MDA content of F. vesiculosus almost doubled during the experiment in the increased temperature treatments (Figure 2 and Table 1). This effect of winter warming, resulting in higher MDA content in F. vesiculosus, was marginally alleviated at increased pCO2 levels; however, this effect was not significant (Figure 2). The MDA content found during the final sampling of F. vesiculosus in the winter experiment revealed an interactive effect of temperature and pCO2 (two-way ANOVA, F = 6.450, df = 1, p < 0.05).
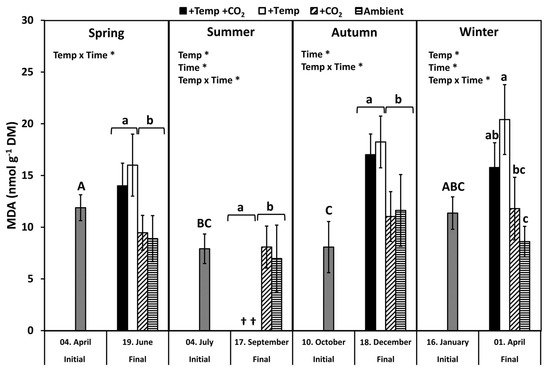
Figure 2. Malondialdehyde (MDA) concentration of initial Fucus vesiculosus growing in its native habitat (n = 12) and at the end of the experiments (n = 3), with controlled temperature and pCO2 conditions during different seasons. Seasons: spring: 4 April–19 June 2013; summer: 4 July–17 September 2013; autumn: 10 October–18 December 2013; winter: 16 January–1 April 2014. Temperature and pCO2 conditions: +Temp +CO2: elevated temperature Δ + 5 °C with elevated pCO2; +Temp: elevated temperature Δ + 5 °C with in situ pCO2; +CO2: in situ Kiel Fjord temperature with elevated pCO2; Ambient: in situ Kiel Fjord temperature and pCO2. Values are means ± SD (standard deviation). *: Significant effects of the tested factors revealed by the repeated-measure ANOVA are marked with an asterisk for each season separately. Different uppercase (comparison of initial values) and lowercase (comparison of final values between treatments) letters specify significant differences (p < 0.05; Tukey-HSD, data were ln-transformed in order to meet assumptions of homogeneity of variance). Cross (†) points to the death of F. vesiculosus in summer under simulated ocean warming.
Table 1. Repeated-measures ANOVA outcome for temperature, CO2, and time effects on malondialdehyde (MDA) concentration in Fucus vesiculosus during the different seasonal experiments (n = 3). Seasons: spring: 4 April–19 June 2013; summer: 4 July–17 September 2013; autumn: 10 October–18 December 2013; winter: 16 January–1 April 2014. p-values < 0.05 are indicated by bold type.
| Source of Variation | DF | F-Value | p-Value |
|---|---|---|---|
| (a) Spring | |||
| Temperature | 1 | 1.078 | 0.33 |
| CO2 | 1 | 0.188 | 0.68 |
| Time | 1 | 0.632 | 0.45 |
| Temp × CO2 | 1 | 4.164 | 0.08 |
| Temp × Time | 1 | 5.712 | 0.04 |
| CO2 × Time | 1 | 0.536 | 0.49 |
| Temp × CO2 × Time | 1 | 0.264 | 0.62 |
| (b) Summer | |||
| Temperature | 1 | 20.979 | 0.002 |
| CO2 | 1 | 1.548 | 0.25 |
| Time | 1 | 22.603 | 0.001 |
| Temp × CO2 | 1 | 0.002 | 0.96 |
| Temp × Time | 1 | 22.663 | 0.001 |
| CO2 × Time | 1 | 0.059 | 0.81 |
| Temp × CO2 × Time | 1 | 2.944 | 0.13 |
| (c) Autumn | |||
| Temperature | 1 | 1.910 | 0.21 |
| CO2 | 1 | 0.113 | 0.75 |
| Time | 1 | 24.796 | 0.002 |
| Temp × CO2 | 1 | 0.717 | 0.43 |
| Temp × Time | 1 | 13.281 | 0.008 |
| CO2 × Time | 1 | 0.409 | 0.54 |
| Temp × CO2 × Time | 1 | 0.708 | 0.33 |
| (d) Winter | |||
| Temperature | 1 | 7.368 | 0.03 |
| CO2 | 1 | 0.016 | 0.93 |
| Time | 1 | 17.644 | 0.003 |
| Temp × CO2 | 1 | 3.047 | 0.12 |
| Temp × Time | 1 | 18.457 | 0.003 |
| CO2 × Time | 1 | 0.381 | 0.55 |
| Temp × CO2 × Time | 1 | 1.989 | 0.19 |
4. Superoxide Dismutase
Superoxide dismutase (SOD) activity of initial F. vesiculosus from its native habitat was significantly higher in July than in April, but it was similar to October and January, respectively (p < 0.05, Tukey’s test; Figure 3). During spring, the SOD activity increased significantly among the measurement dates (Table 2). The activity of SOD was increased under elevated CO2 conditions by 20% at the end of the spring experiment (two-way ANOVA, F = 5.777, df = 1, p < 0.05). SOD activity was significantly decreased by temperature and time under summer conditions, but it was not enhanced by increased pCO2 under ambient temperatures (Figure 3 and Table 2). In autumn, the activity of SOD was not different between the measurement dates under all treatments. However, in winter, SOD activity increased significantly among measurement dates and under warming by 40–60% (Table 2). Maximal SOD activity of 400–500 U SOD mg−1 TSP was reached in winter under increased temperatures (Figure 3).
