The main aim of this work was to create a full-length bispecific antibody (BsAb) as a vehicle for the targeted delivery of interferon-beta (IFN-β) to ErbB2+ tumor cells in the form of non-covalent complex of BsAb and IFN-β. Such a construct is a CrossMab-type BsAb, consisting of an ErbB2-recognizing trastuzumab moiety, a part of chimeric antibody to IFN-β, and human IgG1 Fc domain carrying knob-into-hole amino acid substitutions necessary for the proper assembly of bispecific molecules. The IFN-β- recognizing arm of BsAb not only forms a complex with the cytokine but neutralizes its activity, thus providing a mechanism to avoid the side effects of the systemic action of IFN-β by blocking IFN-β Interaction with cell receptors in the process of cytokine delivery to tumor sites. Enzyme sandwich immunoassay confirmed the ability of BsAb to bind to human IFN-β comparable to that of the parental chimeric mAb.
1. Introduction
The development and research of new therapies for tumors with the overexpression of the ErbB2 receptor are urgent tasks. Recently, research on the use of type I interferons in the treatment of oncological diseases has intensified. Cytokine therapy is aimed at activating the cells of the immune system to fight against tumors, but it has drawbacks that limit its use, which manifest themselves in a number of side effects. Currently, a number of studies are being carried out on the use of IFN-β in oncology aimed at reducing the systemic effect of this cytokine. Among all cancer types, breast cancer is the most common, accounting for 13.1% of the total incidence. As a cause of death, it ranks in5th place, taking 522,000 lives annually, which is 6.4% of total breast cancer cases
[1]. Depending on the presence of oncological markers on the malignant cell surface, three type of breast cancer can be distinguished: ER/PR
+, ErbB2
+ and triple negative. The therapeutic strategies for each of those types differ; in particular, the ER/PR
+ type is treated with hormone therapy
[2]; the triple negative form lacking all three markers can be cured with the use of immune checkpoint inhibitors
[3]. ErbB2
+ tumors are presented in 11–30% of total breast cancer cases; their treatment involves the use of ErbB2-targeted therapeutics
[4].
ErbB2 is a protein from the EGFR family that plays an essential role in cell growth, proliferation and migration
[5]. However, ErbB2 overexpression is associated with oncological processes and poor prognosis. Moreover, an increase of the ErbB2 expression level has also been seen in bladder, pancreas, ovarian, colon, kidney, esophagus, prostate and other types of cancer
[6]. The probability of relapse for ErbB2
+ breast cancer in I–II stages is 2.7 times higher than for ErbB2 negative types, and the chance of metastasis development is 5.3 times higher
[7]. An induced increase in the
ErbB2 transcription level in mice results in the appearance of epithelial-origin tumors and their growth
[8]. Besides that, some somatic mutations of the
ErbB2 gene, termed activating mutations, increasing ErbB2 phosphotyrosine kinase activity or dimerization effectiveness are found in ErbB2
+ breast cancer patients
[9]. The inhibition of phosphatase activities, crucial for ErbB2 signal activity suppression, may also drive malignant cell transformation
[10]. Taking all of these facts in account, both the increase of ErbB2 level expression or erbb2 malfunctioning due to mutagenesis or other mechanisms are important factors in oncogenesis. Nowadays, ErbB2-targeted therapy is one of the most effective in cancer treatment. The breakthrough occurred after the registration of humanized monoclonal antibody trastuzumab (Tz) (trademark Herceptin). This antibody binds to the fourth extracellular domain and disrupts receptor dimerization, preventing the activation of the PI3K-AKT and RAS-MAPK pathways, which are important for cell proliferation and growth
[11]. The other two mechanisms of trastuzumab action are antibody-dependent cellular cytotoxicity and complement system activation
[12]. The combination of trastuzumab and chemotherapy is considered a gold standard for ErbB2
+ breast cancer treatment
[13]. Besides trastuzumab, there are other targeted molecules for ErbB2, for example, pertuzumab, a humanized monoclonal antibody against the second extracellular domain of ErbB2. As well as trastuzumab, pertuzumab disrupts ErbB2 dimerization and involves the immune system to fight tumor
[14]. Despite the effectiveness of the use of anti-ErbB2 antibodies, in some cases tumors become resistant to the therapy
[15]. That is why the research and development of new therapeutics for ErbB2
+ cancer treatment is an important task for scientists around the world.
