
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Youjun Zhang | + 3178 word(s) | 3178 | 2021-12-15 09:39:02 | | | |
| 2 | Lindsay Dong | + 288 word(s) | 3466 | 2022-01-12 07:43:16 | | |
Video Upload Options
Increasing evidence has revealed that the enzymes of several biological pathways assemble into larger supramolecular structures called super-complexes. Indeed, those such as association of the mitochondrial respiratory chain complexes play an essential role in respiratory activity and promote metabolic fitness. Dynamically assembled super-complexes are able to alternate between participating in large complexes and existing in a free state.
1. Introduction
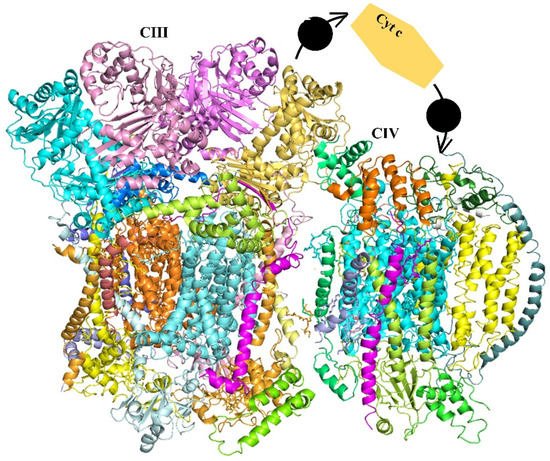
2. Molecular Organization of Different Complexes
2.1. Photosynthesis Complex
2.1.1. Photosystem I Core Complex
Plants and algae have a monomer PSI core complex that connects with different LHCIs to produce the PSI–LHCI super-complex [12][13][14] (Figure 2A). Almost all Lhca/Lhcb proteins from various photosynthetic species have been described structurally. The antenna size, protein content, and related colors of the LHCI proteins varied greatly among species, according to these structural investigations. [15][16]. The structural similarities and differences among various LHCI proteins are explored in the following sections.
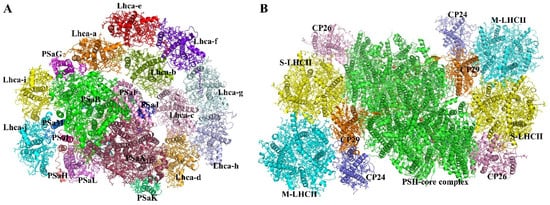
2.1.2. PSII Core Complex
2.1.3. ATPase
2.1.4. Cytochrome b6/f
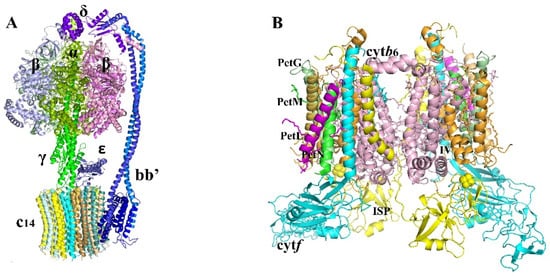
2.2. The Chloroplast Electron Transport Chain
2.3. Calvin–Benson Cycle (CBC)
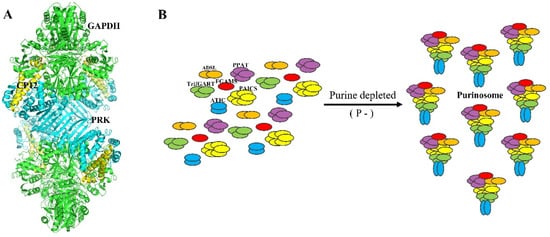
2.4. A Chloroplast Purinosome
References
- Milenkovic, D.; Blaza, J.N.; Larsson, N.G.; Hirst, J. The enigma of the respiratory chain supercomplex. Cell Metab. 2017, 25, 765–776.
- Lobo-Jarne, T.; Ugalde, C. Respiratory chain supercomplexes: Structures, function and biogenesis. Semin. Cell Dev. Biol. 2018, 76, 179–190.
