Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sabrin R. M. Ibrahim | + 1816 word(s) | 1816 | 2021-12-17 05:00:01 | | | |
| 2 | Vivi Li | Meta information modification | 1816 | 2022-01-11 07:30:14 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ibrahim, S. Terretonin. Encyclopedia. Available online: https://encyclopedia.pub/entry/18020 (accessed on 08 February 2026).
Ibrahim S. Terretonin. Encyclopedia. Available at: https://encyclopedia.pub/entry/18020. Accessed February 08, 2026.
Ibrahim, Sabrin. "Terretonin" Encyclopedia, https://encyclopedia.pub/entry/18020 (accessed February 08, 2026).
Ibrahim, S. (2022, January 11). Terretonin. In Encyclopedia. https://encyclopedia.pub/entry/18020
Ibrahim, Sabrin. "Terretonin." Encyclopedia. Web. 11 January, 2022.
Copy Citation
Endophytic fungi are proving to be an excellent source of chemical entities with unique structures and varied bioactivities. Terretonin (TE) and its structurally related derivatives are a class of meroterpenoids, possessing the same unique tetracyclic core skeleton, which have been reported from the Aspergillus genus.
Aspergillus terreus
endophytic fungi
terretonin
anti-inflammatory
LPS
Nrf2
NF-κB
1. Introduction
Acute lung injury (ALI) is a severe ailment with an elevated morbidity rate worldwide. It is characterized by the infiltration of inflammatory PMNs (polymorphonuclear cells) into pulmonary tissue associated with disrupted vascular permeability and pulmonary edema. The exact pathology of ALI remains elusive; however, many pathogenic players such as oxidative stress, lipid peroxidation, and inflammation interact to mediate the development of ALI [1][2]. During ALI, the continuous overproduction of ROS/RNS (reactive oxygen/nitrogen species) overloads the antioxidant capacity of the tissue, causing destruction of the cellular components such as DNA, proteins, and lipids [3][4]. LPS (lipopolysaccharide) is a glycolipid obtained from the bacterial cell wall and is widely utilized to establish a rodent model of ALI [5]. LPS induces the massive generation of ROS/RNS, which causes oxidative damage to pulmonary tissue and stimulates the activation and migration of neutrophils and macrophages into the lung. These activated infiltered cells release a wide variety of pro-inflammatory cytokines such as TNF-α and ILs (interleukins), as well as profibrotic growth factors such as transforming growth factor-beta [6][7]. Simultaneously, the activation of the upstream nuclear transcriptional factor-κB (NF-κB) augments the excessive production of pro-inflammatory cytokines (e.g., ILs and TNF-α) [8][9][10]. In addition to NF-κB, the NOD-like receptor 3 (NLRP3) inflammasome contributes to the inflammatory response via induction and activation of pro-caspase-1 to release caspase-1, which subsequently induces the production of IL-1β and IL-18 from its pro-forms [9][11]. Nrf2 (nuclear factor (erythroid-derived 2)-like 2) is a substantial nuclear transcriptional factor that is activated during oxidative stress. Upon activation, Nrf2 induces the transcription of antioxidant genes (NQO1, GCLm, GCLc, and HO-1) and antioxidant enzymes (GSH and SOD), leading to the neutralization of the oxidative stress and amelioration of the oxidative injury [12][13]. SIRT1 (silent information regulator Sirtuin 1) is a NAD+-dependent histone deacetylase that has a pivotal role in the protection against oxidative inflammatory damage during ALI. Recently, SIRT1 has been shown to suppress pulmonary edema via alleviation of the endothelial tight junction permeability [14]. The activation of SIRT1 has been linked to the modulation of Nrf2 antioxidant signaling [15]. Furthermore, SIRT1 can counteract the inflammatory response via downregulation of the NF-κB signaling pathway [16][17]. The interplay between SIRT1, Nrf2, NF-κB, and NLRP3 pathways regulates the cellular oxidative inflammatory responses, and hence, the agents that promote the activation of SIRT1/Nrf2 and block NF-κB/NLRP3 can control the deleterious effects of LPS and attenuate ALI.
Endophytic fungi constitute a wealthy source of bioactive metabolites for agricultural, industrial, pharmaceutical, environmental, and medical applications [18][19][20][21][22][23][24]. Complex molecular architectures and structural diversity are key features of these metabolites; thus, they are considered as potential candidates for developing beneficial and novel agents [25][26][27][28][29][30][31]. Terpenes are a remarkable group of bio-metabolites, which represent the biggest class of natural metabolites [32]. Meroterpenes are a relatively small group of terpenoids reported from various natural sources such as fungi, insects, terrestrial plants, marine organisms, and lichens [33][34][35]. Terretonins, a class of the fungal meroterpenoids, have mainly been reported from the Aspergillus genus and possess various bioactivities [34][35][36][37][38]. During our ongoing search for exploring the bioactivities of the endophytic fungal metabolites, the protective effect of terretonin (Figure 1) isolated from A. terreus against sepsis-induced ALI was investigated, in addition to its molecular pathways.