Interferons are cytokines with antiproliferative, antiviral, proapoptotic and antiangiogenic activities. Though the effectiveness of antiproliferative and proapoptotic effects depends much on the cell line used for experiments, it is known that interferon-beta (IFN-β) suppresses growth and cell proliferation and induces apoptosis with higher effect than other interferons. It has been observed at least for glioma
[16], hepatocarcinoma
[17] and breast cancer
[18] cell lines. One of the effects described for IFN-β is cell cycle arrest in the S-phase
[19]. IFN-β also increases the level of the expression of proapoptotic proteins Fas, FasL and TRAIL, which are important for apoptosis activation
[20]. Local effects of IFN-β includes tumor infiltration by macrophages
[21], the enhancement of antigen presentation by increasing MHC I expression level
[22], the activation of NK-cells
[23], the generation of NO by macrophages
[24] and angiogenesis suppression
[25]. One of the aims of cytokine therapy is the activation of immune system cells in order to improve tumor recognition and the mediation of cytotoxic reactions. Nevertheless, the practical use of cytokines is limited due to side effects, whose severity depends on the type of a cytokine and its dosage. The use of viral vectors carrying the
IFN-β gene may reduce the systemic side effects of IFN-β during administration
[21]. The construction of immunocytokines is another way to avoid nonspecific reactions and deliver cytokine to the tumor site. In particular, the effectiveness of an immunocytokine, which consists of anti-ErbB2 antibody trastuzumab and IFN-β, was already demonstrated by a group of researchers
[26].
Bispecific antibodies (BsAbs) are engineered to recognize two different antigens at the same time. Due to that property, they are used to crosslink immune system cells, mostly T-killers, to tumor cells and activate them
[27][28]. However, BsAbs could also be considered a vehicle for cytokine delivery for cancer treatment. In the present work, we describe the construction of a molecular complex of IFN-β and bispecific antibody (BsAb) anti-ErbB2 and anti-IFN-β for the targeted therapy of ErbB2
+ solid tumors. To implement this concept, one of the components of the BsAb should bind and neutralize the activity of IFN-β, and the other component specific to ErbB2 will redirect the complex to the tumor site.
2. Analisis of Hybridomas Producing Antibodies to IFN-β
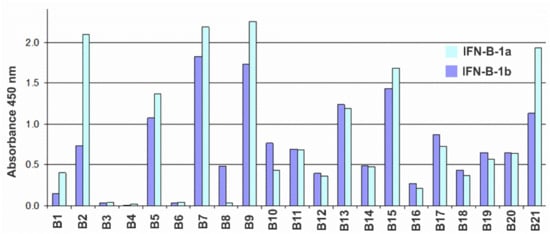
A panel of hybridomas producing murine monoclonal antibodies against recombinant human IFN-β expressed in E. coli cells was prepared according to conventional techniques. Antibodies were tested by ELISA. To detect cross-reactions, E. coli-HuIFN-β, CHO-HuIFN-β, E. coli-HuIFN-α and E. coli-HuIFN-γ were used as antigens for binding to the antibodies under study. As a result of the analysis, it was found that none of the obtained antibodies cross-reacted with either IFN-α or IFN-γ. Testing of antibodies by indirect ELISA for binding to glycosylated (expressed in CHO) and non-glycosylated (expressed in E. coli) forms of IFN-β showed that all antibodies with the exception of B8 recognize non-glycosylated and glycosylated IFN-β forms with comparable binding levels (Figure 1).
Figure 1. Histogram of the reactivity of antibodies B1–B21 against IFN-β according to the indirect ELISA with sorbed CHO-HuIFN-β-1a (blue bars) and E. coli-HuIFN-β-1b (dark blue bars) at an antibody concentration of 30 ng/mL.