- Toleco, M.R.; Naake, T.; Zhang, Y.; Heazlewood, J.L.; R Fernie, A. Plant mitochondrial carriers: Molecular gatekeepers that help to regulate plant central carbon metabolism. Plants 2020, 9, 117.
- Letts, J.A.; Fiedorczuk, K.; Sazanov, L.A. The architecture of respiratory supercomplexes. Nature 2016, 537, 644–648.
- Schäfer, E.; Seelert, H.; Reifschneider, N.H.; Krause, F.; Dencher, N.A.; Vonck, J. Architecture of active mammalian respiratory chain supercomplexes. J. Biol. Chem. 2006, 281, 15370–15375.
- Lenaz, G.; Genova, M.L. Supramolecular organisation of the mitochondrial respiratory chain: A new challenge for the mechanism and control of oxidative phosphorylation. Mitochondrial Oxidative Phosphorylation 2012, 748, 107–144.
- Lapuente-Brun, E.; Moreno-Loshuertos, R.; Acín-Pérez, R.; Latorre-Pellicer, A.; Colás, C.; Balsa, E.; Perales-Clemente, E.; Quirós, P.M.; Calvo, E.; Rodríguez-Hernández, M. Supercomplex assembly determines electron flux in the mitochondrial electron transport chain. Science 2013, 340, 1567–1570.
- Berndtsson, J.; Aufschnaiter, A.; Rathore, S.; Marin-Buera, L.; Dawitz, H.; Diessl, J.; Kohler, V.; Barrientos, A.; Büttner, S.; Fontanesi, F. Respiratory supercomplexes enhance electron transport by decreasing cytochrome c diffusion distance. EMBO Rep. 2020, 21, e51015.
- Althoff, T.; Mills, D.J.; Popot, J.L.; Kuhlbrandt, W. Arrangement of electron transport chain components in bovine mitochondrial supercomplex i1iii2iv1. EMBO J. 2011, 30, 4652–4664.
- Sousa, J.S.; Mills, D.J.; Vonck, J.; Kuhlbrandt, W. Functional asymmetry and electron flow in the bovine respirasome. Elife 2016, 5, e21290.
- Dudkina, N.V.; Kudryashev, M.; Stahlberg, H.; Boekema, E.J. Interaction of complexes I, III, and IV within the bovine respirasome by single particle cryoelectron tomography. Proc. Natl. Acad. Sci. USA 2011, 108, 15196–15200.
- Nelson, N.; Junge, W. Structure and energy transfer in photosystems of oxygenic photosynthesis. Annu. Rev. Biochem. 2015, 84, 659–683.
- Suga, M.; Qin, X.; Kuang, T.; Shen, J.-R. Structure and energy transfer pathways of the plant photosystem I-LHCI supercomplex. Curr. Opin. Struct. Biol. 2016, 39, 46–53.
- Croce, R.; van Amerongen, H. Light-harvesting in photosystem I. Photosynth. Res. 2013, 116, 153–166.
- Busch, A.; Hippler, M. The structure and function of eukaryotic photosystem I. Biochim. Biophys. Acta (BBA)-Bioenerg. 2011, 1807, 864–877.
- Jansson, S. A guide to the lhc genes and their relatives in arabidopsis. Trends Plant Sci. 1999, 4, 236–240.
- Wedel, N.; Soll, J.; Paap, B.K. Cp12 provides a new mode of light regulation of calvin cycle activity in higher plants. Proc. Natl. Acad. Sci. USA 1997, 94, 10479–10484.
- Wei, X.; Su, X.; Cao, P.; Liu, X.; Chang, W.; Li, M.; Zhang, X.; Liu, Z. Structure of spinach photosystem II–LHCII supercomplex at 3.2 å resolution. Nature 2016, 534, 69–74.
- Jackowski, G.; Kacprzak, K.; Jansson, S. Identification of lhcb1/lhcb2/lhcb3 heterotrimers of the main light-harvesting chlorophyll a/b–protein complex of photosystem II (LHC II). Biochim. Biophys. Acta (BBA)-Bioenerg. 2001, 1504, 340–345.