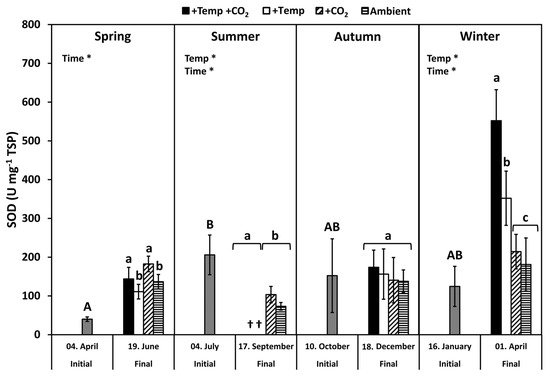
Figure 3. Variations of superoxide dismutase (SOD) activities of initial Fucus vesiculosus growing in its native habitat (n = 12) and at the end of the experiments (n = 3), with controlled temperature and pCO2 conditions over different seasons. Seasons: spring: 4 April–19 June 2013; summer: 4 July–17 September 2013; autumn: 10 October–18 December 2013; winter: 16 January–1 April 2014. Temperature and pCO2 conditions: +Temp +CO2: elevated temperature Δ + 5 °C with elevated pCO2; +Temp: elevated temperature Δ + 5 °C with in situ pCO2; +CO2: in situ Kiel Fjord temperature with elevated pCO2; Ambient: in situ Kiel Fjord temperature and pCO2. Values are means ± SD (standard deviation). *: Significant effects of the tested factors revealed by the repeated-measure ANOVA are marked with an asterisk for each season separately. Different uppercase (comparison of initial values) and lowercase (comparison of final values between treatments) letters specify significant differences (p < 0.05; Tukey-HSD, data were ln-transformed in order to meet assumptions of homogeneity of variance). Cross (†) points to the death of F. vesiculosus in summer under simulated ocean warming.
Table 2. Repeated-measures ANOVA outcome for temperature, CO2, and time effects on superoxide dismutase (SOD) activity in Fucus vesiculosus during the different seasonal experiments (n = 3). Seasons: spring: 4 April–19 June 2013; summer: 4 July–17 September 2013; autumn: 10 October–18 December 2013; winter: 16 January–1 April 2014. p-values < 0.05 are indicated by bold type.