The sub-isotypes of B1–B21 antibodies were also determined by indirect ELISA using commercial typing sera: goat antibodies against mouse immunoglobulins of different isotypes. The vast majority of Ab have a sub-isotype corresponding to the mature immune response of the mouse to protein antigens, namely: IgG1 (6 pcs), IgG2a (7 pcs) and Ig2b (5 pcs). Three Ab have the IgG3 sub-isotype. All L-chains were of the kappa isotype. The activity of antibodies was determined in tests assessing the inhibition of the antiproliferative action of IFN-β on 3 human tumor lines: MCF7 (ATCC HTB-22) breast cancer carcinoma, HT-29 (ATCC HTB-38) colon carcinoma and AGS (ATCC CRL-1739) gastric carcinoma. Analysis of the murine antibodies of a panel of hybridomas for their ability to inhibit the antiproliferative effect of IFN revealed the B16 antibody as a candidate for BsAb construction.
The synthesis of the cDNA coding for the VH and VL variable domains of B16 mAB was performed using total RNA isolated from B16-producing hybridoma cells. Reverse transcription was performed using reverse transcriptase MMuLV (RNase H minus) and oligo (dT18) primer. The synthesis of the second strand of cDNA was carried out using oligonucleotide G-oligo and gene-specific primers complementary to the 5′-end of the antibody constant domain sequences. The amplified and purified fragments of the heavy and light chains were cloned into a linearized pAL2-T vector (Evrogen, Moscow, Russia). In order to determine the consensus nucleotide sequence of the variable domains, at least 10 clones carrying the insert of each of the antibody chain were taken for sequencing. Nucleotide sequence analysis was performed by Chromas, CLC Sequence Viewer and Gene Runner software. As a result, VH and VL consensus nucleotide sequences were determined and compared with the databases of immunoglobulins, IMGT (
www.imgt.org, accessed on 19 December 2021), which confirmed their belonging to the genes of mouse immunoglobulins and made it possible to determine the most-related germ lines. Based on the analysis of the primary sequences using the CLC Sequence Viewer, the amino acid sequences of the VH and VL immunoglobulins were determined. Framework (FR) and hypervariable (CDR) regions of variable domains of B16 were established in accordance with the IMGT rules. Mass spectrometric analysis of the molecular weight of the peptide products of mAb hydrolysis confirmed the correctness of the previously established amino acid sequences VL and VH. The presence of peptides corresponding to the constant domains previously determined by isotyping the mAb panel was also proven (data not shown).
3. Obtaining and Studying the Properties of Chimeric Antibody B16 and Trastuzumab Analogue
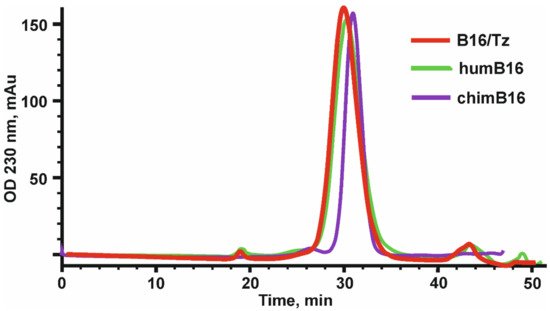
The design, preparation and characterization of chimeric antibodies neutralizing IFN-β were carried out as described in Materials and Methods. The L and H chain genes of the chimeric B16 Ab were cloned into the pcDNA3.4 expression vector under the control of the CMV promoter, the thymidine kinase terminator containing the polyadenylation signal and the WPRE element translation enhancer. For the secretion of antibodies into the culture liquid, leader peptides of the B16 antibody were retained. At the 5′-ends of leader peptides, a NheI restriction site essential for further cloning and Kozak sequence, which is important for RNA efficient translation, were also introduced. For the production of chimeric antibodies, we used a transient expression in CHO cells. After the isolation of antibodies by affinity chromatography, they were analyzed by size-exclusion chromatography (Figure 1).
Figure 1. Elution profile of chimeric (chimB16), bispecific antibody (B16/Tz) and humanized (humB16) antibodies, obtained by size-exclusion chromatography on a Superdex 200 10/300 GL column. Initial protein concentration was equal to 0.5–1.1 mg/mL, and the sample volume was 200–500 µl. Protein separation was performed at a 0.4 mL/min flowrate.