- Caffarri, S.; Croce, R.; Cattivelli, L.; Bassi, R. A look within LHCII: Differential analysis of the Lhcb1−3 complexes building the major trimeric antenna complex of higher-plant photosynthesis. Biochemistry 2004, 43, 9467–9476.
- Standfuss, J.; Kühlbrandt, W. The three isoforms of the light-harvesting complex II: Spectroscopic features, trimer formation, and functional roles. J. Biol. Chem. 2004, 279, 36884–36891.
- Camm, E.L.; Green, B.R. How the chlorophyll-proteins got their names. Photosynth. Res. 2004, 80, 189–196.
- Boekema, E.J.; van Roon, H.; Calkoen, F.; Bassi, R.; Dekker, J.P. Multiple types of association of photosystem II and its light-harvesting antenna in partially solubilized photosystem ii membranes. Biochemistry 1999, 38, 2233–2239.
- Kouřil, R.; Wientjes, E.; Bultema, J.B.; Croce, R.; Boekema, E.J. High-light vs. Low-light: Effect of light acclimation on photosystem ii composition and organization in arabidopsis thaliana. Biochim. Biophys. Acta (BBA)-Bioenerg. 2013, 1827, 411–419.
- Bielczynski, L.W.; Schansker, G.; Croce, R. Effect of light acclimation on the organization of photosystem II super-and sub-complexes in arabidopsis thaliana. Front. Plant Sci. 2016, 7, 105.
- Caffarri, S.; Kouřil, R.; Kereïche, S.; Boekema, E.J.; Croce, R. Functional architecture of higher plant photosystem II supercomplexes. EMBO J. 2009, 28, 3052–3063.
- Nield, J.; Orlova, E.V.; Morris, E.P.; Gowen, B.; van Heel, M.; Barber, J. 3d map of the plant photosystem II supercomplex obtained by cryoelectron microscopy and single particle analysis. Nat. Struct. Biol. 2000, 7, 44–47.
- van Bezouwen, L.S.; Caffarri, S.; Kale, R.S.; Kouřil, R.; Thunnissen, A.-M.W.; Oostergetel, G.T.; Boekema, E.J. Subunit and chlorophyll organization of the plant photosystem II supercomplex. Nat. Plants 2017, 3, 1–11.
- Qin, X.; Suga, M.; Kuang, T.; Shen, J.-R. Structural basis for energy transfer pathways in the plant PSI–LHCI supercomplex. Science 2015, 348, 989–995.
- Mazor, Y.; Borovikova, A.; Nelson, N. The structure of plant photosystem I super-complex at 2.8 å resolution. Elife 2015, 4, e07433.
- Alboresi, A.; Caffarri, S.; Nogue, F.; Bassi, R.; Morosinotto, T. In silico and biochemical analysis of physcomitrella patens photosynthetic antenna: Identification of subunits which evolved upon land adaptation. PLoS ONE 2008, 3, e2033.
- Su, X.; Ma, J.; Wei, X.; Cao, P.; Zhu, D.; Chang, W.; Liu, Z.; Zhang, X.; Li, M. Structure and assembly mechanism of plant c2s2m2-type PSII–LHCII supercomplex. Science 2017, 357, 815–820.
- Kovacs, L.; Damkjær, J.; Kereïche, S.; Ilioaia, C.; Ruban, A.V.; Boekema, E.J.; Jansson, S.; Horton, P. Lack of the light-harvesting complex cp24 affects the structure and function of the grana membranes of higher plant chloroplasts. Plant Cell 2006, 18, 3106–3120.
- Crepin, A.; Caffarri, S. Functions and evolution of lhcb isoforms composing LHCII, the major light harvesting complex of photosystem II of green eukaryotic organisms. Curr. Protein Pept. Sci. 2018, 19, 699–713.
- Nosek, L.; Semchonok, D.; Boekema, E.J.; Ilík, P.; Kouřil, R. Structural variability of plant photosystem II megacomplexes in thylakoid membranes. Plant J. 2017, 89, 104–111.