| Source of variation | DF | F-Value | p-Value |
|---|---|---|---|
| (a) Spring | |||
| Temperature | 1 | 1.078 | 0.33 |
| CO2 | 1 | 0.188 | 0.68 |
| Time | 1 | 5.712 | 0.04 |
| Temp × CO2 | 1 | 4.464 | 0.08 |
| Temp × Time | 1 | 0.632 | 0.45 |
| CO2 × Time | 1 | 0.536 | 0.49 |
| Temp × CO2 × Time | 1 | 0.264 | 0.62 |
| (b) Summer | |||
| Temperature | 1 | 13.536 | 0.006 |
| CO2 | 1 | 0.583 | 0.47 |
| Time | 1 | 14.162 | 0.006 |
| Temp × CO2 | 1 | 0.331 | 0.58 |
| Temp × Time | 1 | 3.284 | 0.11 |
| CO2 × Time | 1 | 1.332 | 0.28 |
| Temp × CO2 × Time | 1 | 0.914 | 0.37 |
| (c) Autumn | |||
| Temperature | 1 | 2.070 | 0.19 |
| CO2 | 1 | 0.028 | 0.87 |
| Time | 1 | 0.000 | 0.99 |
| Temp × CO2 | 1 | 0.212 | 0.66 |
| Temp × Time | 1 | 0.090 | 0.77 |
| CO2 × Time | 1 | 0.019 | 0.89 |
| Temp × CO2 × Time | 1 | 0.014 | 0.91 |
| (d) Winter | |||
| Temperature | 1 | 9.983 | 0.01 |
| CO2 | 1 | 0.012 | 0.92 |
| Time | 1 | 12.226 | 0.008 |
| Temp × CO2 | 1 | 0.044 | 0.84 |
| Temp × Time | 1 | 4.919 | 0.06 |
| CO2 × Time | 1 | 0.897 | 0.371 |
| Temp × CO2 × Time | 1 | 0.470 | 0.512 |
References
- Jueterbock, A.; Tyberghein, L.; Verbruggen, H.; Coyer, J.A.; Olsen, J.L.; Hoarau, G. Climate Change Impact on Seaweed Meadow Distribution in the North Atlantic Rocky Intertidal. Ecol. Evol. 2013, 3, 1356–1373.
- Wernberg, T.; Bennett, S.; Babcock, R.C.; Bettignies, T.D.; Cure, K.; Depczynski, M.; Dufois, F.; Fromont, J.; Fulton, C.J.; Hovey, R.K.; et al. Climate-Driven Regime Shift of a Temperate Marine Ecosystem. Science 2016, 149, 169–172.
- Smale, D.A. Impacts of Ocean Warming on Kelp Forest Ecosystems. New Phytol. 2020, 225, 1447–1454.
- Dring, M. Stress Resistance and Disease Resistance in Seaweeds: The Role of Reactive Oxygen Metabolism. Adv. Bot. Res. 2005, 43, 175–207.
- Potin, P. Oxidative Burst and Related Responses in Biotic Interactions of Algae. In Algal Chemical Ecology; Amsler, C.D., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 245–272. ISBN 978-3-540-74181-7.
- Bischof, K.; Rautenberger, R. Seaweed Responses to Environmental Stress: Reactive Oxygen and Antioxidative Strategies. In Seaweed Biology; Wiencke, C., Bischof, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 109–132. ISBN 978-3-642-28451-9.
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press: New York, NY, USA, 2015; ISBN 978-0-19-871747-8.
- Asada, K. Mechanisms for Scavenging Reactive Molecules Generated in Chloroplasts under Light Stress. In Photoinhibition of Photosynthesis: From Molecular Mechanisms to the Field; Post, A., Baker, N.R., Bowyer, J.R., Eds.; BIOS Scientific Publishers: Oxford, UK, 1994; pp. 128–140.
- Asada, K. Production and Action of Active Oxygen Species in Photosynthetic Tissues. In Causes of Photooxidative Stress and Amelioration of Defence Systems in Plants; Foyer, C., Mullineaux, P.M., Eds.; CRC Press: Boca Raton, FL, USA, 1994; pp. 77–104.
- Collén, J.; Davison, I.R. Seasonality and Thermal Acclimation of Reactive Oxygen Metabolism in Fucus vesiculosus (Phaeophyceae). J. Phycol. 2001, 37, 474–481.
- Aguilera, J.; Dummermuth, A.; Karsten, U.; Schriek, R.; Wiencke, C. Enzymatic Defences against Photooxidative Stress Induced by Ultraviolet Radiation in Arctic Marine Macroalgae. Polar Biol. 2002, 25, 432–441.
- Dummermuth, A.L.; Karsten, U.; Fisch, K.M.; König, G.M.; Wiencke, C. Responses of Marine Macroalgae to Hydrogen-Peroxide Stress. J. Exp. Mar. Biol. Ecol. 2003, 289, 103–121.
- Bischof, K.; Rautenberger, R.; Brey, L.; Pérez-Lloréns, J.L. Physiological Acclimation to Gradients of Solar Irradiance within Mats of the Filamentous Green Macroalga Chaetomorpha linum from Southern Spain. Mar. Ecol. Prog. Ser. 2006, 306, 165–175.
- Lesser, M.P. Oxidative Stress in Marine Environments: Biochemistry and Physiological Ecology. Annu. Rev. Physiol. 2006, 68, 253–278.
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19.
- Valenzuela, A. The Biological Significance of Malondialdehyde Determination in the Assessment of Tissue Oxidative Stress. Life Sci. 1991, 48, 301–309.