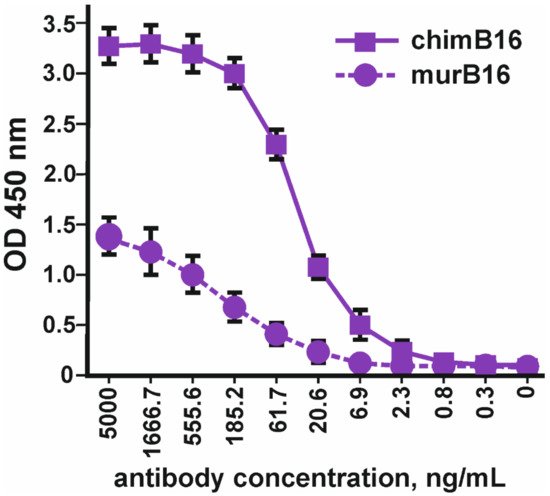
Using the method of indirect ELISA, the binding of the obtained chimeric antibodies to IFN-β (Pharmapark, Russia) was checked in comparison with the prototype mouse antibodies (Figure 2).
Figure 2. Binding of chimeric antibodies (chimB16) with IFN-β-1a versus murine antibodies (murB16) (Pharmapark, Russia). The error bars indicate means ± SD.
It should be noted that the absolute OD values for murine and chimeric antibodies cannot be compared, since different anti-species conjugates were used for the manifestation of binding. The dissociation constant (Kd) of chimeric antibodies (3.2 × 10−9 M) practically coincides with the Kd of the murine prototype (3.8 × 10−9 M).
In parallel with the creation of a chimeric antibody against IFN-β, we designed, obtained and characterized the antibody against the ErbB2 receptor (Tz), which is an analogue of Herceptin. The VL and VH nucleotide sequences of antibodies against the ErbB2 receptor were synthesized by overlapping oligonucleotides based on the amino acid sequences of the trastuzumab antibody
[29], taking into account the optimization of the codon frequency in the Chinese hamster genome. At the 5′-ends of the VL and VH antibody sequences, the Kozak sequence and leader peptide sequences were placed for the secretion of antibodies into the culture liquid.
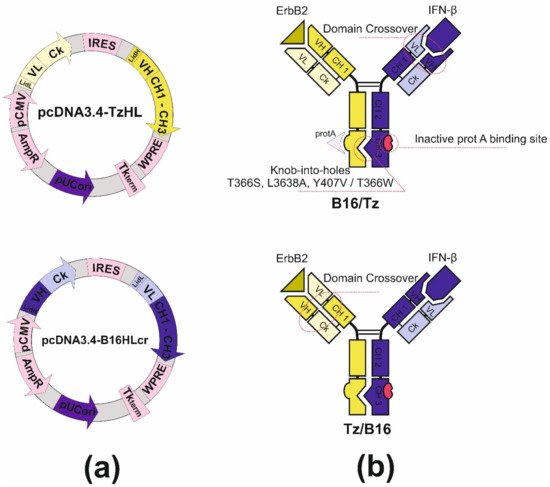
4. Design and Construction of Bispecific Antibodies B16/Tz and Tz/B16
Bispecific antibody construction was started with the introduction of knob-into-holes mutations into IgG1 heavy-chain genes of antibodies by side-directed mutagenesis with primers. For the anti-ErbB2 antibody, we made hole substitutions (T366S, L3638A, Y407V) for the chimB16 knob substitution (T366W). Two other mutations, H457R and Y458F, were also introduced in the gene of the B16 heavy chain to enable BsAb gradient purification. The mutant chains were further used for variable domain recombination, which is called crossover. Two CrossMab antibodies, B16/Tz and Tz/B16, (Figure 3b) were constructed differing only by the location of the crossover of variable domains. We have made a crossover of B16 variable domains for B16/Tz and of the Tz arm for Tz/B16. The crossover of variable domains was performed by a series of SOE-PCRs with the use of gene-specific primers. As a result of cloning, four plasmids were obtained (Table 1). For cell transfection, we combined pcDNA3.4-TzHL to produce a trastuzumab arm of BsAb and pcDNA3.4-B16HLcr to produce a B16HL crossover arm of BsAb (Figure 3a) for B16/Tz biosynthesis, and pcDNA3.4-TzHLcr and pcDNA3.4-B16HL for Tz/B16 biosynthesis, respectively.
