Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Martina Kadoić Balaško | + 3763 word(s) | 3763 | 2021-12-24 08:42:00 | | | |
| 2 | Peter Tang | Meta information modification | 3763 | 2022-01-12 07:31:33 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kadoić Balaško, M. Control Practices in Codling Moth. Encyclopedia. Available online: https://encyclopedia.pub/entry/18047 (accessed on 08 February 2026).
Kadoić Balaško M. Control Practices in Codling Moth. Encyclopedia. Available at: https://encyclopedia.pub/entry/18047. Accessed February 08, 2026.
Kadoić Balaško, Martina. "Control Practices in Codling Moth" Encyclopedia, https://encyclopedia.pub/entry/18047 (accessed February 08, 2026).
Kadoić Balaško, M. (2022, January 11). Control Practices in Codling Moth. In Encyclopedia. https://encyclopedia.pub/entry/18047
Kadoić Balaško, Martina. "Control Practices in Codling Moth." Encyclopedia. Web. 11 January, 2022.
Copy Citation
The codling moth (CM) (Cydia pomonella L.) is a key pest in most pome fruit orchards in Croatia and worldwide. This pest, besides apple, also is a pest of pear, walnut, quince and some stone fruits where it causes economic losses in fruit production. The pest is known for having developed resistance to several chemical groups of insecticides, making its control difficult. The control and management of the codling moth is often hindered by a lack of understanding about its biology and ecology, including aspects of its population genetics.
codling moth
resistance mechanisms
genetics
control strategies
anti-resistance program
geometric morphometrics
SNPs
1. Introduction
Origin and Biology of the Codling Moth, Cydia pomonella
The codling moth (CM) (Cydia pomonella L.) is a key pest in most pome fruit orchards in Croatia and worldwide. This pest, besides apple, also is a pest of pear, walnut, quince and some stone fruits where it causes economic losses in fruit production [1]. Balachowsky and Mesnil [2] were the first to mention CM, and provided data on its origin and damages caused to fruit historically. In Croatia, according to Kovačević [3], CM has been present since ancient times. In North America, it is known that the pest was introduced ca. 1750 [4]. CM was originally from Eurasia, most likely Kazakhstan, but interestingly it was not reported in China until 1953 [5]. Over the last two centuries it dispersed globally with the cultivation of apples and pears. Currently, CM is present in South America, South Africa, Australia and New Zealand [6]. CM occurs in almost every country where apples are grown, and it has achieved a nearly cosmopolitan distribution, being one of the most successful pest insect species known today [7].
CM adults are small (~10 mm in length). They can be distinguished from other moths associated with fruit trees by their dark brown wingtips that have shiny, coppery markings [8]. It overwinters as a fully grown larva within a thick, silken cocoon that can be found under loose scales of bark and in the soil or debris around tree bases [9]. The larvae pupate inside their cocoons in early spring when temperatures exceed 10 °C. Depending on ambient temperature, pupal development occurs within 7–30 days. For the development of adults, the sum of 100 degree-days measured from the 1st of January are required [10]; this value is usually attained at the end of April (i.e., northern hemisphere growing season). For one whole generation of CM, the sum of 610 degrees is required for the complete development of the insect, i.e., from eggs until the appearance of adult moths [10]. A second generation appears after ten days and its flight and egg laying lasts from mid-July to mid-August. Diapausing larvae overwinter in their hibernacula, pupate and then emerge the following spring [11].
The CM has adapted successfully to different habitats by forming various ecotypes, often designated by the term ‘strains’, which differ among each other in several morphological, developmental and physiological features [12]. On apples and pears, larvae penetrate fruit and bore into the core, leaving brown-colored holes in the fruit that are filled with frass (larval droppings) [8]. If chemical treatment is not used during production, CM can cause a decrease in apple harvest from 30% up to 50%. For apples, intensive production tolerates 1% of infested fruit. Producers, with various methods of fruit protection, try to lower that number below 0.5% [1][3].
2. Insecticides Resistance
In apple orchards, 70% of insecticides used are to control CM [6]. CM control is achieved using various neuroactive products such as organophosphates, carbamates, synthetic pyrethroids, neonicotinoids, and insect growth regulators (IGR). The CM is a very plastic species and easily adapts to different climatic conditions including the development of resistance to various groups of synthetic insecticides in the USA and Europe [6][13][14][15]. According to May and Dobson [16], the spread of resistance in insect populations depends on multiple factors, including: the intensity of insecticide selection pressure, the migration ability of individuals, and the fitness costs linked with resistance. In the CM, the first case of resistance recorded was to arsenates in 1928 in the USA [17]. Since then, new cases of resistance have been reported in almost all of the main apple-growing regions worldwide [13][18][19][20]. During the 1980s and 1990s CM control in Europe was achieved using broad spectrum insecticides (pyrethroids and organophosphates [OP]), however, the evolution of pesticide resistance efficacy for these insecticides diminished quickly [13][15][21][22]. Reyes et al. [23] states that insecticide resistance in CM in Europe was first detected ca. 1990 to diflubenzuron (in Italy and southeastern France); further pesticide control failures were observed in Switzerland and Spain. CM populations are now resistant to neonicotinoids including environmentally friendly avermectins [23]. Further, CM has developed resistance to azinphos-methyl and tebufenozide in post-diapausing larval stages, to OP [24] insecticides and more recently to insect growth regulators (IGRs). Resistance is mainly associated with the detoxification system’s mixed-function oxidases (MFO), glutathione-S-transferases (GST) and esterases (EST) [13][23][25]. A kdr mutation in the voltage-dependent sodium channel is involved in resistance to pyrethroids [26] and an acetylcholinesterase (AChE) mutation has been identified in a laboratory strain selected for resistance to azinphos-methyl [27]. Evidently, the last 20 years’ usage of chemical insecticides has modified the development of resistance [6]. An additional problem appeared in the mid-1990s with the development of cross-resistance due to the CM becoming resistant to several chemical groups of insecticides simultaneously [28].