- Cruces, E.; Huovinen, P.; Gómez, I. Phlorotannin and Antioxidant Responses upon Short-Term Exposure to UV Radiation and Elevated Temperature in Three South Pacific Kelps. Photochem. Photobiol. 2012, 88, 58–66.
- Wei, Z.; Long, C.; Yang, F.; Long, L.; Huo, Y.; Ding, D.; Mo, J. Increased Irradiance Availability Mitigates the Physiological Performance of Species of the Calcifying Green Macroalga Halimeda in Response to Ocean Acidification. Algal Res. 2020, 48, 101906.
- Zika, R.G.; Moffett, J.W.; Petasne, R.G.; Cooper, W.J.; Saltzman, E.S. Spatial and Temporal Variations of Hydrogen Peroxide in Gulf of Mexico Waters. Geochim. Cosmochim. Acta 1985, 49, 1173–1184.
- Avery, G.B.; Cooper, W.J.; Kieber, R.J.; Willey, J.D. Hydrogen Peroxide at the Bermuda Atlantic Time Series Station: Temporal Variability of Seawater Hydrogen Peroxide. Mar. Chem. 2005, 97, 236–244.
- Rusak, S.A.; Peake, B.M.; Richard, L.E.; Nodder, S.D.; Cooper, W.J. Distributions of Hydrogen Peroxide and Superoxide in Seawater East of New Zealand. Mar. Chem. 2011, 127, 155–169.
- Szymczak, R.; Waite, T. Generation and Decay of Hydrogen Peroxide in Estuarine Waters. Mar. Freshw. Res. 1988, 39, 289–299.
- Yuan, J.; Shiller, A.M. The Distribution of Hydrogen Peroxide in the Southern and Central Atlantic Ocean. Deep-Sea Res. Part II Top. Stud. Oceanogr. 2001, 48, 2947–2970.
- Twigg, I.M.; Baltar, F.; Hall, J.R.; Hepburn, C.D. Revealing Hydrogen Peroxide as an External Stressor in Macrophyte-Dominated Coastal Ecosystems. Oecologia 2020, 193, 583–591.
- Cooper, W.J.; Zika, R.G.; Petasne, R.G.; Plane, J.M.C. Photochemical Formation of H2O2 in Natural Waters Exposed to Sunlight. Environ. Sci. Technol. 1988, 22, 1156–1160.
- Mopper, K.; Zhou, X. Hydroxyl Radical Photoproduction in the Sea and Its Potential Impact on Marine Processes. Science 1990, 250, 661–664.
- O’Sullivan, D.W.; Neale, P.J.; Coffin, R.B.; Boyd, T.J.; Osburn, C.L. Photochemical Production of Hydrogen Peroxide and Methylhydroperoxide in Coastal Waters. Mar. Chem. 2005, 97, 14–33.
- Garg, S.; Rose, A.L.; Waite, T.D. Photochemical Production of Superoxide and Hydrogen Peroxide from Natural Organic Matter. Geochim. Cosmochim. Acta 2011, 75, 4310–4320.
- Abele-Oeschger, D.; Tüg, H.; Röttgers, R. Dynamics of UV-Driven Hydrogen Peroxide Formation on an Intertidal Sandflat. Limnol. Oceanogr. 1997, 42, 1406–1415.
- Abele, D.; Ferreyra, G.A.; Schloss, I. H2O2 Accumulation from Photochemical Production and Atmospheric Wet Deposition in Antarctic Coastal and Off-Shore Waters of Potter Cove, King George Island, South Shetland Islands. Antarct. Sci. 1999, 11, 131–139.
- Winterbourn, C.C. The Biological Chemistry of Hydrogen Peroxide. In Methods in Enzymology; Abelson, J.N., Simon, M.I., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; Volume 528, pp. 3–25. ISBN 978-0-12-405881-1.
- Badger, M. The Roles of Carbonic Anhydrases in Photosynthetic CO2 Concentrating Mechanisms. Photosynth. Res. 2003, 77, 83–94.
- Parry, M.A.J.; Keys, A.J.; Madgwick, P.J.; Carmo-Silva, A.E.; Andralojc, P.J. Rubisco Regulation: A Role for Inhibitors. J. Exp. Bot. 2008, 59, 1569–1580.
- Tcherkez, G. The Mechanism of Rubisco-Catalysed Oxygenation. Plant. Cell Environ. 2016, 39, 983–997.