Figure 3. (a) Plasmid maps for B16/Tz bispecific antibody production: pcDNA3.4-TzHL and pcDNA3.4-B16HLcr for B16/Tz; (b) structure of B16/Tz and Tz/B16 CrossMab bispecific antibodies.
Table 1. Constructed plasmids for CrossMab production.
| CrossMab |
Plasmid |
Heavy Chain |
Light Chain |
| B16/Tz |
pcDNA3.4-TzHL |
Anti-ErbB2 hole |
Anti-ErbB2 |
| pcDNA3.4-B16HLcr |
B16 knob with domain crossover |
B16 with domain crossover |
| Tz/B16 |
pcDNA3.4-TzHLcr |
Anti-ErbB2 hole with domain crossover |
Anti-ErbB2 with domain crossover |
| pcDNA3.4-B16HL |
B16 knob |
B16 |
3.4. Studying the Properties of Bispecific Antibody B16/Tz
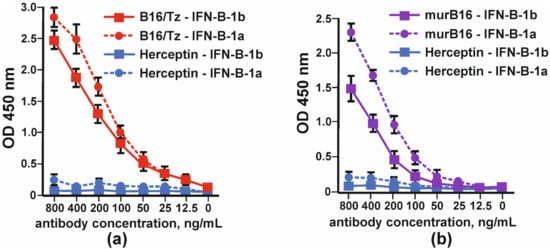
The binding of bispecific antibody B16/Tz versus murine B16 antibody to IFNβ was tested by indirect ELISA. Two forms of interferon, IFN-β1a and IFN-β-1b, were absorbed on each well’s surface. The anti-ErbB2 antibody (Tz) was used as a negative control. Bispecific antibody B16/Tz, like the prototype mouse B16 antibody, binds to both forms of interferon with greater efficiency to the glycosylated form of IFN-β (Figure 5).
Figure 4. Binding of glycosylated (IFN-β-1a) and non-glycosylated (IFN-β-1b) by: (a) CrossMab B16/Tz and (b) murine (murB16) antibodies. Herceptin was used as a negative control. The error bars indicate means ± SD.
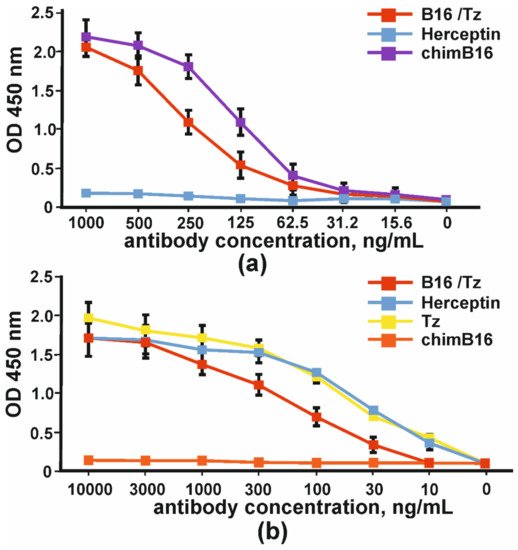
The comparison of the binding capacity of chimeric antibody (chimB16) and CrossMab B16/Tz to IFNβ-1a in one assay demonstrated the lower potency of the BsAb (Figure 5a). In order to prove that bispecific antibodies bind to the ErbB2 receptor, we absorbed SKOV-3 cell lysates (ErbB2+) on plates. The binding curves of B16/Tz, Herceptin and anti-ErbB2 Tz analogue, obtained in this work, are presented in Figure 5. As a negative control, anti-shiga toxin antibody was used. It can be inferred that the binding capacities of Herceptin and its analogue may be considered equal, while CrossMab antibody B16/Tz binds with less capacity (Figure 5b).
Figure 5. The binding of bispecific antibody B16/Tz, chimeric antibody chimB16, trastuzumab analogue Tz to: (a) IFNβ-1a and (b) ErbB2+SKOV-3 lysates. As negative controls, Herceptin and anti-shiga toxin antibodies were used. The error bars indicate means ± SD.
The binding of CrossMab antibodies B16/Tz and Tz/B16 was proven by indirect ELISA with IFNβ and the extracellular part of ErbB2 (Figure 6).