Baculoviruses are insect pathogenic viruses that are widely used as biological control agents of insect pests in agriculture. One of the most important commercially used baculoviruses is the Cydia pomonella granulovirus (CpGV) [29]. For more than 30 years, commercial CpGV products have been successfully applied to control CM in organic and integrated fruit production. For all European CpGV products, the original Mexican isolate described by Tanada in 1964, CpGV-M, has been used [29]. According to Harison and Hoover [30], a granulovirus (GV) was identified from CM cadavers and found to be a type 2 GV that killed larvae in three to four days at higher concentrations. After promising field tests as a control measure in 1968 and 1977 [31][32], CpGV was developed into several control products in Europe and in North America. CpGV is used to control CM on over 100,000 ha of organic and conventional apple orchards in Europe [33][34]. Since 2005, resistance against the widely used isolate CpGV-M has been reported from different countries in Europe [33][35][36]. In a multination monitoring program, Schulze-Bopp and Jehle [37] identified that 70% of CM were resistant or partly resistant to CpGV across multiple orchards in Germany, Austria, Switzerland, Italy, and the Netherlands. The recent research by Sauer et al. [38] described autosomal and dominant inheritance of this resistance and demonstrated cross-resistance to different CpGV genome groups. The same authors report a CM field population with a new type of resistance, which appears to follow a highly complex inheritance in regards to different CpGV isolates [39]. In the European Union (EU) there are no strategic integrated pest management (IPM) programs that solve the current confusion surrounding CM control and resistance. There is a need for new control tools and a fresh approach to CM control and management in the EU.
3. Present Strategies in Codling Moth Suppression
3.1. Mechanical Control
Because of resistance development in CM populations, there is a need for alternatives to insecticides and CpGV. In recent studies, special attention is given to insect exclusion netting systems in apple production. The first netting system was designed in France in 2005 and in 2008 it was introduced in Italy. In both countries, a high level of efficacy of nets was observed against CM, especially for the ‘single-row’ system, which the authors recommend because it was more efficient and more durable than the ‘whole-orchard’ version. Also, this method enables a significant reduction in pesticide use without any major risks for apple production [40]. Pajač Živković et al. [41] tested the effectiveness of insect exclusion netting systems in preventing the attack of CM on apple fruits in Croatia. The authors showed a significant reduction in CM catches and also fruit injury compared to the non-netted control. This is consistent with similar studies in which nets significantly reduced the number of CM catches [42][43].
3.2. Chemical Control
Chemical control of CM is still the main method used in integrated pome fruit production [44]. According to the Insecticide Resistance Action Committee (IRAC) [45] for CM control in most countries, there are 11 modes of action (MoA) available on the market depending on the country. For CM, some insecticides affect the nervous system, or pest growth and development. Acetylcholinesterase inhibitors (carbamates and organophosphates), sodium channel modulators (pyrethroids), nicotinic acetylcholine receptor agonists (neonicotinoids), nicotinic acetylcholine receptor agonists allosteric modulators (spinosyns), chloride channel activators (avermectins), voltage-dependent sodium channel blockers (oxadiazines) and ryanodine receptor modulators (diamides) all affect the pest’s nervous system; these insecticides are fast-acting [45]. Juvenile hormone mimics (phenoxyphenoxy-ethylcarbamate), chitin biosynthesis inhibitors—type 0 (benzonylureas) and ecdysone agonists (diacylhydrazines) all affect pest growth and development [45]. Insect development is controlled by juvenile hormones and ecdysone by directly perturbing cuticle formation/deposition or lipid biosynthesis. Such insect growth regulators are generally slow to moderately-slow acting [45].
The classic model of CM suppression implies the intense application of aggressive chemical preparations, most commonly a wide spectrum of activity. Due to the altered biology of the CM (i.e., more generations/year) insecticides must be applied several times per season [46][47]. Some populations of CM have gained simultaneous resistance to several chemical subgroups of insecticides. In light of this and to delay resistance development, the rotation of compounds from different MoA groups ensures that repeated selection with compounds from any single MoA group is minimized. By rotation of insecticides across all available classes, selection pressure for the evolution of any type of resistance is minimized and the development of resistance will be delayed or prevented. The presence of kdr resistance renders pyrethroids less effective, whereas carbamates and organophosphates can still be used. In addition, the use of larvicides such as the organophosphate in conjunction with pyrethroids can support resistance management through rotation of MoA across different life stages. Effective long-term resistance management is important, but many factors have to be considered (including regional availability of insecticides). Currently, there are eight MoAs for CM control. In practice, it should not be difficult to implement rotation programs because there are enough active substances of insecticides in Europe that have mandated approval for CM. Alternatives to more persistent molecules are being developed [48][49].
3.3. Biological Control
Biological control agents play a key role in most IPM strategies; these include entomopathogens, parasitoids and predators [50]. For augmentative biological control of CM, viruses such as granulovirus and entomopathogenic nematodes (EPNs) (Steinernema carpocapsae, Steinernema feltiae, Heterohabditis spp.) have been used as microbial agents [51].
The most widely used biopesticide is Bacillus thuringiensis (Bt) [52]. For controlling CM, Bt is very limited because of the improbability of ingesting a lethal dose of Bt toxin during feeding by neonate larvae [50]. On the other hand, granulovirus (GV) (Baculoviridae) is one of the most efficient and highly selective pathogens for suppression of CM. Its specificity for CM and safety to non-target organisms is documented by Lacey et al. [53]. It is one of the most virulent baculoviruses known. According to Laing and Jaques (1980) and Huber (1986), the LD50 for neonate larvae has been estimated at 1.2 to 17 granules/larva. The biggest disadvantage of CpGV is its sensitivity to solar radiation [54][55][56], and the need for frequent reapplication.
Parasitoids are insects whose larvae feed and develop within or on the bodies of other arthropods. Each parasitoid larva develops on a single individual and eventually kills that host [44]. Parasitoid wasps from the families Braconidae (Ascogaster quadridentata and Microdes rufipes), Ichneumonidae (Mastrus ridibundus and Liotryphon caudatus) and Trichogrammatidae (Trichogramma sp.) are the best known parasitoid species of CM. The parasitism of entomophagous wasps M. ridibundus and A. quadradentata has been successfully applied in CM control in some US states [50]. Species from Braconidae most commonly parasitize CM larvae, and Ichneumonidae parasitize CM larvae and adults and Trichogrammatidae parasitize eggs of Tortricidae moths. A reduction of 53–84% of CM was achieved by the experimental release of two Trichogramma species (T. dendrolimi and T. embryophagum) in apple orchards in Germany [44]. An additional benefit of the release of parasitoids is the simultaneous control of other pest species in apple orchards. The beneficial organisms alone can play an effective role in IPM but in general, the effect on CM control in economically productive orchards is considered insufficient [57].