- Foyer, C.H.; Lopez-Delgado, H.; Dat, J.F.; Scott, I.M. Hydrogen Peroxide- and Glutathione-Associated Mechanisms of Acclimatory Stress Tolerance and Signalling. Physiol. Plant. 1997, 100, 241–254.
- Collén, J.; Davison, I.R. Reactive Oxygen Production and Damage in Intertidal Fucus Spp. (Phaeophyceae). J. Phycol. 1999, 35, 54–61.
- Carvalho, A.M.; Neto, A.M.P.; Tonon, A.P.; Pinto, E.; Cardozo, K.H.M.; Brigagão, M.R.P.L.; Barros, M.P.; Torres, M.A.; Magalhães, P.; Cg, S. Circadian Protection against Oxidative Stress in Marine Algae. Hypnos 2004, 1, 142–157.
- Takahashi, M.-A.; Asada, K. Superoxide Anion Permeability of Phospholipid Membranes and Chloroplast Thylakoids. Arch. Biochem. Biophys. 1983, 226, 558–566.
- Ross, C.; Alstyne, K.L.V. Intraspecific Variation in Stress-Induced Hydrogen Peroxide Scavenging by the Ulvoid Macroalga Ulva lactuca. J. Phycol. 2007, 43, 466–474.
- Dittami, S.M.; Scornet, D.; Petit, J.-L.; Ségurens, B.; Da Silva, C.; Corre, E.; Dondrup, M.; Glatting, K.-H.; König, R.; Sterck, L.; et al. Global Expression Analysis of the Brown Alga Ectocarpus siliculosus (Phaeophyceae) Reveals Large-Scale Reprogramming of the Transcriptome in Response to Abiotic Stress. Genome Biol. 2009, 10, R66.
- Liu, F.; Wang, W.; Sun, X.; Liang, Z.; Wang, F. RNA-Seq Revealed Complex Response to Heat Stress on Transcriptomic Level in Saccharina japonica (Laminariales, Phaeophyta). J. Appl. Phycol. 2014, 26, 1585–1596.
- Li, H.; Monteiro, C.; Heinrich, S.; Bartsch, I.; Valentin, K.; Harms, L.; Glöckner, G.; Corre, E.; Bischof, K. Responses of the Kelp Saccharina latissima (Phaeophyceae) to the Warming Arctic: From Physiology to Transcriptomics. Physiol. Plant. 2020, 168, 5–26.
- Collén, J.; Guisle-Marsollier, I.; Léger, J.J.; Boyen, C. Response of the Transcriptome of the Intertidal Red Seaweed Chondrus crispus to Controlled and Natural Stresses. New Phytol. 2007, 176, 45–55.
- Pearson, G.A.; Hoarau, G.; Lago-Leston, A.; Coyer, J.A.; Kube, M.; Reinhardt, R.; Henckel, K.; Serrão, E.A.; Corre, E.; Olsen, J.L. An Expressed Sequence Tag Analysis of the Intertidal Brown Seaweeds Fucus serratus (L.) and F. vesiculosus (L.) (Heterokontophyta, Phaeophyceae) in Response to Abiotic Stressors. Mar. Biotechnol. 2010, 12, 195–213.
- Heinrich, S.; Valentin, K.; Frickenhaus, S.; John, U.; Wiencke, C. Transcriptomic Analysis of Acclimation to Temperature and Light Stress in Saccharina latissima (Phaeophyceae). PLoS ONE 2012, 7, e44342.
- Kautsky, H.; Kautsky, L.; Kautsky, N.; Kautsky, U.; Lindblad, C. Studies on the Fucus vesiculosus Community in the Baltic Sea. In Phycological Studies of Nordic Coastal Waters; Wallentinus, I., Snoeijs, P., Eds.; Svenska växtgeografiska sällskapet: Uppsala, Sweden, 1992; pp. 33–48.
- Rönnbäck, P.; Kautsky, N.; Pihl, L.; Troell, M.; Söderqvist, T.; Wennhage, H. Ecosystem Goods and Services from Swedish Coastal Habitats: Identification, Valuation, and Implications of Ecosystem Shifts. Ambio 2007, 36, 534–544.
- Wahl, M.; Shahnaz, L.; Dobretsov, S.; Saha, M.; Symanowski, F.; David, K.; Lachnit, T.; Vasel, M.; Weinberger, F. Ecology of Antifouling Resistance in the Bladder Wrack Fucus vesiculosus: Patterns of Microfouling and Antimicrobial Protection. Mar. Ecol. Prog. Ser. 2010, 411, 33–48.