- Crepin, A.; Caffarri, S. The specific localizations of phosphorylated lhcb1 and lhcb2 isoforms reveal the role of lhcb2 in the formation of the PSI–LHCIi supercomplex in arabidopsis during state transitions. Biochim. Biophys. Acta (BBA)-Bioenerg. 2015, 1847, 1539–1548.
- Longoni, P.; Douchi, D.; Cariti, F.; Fucile, G.; Goldschmidt-Clermont, M. Phosphorylation of the light-harvesting complex II isoform lhcb2 is central to state transitions. Plant Physiol. 2015, 169, 2874–2883.
- Pietrzykowska, M.; Suorsa, M.; Semchonok, D.A.; Tikkanen, M.; Boekema, E.J.; Aro, E.-M.; Jansson, S. The light-harvesting chlorophyll a/b binding proteins lhcb1 and lhcb2 play complementary roles during state transitions in arabidopsis. Plant Cell 2014, 26, 3646–3660.
- Pan, X.; Ma, J.; Su, X.; Cao, P.; Chang, W.; Liu, Z.; Zhang, X.; Li, M. Structure of the maize photosystem I supercomplex with light-harvesting complexes I and II. Science 2018, 360, 1109–1113.
- Jenny, A.; Mark, W.; Robin, G.W.; Caroline, A.H.; Alexander, V.R.; Peter, H.; Stefan, J. Absence of the lhcb1 and lhcb2 proteins of the light-harvesting complex of photosystem II–effects on photosynthesis, grana stacking and fitness. Plant J. 2003, 35, 350–361.
- Ruban, A.V.; Wentworth, M.; Yakushevska, A.E.; Andersson, J.; Lee, P.; Keegstra, W.; Dekker, J.; Boekema, E.; Jansson, S.; Horton, P. Plants lacking the main light-harvesting complex retain photosystem ii macro-organization. Nature 2003, 421, 648–652.
- Van Oort, B.; Murali, S.; Wientjes, E.; Koehorst, R.B.; Spruijt, R.B.; van Hoek, A.; Croce, R.; van Amerongen, H. Ultrafast resonance energy transfer from a site-specifically attached fluorescent chromophore reveals the folding of the n-terminal domain of cp29. Chem. Phys. 2009, 357, 113–119.
- Lolkema, J.S.; Boekema, E.J. The a-type atp synthase subunit k of methanopyrus kandleri is deduced from its sequence to form a monomeric rotor comprising 13 hairpin domains. FEBS Lett. 2003, 543, 47–50.
- Seelert, H.; Poetsch, A.; Dencher, N.A.; Engel, A.; Stahlberg, H.; Müller, D.J. Proton-powered turbine of a plant motor. Nature 2000, 405, 418–419.
- Abrahams, J.P.; Leslie, A.; Walker, J. Structure at 2.8 a resolution of f1-atpasem. Nature 1994, 370, 25.
- Groth, G.; Pohl, E. The structure of the chloroplast f1-atpase at 3.2 å resolution. J. Biol. Chem. 2001, 276, 1345–1352.
- Arnold, I.; Pfeiffer, K.; Neupert, W.; Stuart, R.A.; Schägger, H. Yeast mitochondrial f1f0-atp synthase exists as a dimer: Identification of three dimer-specific subunits. EMBO J. 1998, 17, 7170–7178.
- Zhang, H.; Whitelegge, J.P.; Cramer, W.A. Ferredoxin: Nadp+ oxidoreductase is a subunit of the chloroplast cytochrome b6fcomplex. J. Biol. Chem. 2001, 276, 38159–38165.
- Johnson, G.N. Physiology of PSI Cyclic Electron Transport in Higher Plants Bioenergetics. Biochim. Biophys. Acta (BBA)-Bioenerg. 2011, 1807, 384–389.
- Rochaix, J.-D. Reprint of: Regulation of photosynthetic electron transport. Biochim. Biophys. Acta (BBA)-Bioenerg. 2011, 1807, 878–886.
- Iwai, M.; Takizawa, K.; Tokutsu, R.; Okamuro, A.; Takahashi, Y.; Minagawa, J. Isolation of the elusive supercomplex that drives cyclic electron flow in photosynthesis. Nature 2010, 464, 1210–1213.