For biological control, the most promising EPN species for suppression of CM are from the families Steinernematidae and Heterorhabditidae [58]. Species from both families are obligatorily associated with symbiotic bacteria (Xenorhabdis spp. and Photorhabdis spp., respectively) which are known for quickly killing its host insect. The most promising results for CM control have been with Steinernema feltiae and Steinernema carpocapsae [59]. Cocooned overwintering CM larva is the life stage most practical to control using EPNs. That life stage occurs between late summer and early spring in cryptic habitats, such as underneath loose pieces of bark or in pruning wounds on trees [59]. Eliminating cocooned larvae would protect fruit from damage in the following growing season [60]. The main obstacles for successful CM control with EPNs are low fall temperatures and desiccation of the infective juvenile stage of EPNs before they have penetrated the host’s cocoon.
3.4. Population Genetic Monitoring
Analysis of population genetic structure is a key aspect in understanding insect pest population dynamics in agriculture [61]. The development of effective pest management strategies relies on a multidisciplinary approach [62] and one component of this is knowledge of the population genetics of the pest. Genetic structure and patterns of dispersal at the local and landscape scale are important for establishing a control strategy for insect pests [63]. Understanding the population genetics of CM invasions enables identification of the geographic origin, number of introduction events and the spread of the infestation [64]. According to Keil et al. [65] CM populations are composed of mobile and sedentary genotypes and this has direct consequences for the local observable population dynamics of the species as well as the implementation of new behavior-based pest management measures (e.g., mating disruption, attract-and-kill and SIT technique) [66]. The first attempt to elucidate the population genetic structure of CM on a global geographic scale (i.e., inter-continental) using allozymes was conducted by Pashley and Bush [67]. These authors showed that CM populations were not differentiated among countries investigated (FST: 0.05). Following this, Bues and Toubon [68] used the same approach to study populations in Switzerland and France. More recently, Timm et al. [69] and Thaler et al. [7] used amplified fragment length polymorphism (AFLP) markers to study the molecular phylogeny and genetic structure of CM where they found large differences among these populations (FST: 0.70). More recently, co-dominant microsatellite markers from CM were developed by Zhou et al. [70] who characterized 17 loci. An additional 24 microsatellite loci were characterized by Frank et al. [71], with these loci most frequently used in population genetic studies worldwide [6][72][61][63][73].
They found ascertained loss of genetic diversity and important structuring related to distribution, however no important correlation between genetic distance and geographic distance among populations (FST: 0.22091) was found. Voudouris et al. [74] used 11 microsatellite loci to analyze nine CM populations from Greece and six from France for comparison. Results from Bayesian clustering and genetic distance analyses separated CM populations in two genetic clusters. In agreement with previous published studies FST values showed low genetic differentiation among populations (Greek populations FST: 0.009 and FST: 0.0150 French populations).
Dispersal of fertilized females is important because it directly affects the effectiveness of pest control programs. Margaritopoulos et al. [75] used the mark-release-recapture (MRR) method on male and female individuals from two laboratory and one wild CM populations. Kinship analysis was made on 303 genotyped individuals (11 microsatellite loci) from two contiguous apple orchards to see the dispersal patterns in the Greek CM populations. The collected data confirm the view of the sedentary nature of CM and indicate that genotypes able to migrate at long distances are not present in the studied area. The information obtained could be fundamental for determining the dynamics and genetics of the pest populations and for developing efficient management programs. Results about the dispersal pattern of codling moths might have practical applications in mating disruption or mass trapping pest control programs.
3.5. Area-Wide Integrated Pest Management
The 5-year CAMP (CM Area-Wide Management Program) was the first of the area-wide programs initiated by the US Department of Agriculture [76]. Demonstration of this was initiated in 1995 in a multi-institutional program created through the collaboration of university and government researchers in Washington, Oregon and California. The goal of this program was to implement, assess, research and educate industry users about promising new IPM technologies. CAMP was highly successful in fueling the rapid adoption of a new paradigm in orchard pest management that resulted in significant reduction in fruit injury using nearly 80% less broad-spectrum insecticides [77].
IPM is based on environmentally and toxicological acceptable treatments. Using pheromones, attract-and-kill methods and mating disruption results in a promising way of controlling CM. According to Witzgall et al. [78], orchard treatments with up to 100 g of synthetic pheromone per hectare effectively control CM populations over the entire growing season. The disadvantage of these techniques is that females are not affected [79].
Mass trapping, as one of the first mating control strategies, can significantly reduce CM damage levels. However, several intensive field studies have shown that it is not effective enough for CM control because of the low damage thresholds (no more than 1–2% of the crop) required in commercial apple growing. Since adequate control cannot be achieved by using only mass trapping, there is a need for combining it with other control measures [80]. Another problem is the cost and practical difficulties of deploying sufficient trapping stations. If droplets containing sex pheromones and a fast-acting insecticide are used instead of traps [81], then the costs can be substantially reduced. The potential strength of the approach is that males have been removed from the system, stopping their ability to find a mate.
The attract-and-kill method, in its technically simplest form is the attractant applied as a ‘tank-mix’ with an insecticide. This method uses the same attractants as mass trapping but in an envelope impregnated with an insecticide on the outside. This technology has shown efficacy in the control of several important lepidopteran pests including pink bollworm, Pectinophora gossypiella (Saunders), light brown apple moth, Epiphyas postvittana (Walker), and CM [82]. In both systems, mass trapping and attract-and-kill, chemicals are utilized only when the population increases considerably [83].
For AW-IPM the integration of sterile insects is a very effective and environmentally friendly control tactic that can be combined with other control practices and offers great potential [84][85]. Sterile insect technique (SIT) is non-destructive to the environment, does not affect non-target organisms, and can easily be integrated with other biological control methods such as parasitoids, predators and pathogens [86]. The technique has gained traction in the last few decades [87][88]. SIT is an autocidal pest control technique that controls pests with a form of birth control [86]. The target pest species is mass-reared, sterilized through the use of gamma radiation and then released in the target area in high numbers. After release, sterile males will locate and mate with wild females and transfer the infertile sperm thus reducing the wild population. Another method of sterilization is genetic manipulation or sexing strains, where lethal mutations are incorporated into sperm [86]. The SIT, together with mating disruption, granulosis virus and EPNs, are the options that offer great potential as cost-effective additions to accessible management techniques for AW-IPM approaches.