Figure 6. The binding of IFNβ and the extracellular part of ErbB2 by CrossMab antibodies: (a) B16/Tz and (b) Tz/B16. The error bars indicate means ± SD.
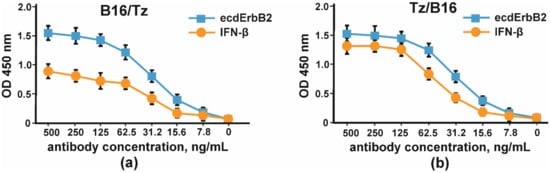
In order to prove the ability of B16/Tz to bind two different antigens, which is essential for bispecific antibodies, we proposed a sandwich ELISA system with two antigens. IFN-β was used to immobilize CrossMab through its anti-interferon arm, and biotinylated-ecdErbB2 recombinant protein was used to detect BsAbs through their ErbB2 binding activity. The result of the experiment is shown in Figure 7.
Figure 7. Binding curves of simultaneous binding of two antigens: IFN-β and ecdErbB2 in sandwich ELISA. The error bars indicate means ± SD.
6. Humanization of Antibody B16
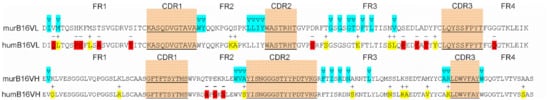
For murine antibody humanization, the CDR-grafting method was chosen. We decided to use the IMGT database containing human germline Ig genes for the Ig FR-donor search. Among a wide diversity of sequences, we found IGHV3-23*04 and IGKV1-9*01 genes with a homology degree of 80% and 65% to murine B16 heavy- and light-chain variable domains, respectively. The comparison of humanized and murine variable domain sequences resulted in discovering 10 critical substitutions and 9 conservative ones in light-chain variable domain and 3 critical substitutions and 10 conservative ones in heavy-chain variable domain. The analysis of Vernier zone residues showed no critical replacements there; therefore, no reverse mutations were introduced (Figure 8).
Figure 8. Amino acid sequences of murB16 and hum B16 variable domains. CDRs are underlined, blue for Vernier zones, red and yellow for substitutions.
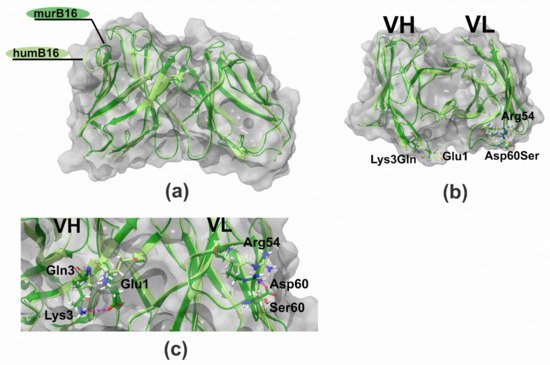
To make sure that the proposed method of humanization by CDR-grafting did not significantly affect the structure of the antibody, we created 3D-structure models of murine and humanized B16 variable domains using Rosetta Antibody web service. A few steps were performed: (1) searching for antibodies with known spatial structure and similarity to humB16 amino acid sequences, (2) building initial models using conservative template fragments and humB16 VL and VH query sequences and (3) improving the initial models by selecting optimal conformations of the main chain and amino acid residues. Then the obtained models were optimized by taking into account the influence of the solvent, and molecular dynamics calculations were performed in Gromacs and Charmm36 force field. The solvent was H2O and the parameters: 300 °K, physiological concentrations of NaCl, simulation length 10 ns. After that MD trajectories were subjected to cluster analysis, the most representative of those were selected as final models.
The comparison and superposition of murine and humanized variable domain models showed no crucial displacement in the backbone chain (Figure 9a). In particular, some mutations like H8P and R44G led to the noticeable local displacement of the backbone chain with no effect on the structure of the antibody paratope. More detailed analysis of models with the research of the positioning of residues made it possible to formulate a number of recurrent substitutions in framework regions of the humanized antibody. Two mutations, K3Q in the heavy chain and D60S in the light chain, may lead to surface changes of the surroundings of the paratope (Figure 9b). In murine B16, heavy-chain K3 forms a salt bridge with E1, and the replacement of lysine by glutamine disrupts the interaction between those two charged residues. As a result, the glutamate side chain changes its position toward the paratope surface. The replacement of Asp60 by Ser in light chain makes impossible the formation of an interaction with Arg54, which changes the orientation of this CDR2 residue (Figure 9c). Both mutations K3Q and D60S change the location of charged residues near the environment of the paratope, which may influence negatively on the Kd value of the antibody.