- Wahl, M.; Jormalainen, V.; Eriksson, B.K.; Coyer, J.A.; Molis, M.; Schubert, H.; Dethier, M.; Karez, R.; Kruse, I.; Lenz, M.; et al. Stress Ecology in Fucus: Abiotic, Biotic and Genetic Interactions. In Advances in Marine Biology; Lesser, M., Ed.; Elsevier Ltd.: Oxford, UK, 2011; Volume 59, pp. 37–105. ISBN 978-0-12-385536-7.
- Kautsky, H.; van der Maarel, E. Multivariate Approaches to the Variation in Phytobenthic Communities and Environmental Vectors in the Baltic Sea. Mar. Ecol. Prog. Ser. 1990, 60, 169–184.
- Jormalainen, V.; Wikström, S.A.; Honkanen, T. Fouling Mediates Grazing: Intertwining of Resistances to Multiple Enemies in the Brown Alga Fucus vesiculosus. Oecologia 2008, 155, 559–569.
- Werner, F.J.; Graiff, A.; Matthiessen, B. Temperature Effects on Seaweed-Sustaining Top-down Control Vary with Season. Oecologia 2016, 180, 889–901.
- Torn, K.; Krause-Jensen, D.; Martin, G. Present and Past Depth Distribution of Bladderwrack (Fucus vesiculosus) in the Baltic Sea. Aquat. Bot. 2006, 84, 53–62.
- Takolander, A.; Cabeza, M.; Leskinen, E. Climate Change Can Cause Complex Responses in Baltic Sea Macroalgae: A Systematic Review. J. Sea Res. 2017, 123, 16–29.
- Elken, J.; Lehmann, A.; Myrberg, K. Recent Change-Marine Circulation and Stratification. In Second Assessment of Climate Change for the Baltic Sea Basin; The BACC II Author Team, Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 131–144. ISBN 978-3-319-16005-4.
- Hobday, A.J.; Alexander, L.V.; Perkins, S.E.; Smale, D.A.; Straub, S.C.; Oliver, E.C.J.; Benthuysen, J.A.; Burrows, M.T.; Donat, M.G.; Feng, M.; et al. A Hierarchical Approach to Defining Marine Heatwaves. Prog. Oceanogr. 2016, 141, 227–238.
- HELCOM. Climate Change in the Baltic Sea Area: HELCOM Thematic Assessment in 2013. In Baltic Sea Environment Proceedings; Helsinki Commission: Helsinki, Finland, 2013; Volume 137, ISBN 0357-2994.
- Müller, J.D.; Schneider, B.; Rehder, G. Long-Term Alkalinity Trends in the Baltic Sea and Their Implications for CO2-Induced Acidification. Limnol. Oceanogr. 2016, 61, 1984–2002.
- Omstedt, A.; Edman, M.K.; Claremar, B.; Frodin, P.; Gustafsson, E.; Humborg, C.; Hägg, H.; Mörth, M.; Rutgersson, A.; Schurgers, G.; et al. Future Changes in the Baltic Sea Acid-Base (PH) and Oxygen Balances. Tellus 2012, 64, 19586.
- BACC II Author Team. Second Assessment of Climate Change for the Baltic Sea Basin; Bolle, H.-J., Menenti, M., Rasool, S.I., Eds.; Springer International Publishing AG Switzerland: Gesthacht, Switzerland, 2015; ISBN 978-3-319-16005-4.
- Schneider, B.; Eilola, K.; Lukkari, K.; Müller-Karulis, B.; Neumann, T. Environmental Impacts—Marine Biogeochemistry. In Second Assessment of Climate Change for the Baltic Sea Basin; The BACC II Author Team, Ed.; Springer International Publishing: Berlin, Germany, 2015.
- Wahl, M.; Buchholz, B.; Winde, V.; Golomb, D.; Guy-Haim, T.; Müller, J.; Rilov, G.; Scotti, M.; Böttcher, M.E. A Mesocosm Concept for the Simulation of Near-Natural Shallow Underwater Climates: The Kiel Outdoor Benthocosms (KOB). Limnol. Oceanogr. Methods 2015, 13, 651–663.
- Graiff, A.; Liesner, D.; Karsten, U.; Bartsch, I. Temperature Tolerance of Western Baltic Sea Fucus vesiculosus—Growth, Photosynthesis and Survival. J. Exp. Mar. Biol. Ecol. 2015, 471, 8–16.
More
Information
Subjects:
Marine & Freshwater Biology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
778
Revisions:
2 times
(View History)
Update Date:
12 Jan 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