4. Resistance Management Strategies
The most effective strategy to combat insecticide resistance is to do everything possible to prevent it from occurring in the first place. To this end, crop specialists recommend insect resistance management (IRM) programs as one part of a larger (IPM) approach covering three basic components: monitoring pest complexes in the field for changes in population density, focusing on economic injury levels and integrating multiple control strategies. IRM is the scientific approach of managing pests long term and preventing or delaying pest evolution towards pesticide resistance and minimizing the negative impacts of resistance on agriculture [89]. The basic strategy for IRM is to incorporate as many different control strategies as possible for particular pests including the use of synthetic insecticides, biological insecticides, beneficial insects (predators/parasitoids), cultural practices, transgenic plants (where allowed), crop rotation, pest-resistant crop varieties, and chemical attractants or deterrents. The establishment of an anti-resistance program in perennial crops is slightly more difficult than in arable crops where crop rotation is possible. If non-chemical methods provide satisfactory pest control, preference should be given to them over chemical methods. Key insect pests of apple and grape such as CM and grapevine moths are effectively controlled via mating disruption. In Switzerland, mating disruption is in use in 50% of the apple orchards and 60% of vineyards, and this has enabled a reduction of synthetic pesticide use by two thirds [90].
Insecticides, if necessary, must be selected with care and their impact on future pest populations considered. Broad-spectrum insecticides should always be avoided when a more specific insecticide will suffice. Even cultural practices, such as irrigation for destroying overwintering stages (e.g., cotton bollworm, Helicoverpa armigera) of pests can play a role in managing resistance [91]. When insecticide is applied it should be timed correctly and for the best efficacy, it should target the most vulnerable life stage of the insect pest. It is important to mix and apply insecticides carefully. With the increasing problem of resistance, there is no space for error in terms of insecticide dose, timing, coverage, etc.
Reducing doses, application frequency, and resorting to the partial application of pesticides contribute to the IPM goal of reducing or minimizing risks to human health and the environment. Regular monitoring for insecticide resistance is essential to react proactively to prevent insecticide resistance from compromising control [92].
Before applying any CM control action, it is necessary to monitor CM occurrence and early infestation of apples. Pheromone traps are used in orchards to determine the present amount of adult male moths. For estimating the potential infestation risk of the second generation, it is recommended to examine 1000 young apples in June for damage or the presence of CM [93]. Spray thresholds are also based on the number of moths in the pheromone traps or on infestation rates detected in the harvest of the current or last season. For apples, the economic threshold for the CM is 1% of infested fruit [94].
Figure 1 shows recommendations for effective CM control and resistance management based on current knowledge: I. to monitor; II. application of ecotoxicological favorable protection measures like mating disruption (when CM population levels are low); III. application of chemical control measurements (if necessary); and IV. control of overwintering stages by applying biological agents (e.g., CpGV, nematodes) to reduce the late summer and fall CM population in order to minimize the population in the following growing season. It is an effective example of how resistance management should work in orchards (Figure 1).
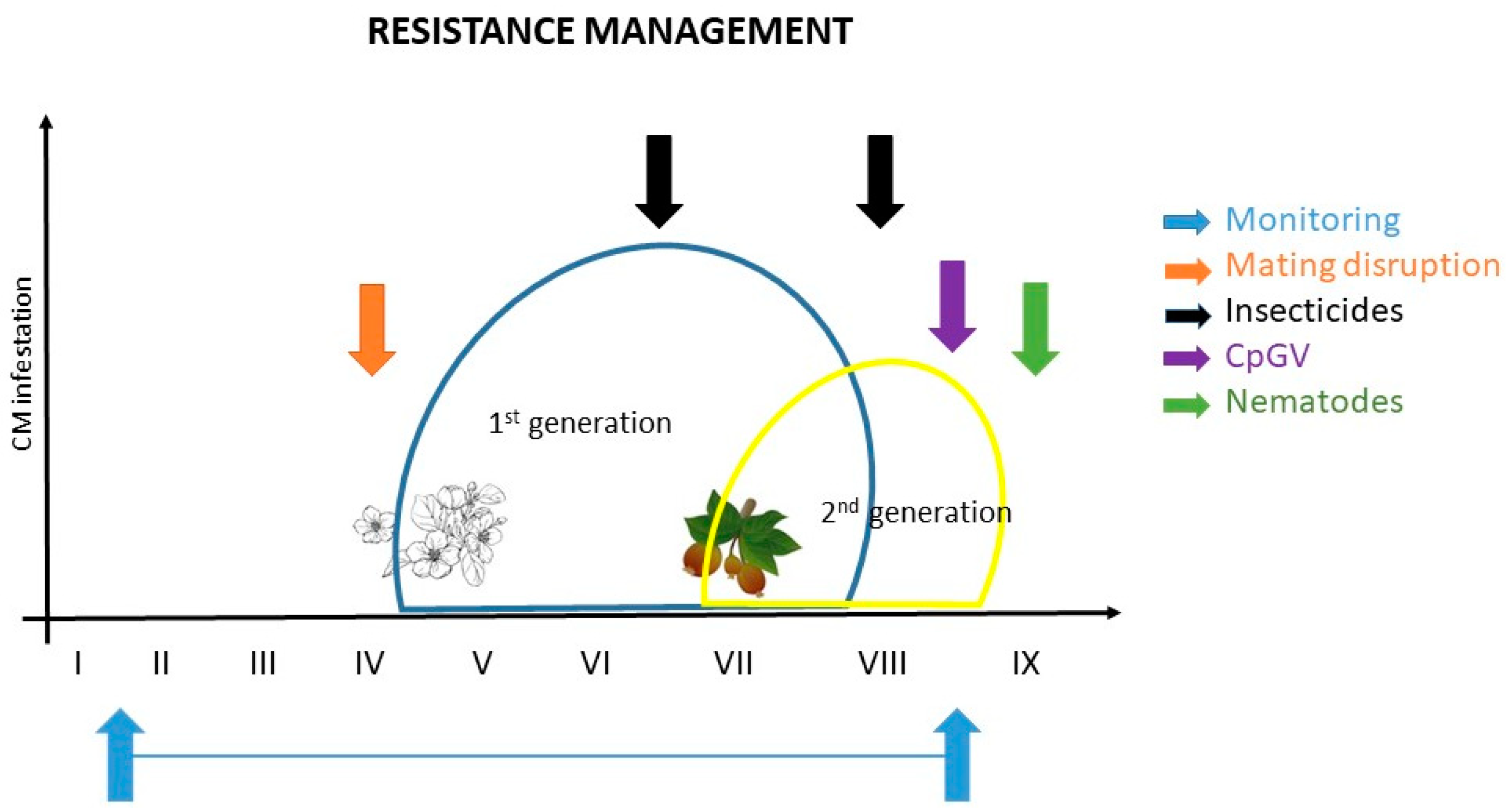
Figure 1. Example of resistance management for codling moth; the ideal control is a combination of different measures (modified by Martina Kadoić Balaško).