Figure 9. Structure of B16 wild-type and humanized variable domains: (a) superimposed structures of humB16 and murB16 variable domains; (b) location of K3Q and D60S mutations on superimposed structures of humB16 and murB16 variable domains, view from the top; (c) change of position of G1 (VH) and R54 (VL) after introducing K3Q (VH) and D60S (VL) mutations in variable domains. Light green and dark green colors of main chains were used for murB16 and humB16 variable domains, for side chains red, blue, white and green colors were used for oxygen, nitrogen, hydrogen and carbon atoms. Pink dashed line – hydrogen bonds and salt bridges.
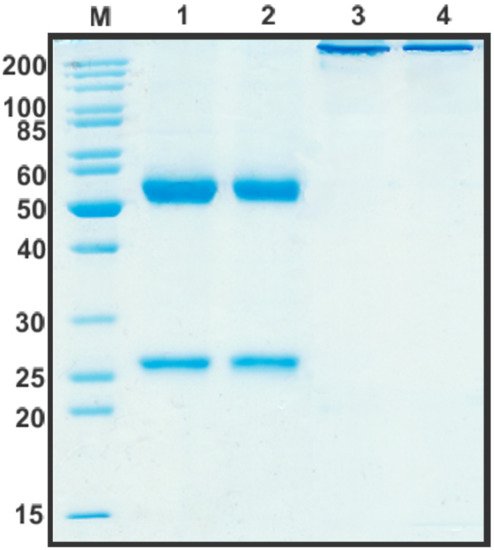
After the construction of humB16 genes and cloning them into the expression vector, both humanized and murine B16 antibodies were produced in CHO cells and purified, and their properties were studied. The yields of purified proteins were measured and counted up to 330 mg/L for chimB16 and 353 mg/L for humB16. Elution profiles obtained by the size-exclusion chromatography of humB16 and chimB16 have similar character, with the retention time of both antibodies ranging from 31–32 min, corresponding to the monomeric form of IgG with a calculated molecular weight 162–165 kDa (Figure 1). The purity of the obtained antibodies is also confirmed by SDS-PAGE (Figure 10); under non-reducing conditions, immunoglobulins migrate as a single band, but after disulfide bond reduction with mercaptoethanol, both humanized and murine antibodies migrate as two bands corresponding to 25 kDa light and 50 kDa heavy chains.
Figure 10. SDS-PAGE in 12% gel of purified by affinity chromatography chimB16 (lane 1, 3) and humB16 antibodies (lane 2, 4). Lane 1, 2—reducing conditions, lane 3, 4—non-reducing conditions.
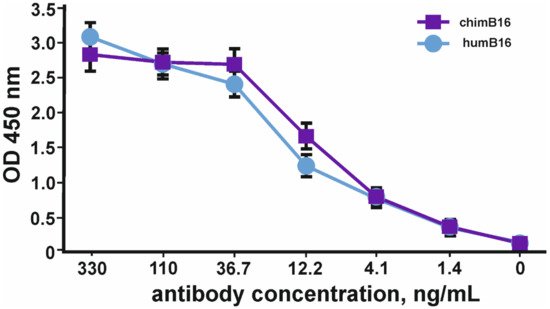
Binding curves to IFN-β of both antibodies (Figure 11) characterize them as antibodies with a high degree of affinity, which is confirmed by Kd values 3.2 × 10−9 M for chimB16 and 3.3 × 10−9 M for humB16.
Figure 11. IFN-β binding curves by humB16 and chimB16 antibodies. The error bars indicate means ± SD.