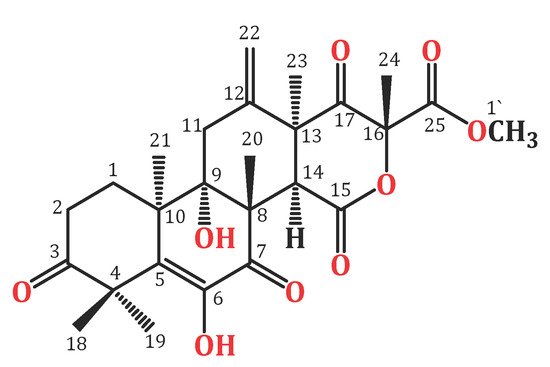
Figure 1. Chemical structure of terretonin (TE).
2. TE Lowered LPS-Induced Lung Edema and LDH Activity in BALF and Improved LPS-Induced Lung Histopathological Damage
LPS challenge induced remarkable pulmonary edema as there was a notable increase in the lung W/D ratio and protein content of BALF compared to normal mice (Figure 3I(A,B)). Additionally, LPS caused an elevation in LDH activity in BALF (Figure 3I(C)). However, TE pretreatment reduced the lung W/D ratio, protein content, and LDH activity compared to the LPS group.
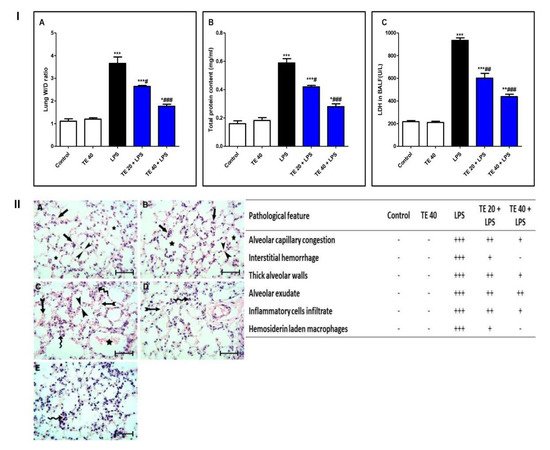
Figure 3. Terretonin (TE) attenuated biochemical and histopathological indices of lipopolysaccharide (LPS)-induced ALI. (I) (A) Lung W/D ratio; (B) total protein content; and (C) lactate dehydrogenase (LDH) activity in BALF. (II) Lung sections of: (A) Control and (B) TE 40 groups showed normal lung histology (normal alveolar capillaries (black arrows), thin alveolar walls (between arrow heads), and clear alveoli (star)). (C) Lungs of the LPS group exhibited many pathological features such as congested alveolar capillaries and alveolar hemorrhage (tailed black arrows), thick edematous alveolar walls (between arrow heads), edema fluid exudates in the alveoli (star), infiltration of inflammatory cells (curved arrows). (D) TE 20 + LPS and (E) TE 40 + LPS groups showed much improvement of all LPS-induced pathological changes in the lung. H&E stain ×400, scale bar 25 µm. Semiquantitative assessment of pathological changes in lung tissue in all groups. Data are the mean ± SE (n = 8). * p < 0.05, ** p < 0.01, *** p < 0.001 vs. control group; # p < 0.05, ## p < 0.01, ### p < 0.001 vs. LPS group (one-way ANOVA followed by Tukey’s Kramer multiple comparisons test).
Histopathological examination of the lung specimen of the control exhibited normal histology with no mark of any pathological change (Figure 3II). Lungs of the LPS group showed extensive injury in the form of congested alveolar capillaries accompanied by alveolar hemorrhage. There were edematous thick alveolar walls, marked inflammatory cells’ infiltration, edema fluid exudates in the alveoli, and hemosiderin-laden macrophages. On the contrary, TE + LPS-treated groups appeared protected from the injurious effects of LPS. The signs and scores of lesions were remarkably ameliorated.
3. TE Ameliorated LPS-Induced Inflammatory Cell Infiltration into the Lung and Repressed MPO Activity
As shown in Figure 4, LPS significantly intensified the inflammatory cells’ infiltration into the pulmonary tissue as there was an elevation in the differential and total cell counts in BALF compared to control mice. In addition, MPO activity was enhanced in the lungs of the LPS group. On the other hand, pretreatment with TE significantly reduced the total and differential cell counts, besides MPO activity compared to the LPS group.