References
- Ciglar, I. Integrirana Zaštita Voćaka i Vinove Loze, 1st ed.; Zrinski: Čakovec, Croatia, 1998; pp. 82–87.
- Balachowsky, A.; Mesnil, L. Les Insectes Nuisibles Aux Plantes Cultivées; Ministère de L’ Agriculture: Paris, France, 1935; pp. 130–158.
- Kovačević, Ž. Applied Entomology, 2nd ed.; University of Zagreb: Zagreb, Croatia, 1952; pp. 312–319.
- Slingerland, M.V. Codling moth in New England in 1750. N. Y. Agric. Exp. 1898, 142, 85–155.
- Zhang, X.Z. Taxonomic notes on the codling moth, Carpocapsa pomonella L. In Sinkiang. Acta Entomol. Sin. 1957, 7, 467–472.
- Franck, P.; Reyes, M.; Olivares, J.; Sauphanor, B. Genetic architecture in codling moth populations: Comparison between microsatellite and insecticide resistance markers. Mol. Ecol. 2007, 16, 3554–3564.
- Thaler, R.; Brandstätter, A.; Meraner, A.; Chabicovski, M.; Parson, W.; Zelger, R.; Dalla Via, J.; Dallinger, R. Molecular phylogeny and population structure of the codling moth (Cydia pomonella) in Central Europe: II. AFLP analysis refl ects human-aided local adaptation of a global pest species. Mol. Phylogenet. Evol. 2008, 48, 838–849.
- Maceljski, M. Jabučni savijač (Cydia/Laspeyresia, Carpocapsa, Grapholita/ pomonella L.). In Poljoprivredna Entomologija, 2nd ed.; Zrinski: Čakovec, Croatia, 2002; pp. 302–309.
- Alford, D.V. A Color Atlas of Fruit Pests Their Recognition, Biology, and Control; Wolfe Publishing: Prescott, AZ, USA, 1984.
- Wildbolz, T. Über Möglichkeiten der Prognose und Befallsüberwachung und Über Toleranzgrenzen bei der Integrierten Schädlingsbekämpfung im Obstbau. Entomophaga 1962, 7, 273–283.
- Higbee, B.S.; Calkins, C.O.; Temple, C.A. Overwintering of codling moth (Lepidoptera: Tortricidae) larvae in apple harvest bins and subsequent moth emergence. J. Econ. Entomol. 2001, 94, 1511–1517.
- Meraner, A.; Brandstätter, A.; Thaler, R.; Aray, B.; Unterlechner, M.; Niederstätter, H.; Parson, W.; Zelger, R.; Dalla Via, J.; Dallinger, R. Molecular phylogeny and population structure of the codling moth (Cydia pomonella) in Central Europe: I. Ancient clade splitting revealed by mitochondrial haplotype markers. Mol. Phylogenet. Evol. 2008, 48, 825–837.
- Sauphanor, B.; Brosse, V.; Bouvier, J.C.; Speich, P.; Micoud, A.; Martinet, C. Monitoring resistance to diflubenzuron and deltamethrin in French codling moth populations (Cydia pomonella). Pest Manag. Sci. 2000, 56, 74–82.
- Boivin, T.; Chabert D’Hières, C.; Bouvier, J.C.; Beslay, D.; Sauphanor, B. Pleiotropy of insecticide resistance in the codling moth, Cydia pomonella. Entomol. Exp. Appl. 2001, 99, 381–386.
- Bouvier, J.C.; Buès, R.; Boivin, T.; Boudinhon, L.; Beslay, D.; Sauphanor, B. Deltamethrin resistance in the codling moth (Lepidoptera: Tortricidae): Inheritance and number of genes involved. Heredity 2001, 87, 456–462.
- May, R.M.; Dobson, A.P. Population dynamics and the rate of evolution of pesticide resistance. In Pesticide Resistance: Strategies and Tactics for Management; National Academy Press: Washington, DC, USA, 1986; pp. 170–193.
- Hough, W.S. Relative resistance to arsenical poisoning of two codling moth strains. J. Econ. Entomol. 1928, 21, 325–329.
- Thwaite, W.G.; Williams, D.G.; Hately, A.M. Extent and significance of azinphos-methyl resistance in codling moth in Australia. Pest Control. Sustain. Agric. 1993, 93, 166–168.
- Sauphanor, B.; Bouvier, J.C.; Brosse, V. Spectrum of insecticide resistance in Cydia pomonella (Lepidoptera: Tortricidae) in South-eastern France. J. Econ. Entomol. 1998, 91, 1225–1231.
- Reuveny, H.; Cohen, E. Resistance of the codling moth Cydia pomonella (L.) (Lep Tortricidae) to pesticides in Israel. J. Appl. Entomol. 2004, 128, 645–651.
- Sauphanor, B.; Cuany, A.; Bouvier, J.C.; Brosse, V.; Amichot, M.; Berge, J.B. Mechanism of resistance to deltametrin in Cydia pomonella (L.) (Lepidoptera: Tortricidae). Pestic. Biochem. Physiol. 1997, 58, 109–117.
- Stara, J.; Naďova, K.; Kocourek, F. Insecticide resistance in the codling moth (Cydia pomonella). J. Fruit Ornam. Plant Res. 2006, 14, 99–106.