7. Study of the IFN-β Neutralization Capacity of Chimeric ChimB16 and Bispecific B16/Tz Antibodies
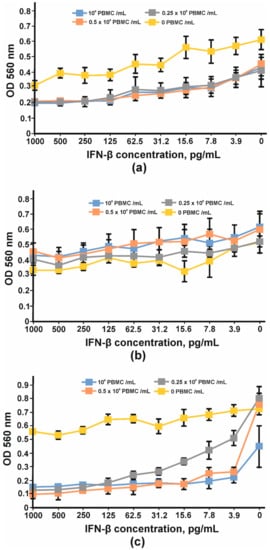
In order to find the appropriate cell line for IFN-β antiproliferative assay, three cell lines, HT29, SKOV-3 and BT474, were tested. After four days of cultivation with serial dilutions of IFN-β and PBMC, the viabilities of the cells were determined using an MTT test. Neither interferon nor PBMC had any impact on BT474 cells (Figure 12b). A more pronounced antiproliferative effect of IFNβ was observed for SKOV-3 cells (Figure 12a); however, the addition of PBMC decreased the slope of the curve and the maximum OD value so that the detection of the growth inhibition effect could be problematic. No significant antiproliferative effect mediated by IFN-β was observed for HT29 cells; nevertheless, the combination of interferon and PBMCs greatly decreased HT29 cell growth. The viability of HT29 cells cultivated without interferon did not change substantially with the addition of 25,000 cells (0.25 × 106 PBMC/mL) or 50,000 cells (0.5 × 106PBMC/mL) per well but decreased by at least 50% when 100,000 PMBCs were added (106 PBMC/mL) (Figure 12c). That is why 100,000 PMBCs/well were unsuitable for further antibody IC measurement. It should be noted that interferon dilutions for the remaining two curves decreased more for 0.5 × 106 PBMC/mL than for 0.25 × 106 PBMC/mL. The maximum antiproliferative effect for 0.5 × 106 PBMC/mL was observed at 15.62 pg/mL of IFN-β and did not change significantly at higher IFN-β concentrations.
Figure 12. Inhibition of cell proliferation by IFNβ and PBMC: (a) SKOV-3, (b) BT474 and (c) HT29 cell lines. The error bars indicate means ± SD.
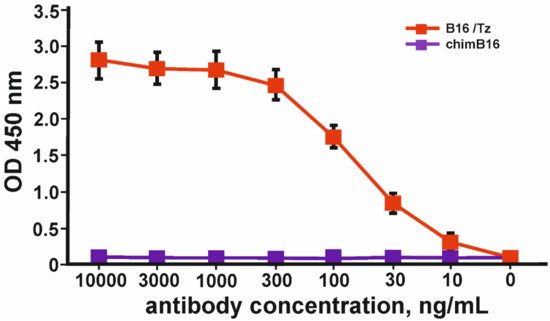
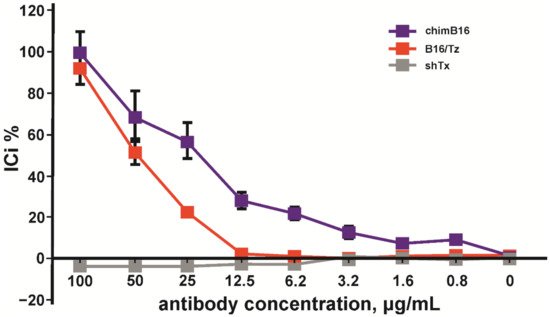
Considering the above-mentioned facts, we chose the HT29 cell line for IC determination. A total of 100 pg/mL of IFN-β, 0.5 × 106 PBMC/mL, was added to adherent cells. The inhibition of the IFN-β antiproliferative effect by anti-IFN-β antibodies was performed in a concentration range of 0.5–100 μg/mL. The inhibition of the antiproliferative activity of IFN-β was observed for chimB16 starting from 3.1 μg/mL and for CrossMab B16/Tz from 25 μg/mL. The IC50 values for both antibodies were determined and turned out to be 21.8 μg/mL for B16 and 49.3 μg/mL for B16/Tz (Figure 13). As a negative control, anti-shiga toxin antibody was used. No inhibition effect on IFN-β was observed for the control antibodies. Taking all that is mentioned above into account, we can state that both B16/Tz and B16 possess the ability to neutralize IFN-β.
Figure 13. Inhibition of IFN-β antiproliferative activity by chimB16 and B16/Tz antibodies, shown on HT 29 cells. An anti-shiga toxin antibody was used as a negative control. The error bars indicate means ± SD.