- Johnson, M.P. Correction: Photosynthesis. Essays Biochem. 2017, 61, 429.
- Buchanan, B.B. Regulation of co2 assimilation in oxygenic photosynthesis: The ferredoxin/thioredoxin system: Perspective on its discovery, present status, and future development. Arch. Biochem. Biophys. 1991, 288, 1–9.
- Dai, S.; Johansson, K.; Miginiac-Maslow, M.; Schürmann, P.; Eklund, H. Structural basis of redox signaling in photosynthesis: Structure and function of ferredoxin: Thioredoxin reductase and target enzymes. Photosynth. Res. 2004, 79, 233–248.
- Howard, T.P.; Metodiev, M.; Lloyd, J.C.; Raines, C.A. Thioredoxin-mediated reversible dissociation of a stromal multiprotein complex in response to changes in light availability. Proc. Natl. Acad. Sci. USA 2008, 105, 4056–4061.
- Lopez-Calcagno, P.E.; Howard, T.P.; Raines, C.A. The cp12 protein family: A thioredoxin-mediated metabolic switch? Front. Plant Sci. 2014, 5, 9.
- Graciet, E.; Gans, P.; Wedel, N.; Lebreton, S.; Camadro, J.-M.; Gontero, B. The small protein cp12: A protein linker for supramolecular complex assembly. Biochemistry 2003, 42, 8163–8170.
- Marri, L.; Trost, P.; Pupillo, P.; Sparla, F. Reconstitution and properties of the recombinant glyceraldehyde-3-phosphate dehydrogenase/cp12/phosphoribulokinase supramolecular complex of arabidopsis. Plant Physiol. 2005, 139, 1433–1443.
- Marri, L.; Trost, P.; Trivelli, X.; Gonnelli, L.; Pupillo, P.; Sparla, F. Spontaneous assembly of photosynthetic supramolecular complexes as mediated by the intrinsically unstructured protein cp12. J. Biol. Chem. 2008, 283, 1831–1838.
- Wedel, N.; Soll, J. Evolutionary conserved light regulation of calvin cycle activity by nadph-mediated reversible phosphoribulokinase/cp12/glyceraldehyde-3-phosphate dehydrogenase complex dissociation. Proc. Natl. Acad. Sci. USA 1998, 95, 9699–9704.
- Marri, L.; Zaffagnini, M.; Collin, V.; Issakidis-Bourguet, E.; Lemaire, S.D.; Pupillo, P.; Sparla, F.; Miginiac-Maslow, M.; Trost, P. Prompt and easy activation by specific thioredoxins of calvin cycle enzymes of arabidopsis thaliana associated in the gapdh/cp12/prk supramolecular complex. Mol. Plant 2009, 2, 259–269.
- Chan, C.Y.; Zhao, H.; Pugh, R.J.; Pedley, A.M.; French, J.; Jones, S.A.; Zhuang, X.; Jinnah, H.; Huang, T.J.; Benkovic, S.J. Purinosome formation as a function of the cell cycle. Proc. Natl. Acad. Sci. USA 2015, 112, 1368–1373.
- Zhao, H.; French, J.B.; Fang, Y.; Benkovic, S.J. The purinosome, a multi-protein complex involved in the de novo biosynthesis of purines in humans. Chem. Commun. 2013, 49, 4444–4452.
- Pedley, A.M.; Benkovic, S.J. A new view into the regulation of purine metabolism: The purinosome. Trends Biochem. Sci. 2017, 42, 141–154.
- Sweetlove, L.J.; Fernie, A.R. The role of dynamic enzyme assemblies and substrate channelling in metabolic regulation. Nat. Commun. 2018, 9, 2136.
- Baresova, V.; Skopova, V.; Sikora, J.; Patterson, D.; Sovova, J.; Zikanova, M.; Kmoch, S. Mutations of atic and adsl affect purinosome assembly in cultured skin fibroblasts from patients with aica-ribosiduria and ADSL deficiency. Hum. Mol. Genet. 2011, 21, 1534–1543.