- Reyes, M.; Franck, P.; Charmillot, P.J.; Ioriatti, C.; Olivares, J.; Pasqualini, E.; Sauphanor, B. Diversity of insecticide resistance mechanisms and spectrum in European populations of the codling moth, Cydia pomonella. Pest Manag. Sci. 2007, 63, 890–902.
- Reyes, M.; Barros-Parada, W.; Ramírez, C.C.; Fuentes-Contreras, E. Organophosphate resistance and its main mechanism in populations of codling moth (Lepidoptera: Tortricidae) from Central Chile. J. Econ. Entomol. 2015, 108, 277–285.
- Bush, M.R.; Abdel-All, Y.A.; Rock, G.C. Parathion resistance and esterase activity in codling moth (Lepidoptera: Tortricidae) from North Carolina. J. Econ. Entomol. 1993, 86, 660–666.
- Brun-Barale, A.; Bouvier, J.C.; Pauron, D.; Bergé, J.B.; Sauphanor, B. Involvement of a sodium channel mutation in pyrethroid resistance in Cydia pomonella L., and development of a diagnostic test. Pest Manag. Sci. 2005, 61, 549–554.
- Cassanelli, S.; Reyes, M.; Rault, M.; Manicardi, G.C.; Sauphanor, B. Acetylcholinesterase mutation in an insecticide-resistant population of the codling moth Cydia pomonella (L.). Insect Biochem. Mol. Biol. 2006, 36, 642–653.
- Pajač, I.; Barić, B.; Šimon, S.; Mikac, K.M.; Pejić, I. An initial examination of the population genetic structure of Cydia pomonella (Lepidoptera: Tortricidae) in Croatian apple orchards. J. Food Agric. Environ. 2011, 9, 459–464.
- Herniou, E.A.; Luque, T.; Chen, X.; Vlak, J.M.; Winstanley, D.; Cory, J.S.; O’Reilly, D.R. Use of whole genome sequence data to infer baculovirus phylogeny. J. Virol. 2001, 75, 8117–8126.
- Harrison, R.L.; Hoover, K. Baculoviruses and other occluded insect viruses. In Insect Pathology, 2nd ed.; Vega, F.E., Kaya, H.K., Eds.; Academic Press: London, UK, 2012; pp. 73–131.
- Falcon, L.A.; Kane, W.R.; Bethell, R.S. Preliminary evaluation of a granulosis virus for control of the codling moth. J. Econ. Entomol. 1968, 61, 1208–1213.
- Huber, J.; Dickler, E. Codling moth granulosis virus: It’s efficiency in the field in comparison with organophosphorus insecticides. J. Econ. Entomol. 1977, 70, 557–561.
- Asser-Kaiser, S.; Fritsch, E.; Undorf-Spahn, K.; Kienzle, J.; Eberle, K.E.; Gund, N.A.; Reineke, A.; Zebitz, C.P.W.; Heckel, D.G.; Huber, J.; et al. Rapid emergence of baculovirus resistance in codling moth due to dominant, sex-linked inheritance. Science 2007, 317, 1916–1918.
- Eberle, K.E.; Jehle, J.A. Field resistance of codling moth against Cydia pomonella granulovirus (CpGV) is autosomal and incompletely dominant inherited. J. Invertebr. Pathol. 2006, 93, 201–206.
- Schmitt, A.; Bisutti, I.L.; Ladurner, E.; Benuzzi, M.; Sauphanor, B.; Kienzle, J.; Zingg, D.; Undorf-Spahn, K.; Fritsch, E.; Huber, J.; et al. The occurrence and distribution of resistance of codling moth to Cydia pomonella granulovirus in Europe. J. Appl. Entomol. 2013, 137, 641–649.
- Zichová, T.; Stará, J.; Kundu, J.K.; Eberle, K.E.; Jehle, J.A. Resistance to Cydia pomonella granulovirus follows a geographically widely distributed inheritance type within Europe. Biocontrol 2013, 58, 525–534.
- Schulze-Bopp, S.; Jehle, J.A. Development of a direct test of baculovirus resistance in wild codling moth populations. J. Appl. Entomol. 2013, 137, 153–160.
- Sauer, A.J.; Fritsch, E.; Undorf-Spahn, K.; Nguyen, P.; Marec, F.; Heckel, D.G.; Jehle, J.A. Novel resistance to Cydia pomonella granulovirus (CpGV) in codling moth shows autosomal and dominant inheritance and confers cross-resistance to different CpGV genome groups. PLoS ONE 2017, 12, e0179157.
- Sauer, A.J.; Schulze-Bopp, S.; Fritsch, E.; Undorf-Spahn, K.; Jehle, J.A. A third type of resistance to Cydia pomonella granulovirus in codling moths shows a mixed Z-linked and autosomal inheritance pattern. Appl. Environ. Microbiol. 2017, 83, e01036-17.
- Alaphilippe, A.; Capowiez, Y.; Severac, G.; Simon, S.; Saudreau, M.; Caruso, S.; Vergnani, S. Codling moth exclusion netting: An overview of French and Italian experiences. IOBC-WPRS Bull. 2016, 112, 31–35.
- Pajač Živković, I.; Jemrić, T.; Fruk, M.; Buhin, J.; Barić, B. Influence of different netting structures on codling moth and apple fruit damages in northwest Croatia. Agric. Conspec. Sci. 2016, 81, 99–102.
- Tasin, M.; Demaria, D.; Ryne, C.; Cesano, A.; Galliano, A.; Anfora, G.; Ioriatti, C.; Alma, A. Effect of anti-hail nets on Cydia pomonella behavior in apple orchards. Entomol. Exp. Appl. 2008, 129, 32–36.
- Sauphanor, B.; Severac, G.; Maugin, S.; Toubon, J.F.; Capowiez, Y. Exclusion netting may alter reproduction of the codling moth (Cydia pomonella) and prevent associated fruit damage to apple orchards. Entomol. Exp. Appl. 2012, 145, 134–142.
- Sauer, A.J. Novel Types of Resistance of Codling Moth to Cydia pomonella Granulovirus. Ph.D. Thesis, Technische Universität, Darmstadt, Germany, 2017.
- Insecticide Resistance Action Committee (IRAC). Codling Moth, Cydia pomonella. Available online: https://www.irac-online.org/pests/cydia-pomonella (accessed on 20 August 2019).
- Gonzalez, D.C. Cydia pomonella (L.) Behavior and Responses to Host Volatiles. Ph.D. Thesis, Department de Quimica, Universitat de Lleida, Lleida, Spain, 2007.
- Lacey, L.A.; Thomson, D.; Vincent, C.; Arthurs, S.P. Codling moth granulovirus: A comprehensive review. Biocontrol Sci. Technol. 2008, 18, 639–663.
- Czaja, K.; Góralczyk, K.; Struciński, P.; Hernik, A.; Korcz, W.; Minorczyk, M.; Łyczewska, M.; Ludwicki, J.K. Biopesticides-towards increased consumer safety in the European Union. Pest Manag. Sci. 2015, 71, 3–6.
- Gerwick, B.C.; Sparks, T.C. Natural products for pest control: An analysis of their role, value and future. Pest Manag. Sci. 2014, 70, 1169–1185.
- Lacey, L.A.; Unruh, T.R. Biological control of codling moth (Cydia pomonella, Lepidoptera: Tortricidae) and its role in integrated pest management, with emphasis on entomopathogens. Vedalia 2005, 12, 33–60.
- Bassi, A.; Rison, J.L.; Wiles, J.A. Chlorantraniliprole (DPX-E2Y45, Rynaxypyr®, Coragen®), a new diamide insecticide for control of codling moth (Cydia pomonella), Colorado potato beetle (Leptinotarsa decemlineata) and European grapevine moth (Lobesia botrana). Nova Goric. 2009, 4, 39–45.
- Lacey, L.A.; Frutos, R.; Kaya, H.K.; Vail, P. Insect pathogens as biological control agents: Do they have a future? Biol. Contr. 2001, 21, 230–248.
- Lacey, L.A.; Arthurs, S.P.; Headrick, H. Comparative activity of the codling moth granulovirus against Grapholita molesta and Cydia pomonella (Lepidoptera: Tortricidae). J. Entomol. Soc. Br. Columbia 2005, 102, 79–80.
- Arthurs, S.P.; Lacey, L.A. Field evaluation of commercial formulations of the codling moth granulovirus (CpGV): Persistence of activity and success of seasonal applications against natural infestations in the Pacific Northwest. Biol. Control 2004, 31, 388–397.
- Lacey, L.A.; Arthurs, S.P.; Knight, A.; Becker, K.; Headrick, H. Efficacy of codling moth granulovirus: Effect of adjuvants on persistence of activity and comparison with other larvicides in a Pacific Northwest apple orchard. J. Entomol. Sci. 2004, 39, 500–513.
- Lacey, L.A.; Arthurs, S.P. New method for testing solar sensitivity of commercial formulations of the granulovirus of codling moth (Cydia pomonella, Tortricidae: Lepidoptera). J. Invertebr. Pathol. 2005, 90, 85–90.
- Thorpe, P.T.; Pryke, J.S.; Samways, M.J. Review of ecological and conservation perspectives on future options for arthropod management in Cape Floristic Region pome fruit orchards. Afr. Entomol. 2016, 24, 279–306.
- Georgis, R.; Koppenhöfer, A.; Lacey, L.; Belair, G.; Duncan, L.; Grewal, P.; Samish, M.; Torr, P.; van Tol, R. Successes and failures of entomopathogenic nematodes. Biol. Contr. 2006, 38, 103–123.
- Lacey, L.A.; Unruh, T.R. Entomopathogenic nematodes for control of codling moth: Effect of nematode species, dosage, temperature and humidity under laboratory and simulated field conditions. Biol. Contr. 1998, 13, 190–197.
- Lacey, L.A.; Granatstein, D.; Arthurs, S.P.; Headrick, H.; Fritts, J.R. Use of entomopathogenic nematodes (Steinernematidae) in conjunction with mulches for control of overwintering codling moth (Lepidoptera: Tortricidae). J. Entomol. Sci. 2006, 41, 107–119.
- Franck, P.; Timm, A.E. Population genetic structure of Cydia pomonella: A review and case study comparing spatiotemporal variation. J. Appl. Entomol. 2010, 134, 191–200.
- Blommers, L.H. Integrated pest management in European apple orchards. Annu. Rev. Entomol. 1994, 39, 213–241.
- Fuentes-Contreras, E.; Espinoza, J.L.; Lavandero, B.; Ramírez, C.C. Population genetic structure of codling moth (Lepidoptera: Tortricidae) from apple orchards in central Chile. J. Econ. Entomol. 2008, 101, 190–198.
- Roderick, G.K. Geographic structure of insect populations: Gene flow, phylogeography, and their uses. Annu. Rev. Entomol. 1996, 41, 325–352.
- Keil, S.; Gu, H.; Dorn, S. Response of Cydia pomonella to selection on mobility: Laboratory evaluation and field verifi caion. Ecol. Entomol. 2001, 26, 495–501.
- Dorn, S.; Schumacher, P.; Abivardy, C.; Meyhofer, R. Global and regional pest insects and their antagonists in orchards: Spatial dynamics. Agric. Ecosyst. Environ. 1999, 73, 111–118.
- Pashley, D.P.; Bush, G.L. The use of allozymes in studying insect movement with special reference to the codling moth, Laspeyresia pomonella. In Movement of Highly Mobile Insects: Concepts and Methodology in Research, 1st ed.; Rabb, R.L., Kennedy, G.G., Eds.; North Carolina State University Press: Raleigh, NC, USA, 1979; pp. 333–341.
- Bues, R.; Toubon, J.F.; Poitout, H.S. Variabilite ecophysiologique et enzymatique de Cydia pomonella L. en fonction de l’origine geographique et de la plante hote. Agronomie 1995, 15, 221–231.
- Timm, A.E.; Geertsema, H.; Warnich, L. Gene flow among Cydia pomonella (Lepidoptera: Tortricidae) geographic and host populations in South Africa. J. Econ. Entomol. 2006, 99, 341–348.
- Zhou, Y.H.; Gu, H.N.; Dorn, S. Isolation of microsatellite loci in the codling moth Cydia pomonella (Lepidoptera: Tortricidae). Mol. Ecol. Notes 2005, 5, 226–227.
- Franck, P.; Guérin, F.; Loiseau, A.; Sauphanor, B. Isolation and characterization of microsatellite loci in the codling moth Cydia pomonella (Lepidoptera: Tortricidae). Mol. Ecol. Notes 2005, 5, 99–102.
- Pajač, I.; Barić, B.; Mikac, K.M.; Pejić, I. New insights into the biology and ecology of Cydia pomonella from apple orchards in Croatia. Bull. Insectol. 2012, 65, 185–193.
- Chen, M.H.; Dorn, S. Microsatellites reveal genetic differentiation among populations in an insect species with high genetic variability in dispersal, the codling moth, Cydia pomonella (L.) (Lepidoptera: Tortricidae). Bull. Entomol. Res. 2010, 100, 75–81.
- Voudouris, C.; Franck, P.; Olivares, J.; Sauphanor, B.; Mamuris, Z.; Tsitsipis, J.; Margaritopoulos, J. Comparing the genetic structure of codling moth Cydia pomonella (L.) from Greece and France: Long distance gene-flow in a sedentary pest species. Bull. Entomol. Res. 2012, 102, 185–198.
- Margaritopoulos, J.T.; Voudouris, C.C.; Olivares, J.; Sauphanor, B.; Mamuris, Z.; Tsitsipis, J.A.; Franck, P. Dispersal ability in codling moth: Mark-release-recapture experiments and kinship analysis. Agric. For. Entomol. 2012, 14, 399–407.
- Knight, A.L. Codling moth areawide integrated pest management. In Areawide Pest Management: Theory and Implementation, 1st ed.; Koul, O., Cuperus, G., Elliott, N., Eds.; CAB International: Oxfordshire, UK, 2008; pp. 159–190.
- Men, Q.L.; Chen, M.H.; Zhang, Y.L.; Feng, J.N. Genetic structure and diversity of a newly invasive species, the codling moth, Cydia pomonella (L.) (Lepidoptera: Tortricidae) in China. Biol. Invasions 2013, 15, 447–458.
- Witzgall, P.; Stelinski, L.; Gut, L.; Thomson, D. Codling moth management and chemical ecology. Annu. Rev. Entomol. 2008, 53, 503–522.
- Yan, F.; Bengtsson, M.; Witzgall, P. Behavioral response of female Codling Moths, Cydia Pomonella, to apple volatiles. J. Chem. Ecol. 1999, 25, 1343–1351.
- Lösel, P.M.; Penners, G.; Potting, R.P.; Ebbinghaus, D.; Elbert, A.; Scherkenbeck, J. Laboratory and field experiments towards the development of an attract and kill strategy for the control of the codling moth, Cydia pomonella. Entomol. Exp. Appl. 2000, 95, 39–46.
- Charmillot, P.J.; Pasquier, D.; Scalco, A.; Hofer, D. Studies on the control of the codling moth Cydia pomonella L. using attractant-insecticide. Mitt. Schweiz. Entomol. Ges. 1996, 69, 431–439.
- Mansour, M. Attract and kill for codling moth Cydia pomonella (Linnaeus) (Lepidoptera: Tortricidae) control in Syria. J. Appl. Entomol. 2010, 134, 234–242.
- Damos, P.; Colomar, L.A.; Ioriatti, C. Integrated fruit production and pest management in Europe: The apple case study and how far we are from the original concept? Insects 2015, 6, 626–657.
- Klassen, W. Area-wide integrated pest management and the sterile insect technique. In Sterile Insect Technique. Principles and Practice in Area-wide Integrated Pest Management, 1st ed.; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 39–68.
- Vreysen, M.J.B.; Robinson, A.S.; Hendrichs, J. Areawide control of insect pests. From research to field implementation. In Sterile Insect Technique. Principles and Practice in Area-Wide Integrated Pest Management, 1st ed.; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 351–353.
- Vreysen, M.J.B.; Carpenter, J.E.; Marec, F. Improvement of the sterile insect technique for codling moth Cydia pomonella (Linnaeus) (Lepidoptera Tortricidae) to facilitate expansion of field application. J. Appl. Entomol. 2010, 134, 165–181.
- Bloem, K.A.; Bloem, S.; Carpenter, J.E. Impact of moth suppression/eradication programmes using the sterile insect technique or inherited sterility. In Sterile Insect Technique. PRINCIPLES and Practice in Area-Wide Integrated Pest Management, 1st ed.; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 677–700.
- Dyck, V.A.; Hendrichs, J.; Robinson, A.S. Public Relations and Political Support in Area-Wide Integrated Pest Management Programmes that Integrate the Sterile Insect Technique. In Sterile Insect Technique. Principles and Practice in Area-Wide Integrated Pest Management, 1st ed.; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 524–545.
- Joshi, N.K.; Rajotte, E.G.; Naithani, K.J.; Krawczyk, G.; Hull, L.A. Population dynamics and flight phenology model of codling moth differ between commercial and abandoned apple orchard ecosystems. Front. Physiol. 2016, 7, 408.
- Onstad, D. Major Issues in Insect Resistance Management. In Insect Resistance Management, Biology, Economics and Prediction, 2nd ed.; Onstad, D., Ed.; Academic Press: Cambridge, MA, USA, 2007; pp. 1–16.
- Yu, F.L.; Wu, G.; Liu, T.J.; Zhai, B.P.; Chen, F.J. Effects of irrigation on the performance of cotton bollworm, Helicoverpa armigera (Hübner) during different pupal stages. Int. J. Pest Manag. 2008, 54, 137–142.
- Barzman, M.; Bàrberi, P.; Birch, A.N.E.; Boonekamp, P.; Dachbrodt-Saaydeh, S.; Graf, B.; Hommel, B.; Jensen, J.E.; Kiss, J.; Kudsk, P.; et al. Eight principles of integrated pest management. Agron. Sustain. Dev. 2015, 35, 1199–1215.
- Insecticide Resistance Action Committee (IRAC). Resistance Management for Sustainable Agriculture and Improved Public Health, 2nd ed. 2010. Available online: http://www.irac-online.org/wp-content/uploads/2009/09/VM-Layout-v2.6_LR.pdf (accessed on 20 August 2019).
- Beers, E.H.; Stuckling, D.M.; Prokopy, R.J.; Avila, J. Ecology and management of apple arthropod pests. In Apples: Botany, Production and Uses, 1st ed.; Ferree, D.C., Warrington, I.J., Eds.; CABI Publishing: Wallingford, UK, 2003; pp. 489–514.
More
Information
Subjects:
Agriculture, Dairy & Animal Science
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
12 Jan 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