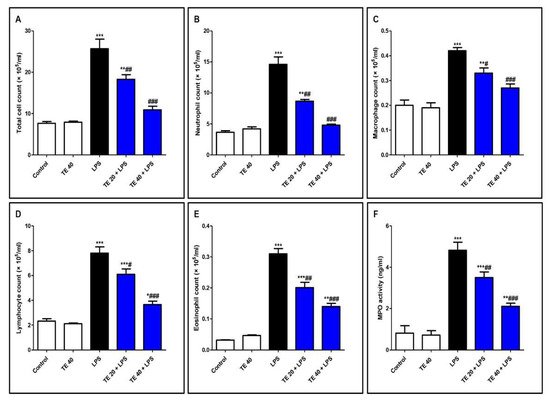
Figure 4. Terretonin (TE) ameliorated lipopolysaccharide (LPS)-induced inflammatory cell infiltration into the lung and suppressed myeloperoxidase (MPO) activity. (A–E) Total and differential cell counts in bronchoalveolar lavage fluid (BALF). (F) MPO activity in lung tissue. Data are the mean ± SE (n = 8). * p < 0.05, ** p < 0.01, *** p < 0.001 vs. control group; # p < 0.05, ## p < 0.01, ### p < 0.001 vs. LPS group (one-way ANOVA followed by Tukey’s Kramer multiple comparisons test).
4. TE Attenuated LPS-Produced Lipid Peroxidation and Augmented Antioxidants
Injecting LPS significantly exaggerated the lipid peroxidative parameters, 4-HNE, MDA, and PC in the pulmonary tissue in contrast to the control group (Figure 5A–C). Furthermore, LPS depressed pulmonary antioxidants (GSH, SOD, CAT, and TAC) in comparison to the control group (Figure 5D–G). Notably, TE pretreatment produced a significant increase in the antioxidants, as well as a significant decrease in the lipid peroxidative parameters compared to the LPS group.
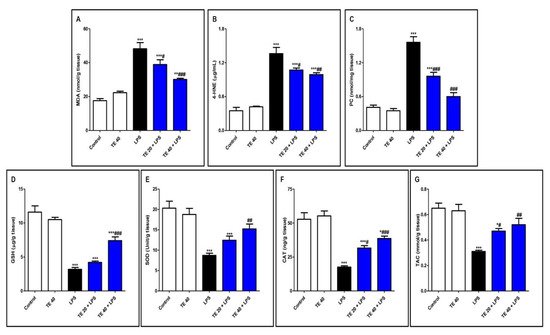
Figure 5. Terretonin (TE) attenuated lipopolysaccharide (LPS)-induced lipid peroxidation and augmented antioxidants in the lungs. (A) Malondialdehyde (MDA); (B) 4-hydroxynonenal (4-HNE); (C) protein carbonyl (PC); (D) reduced glutathione (GSH); (E) superoxide dismutase (SOD); (F) catalase (CAT); (G) total antioxidant capacity (TAC). Data are the mean ± SE (n = 8). * p < 0.05, ** p < 0.01, *** p < 0.001 vs. control group; # p < 0.05, ## p < 0.01, ### p < 0.001 vs. LPS group (one-way ANOVA followed by Tukey’s Kramer multiple comparisons test).
5. TE Counteracted LPS-Induced Suppression of SIRT1/Nrf2 Signaling and Targeted Genes in Lung
As demonstrated in Figure 6, LPS induced a remarkable reduction in the mRNA expressions of SIRT1, Nrf2, and its targeted genes (GCLm, NQO1, and HO-1) compared with the control group. Consequently, LPS significantly attenuated the Nrf2 binding activity and the level of HO-1 (Figure 6II). TE pretreatment significantly boosted the mRNA expression of SIRT1, GCLm, Nrf2, NQO1, and HO-1 in comparison to the LPS group. Additionally, TE augmented the Nrf2 binding activity and increased the HO-1 level, compared to the LPS group.
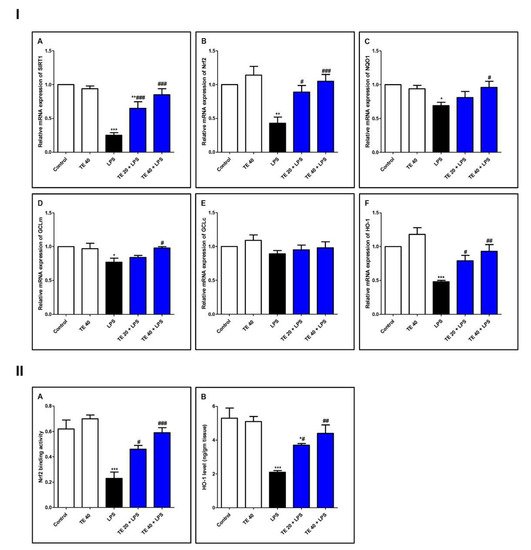
Figure 6. Terretonin (TE) counteracted the LPS-induced suppression of SIRT1/Nrf2 signaling and targeted genes in the lung. (I) (A–F) Gene expressions of SIRT1, Nrf2, NQO1, GCLm, GCLc, and HO-1. (II) (A) Nrf2 binding activity; (B) HO-1 level in the lung. Data are the mean ± SE (n = 8). * p < 0.05, ** p < 0.01, *** p < 0.001 vs. control group; # p < 0.05, ## p < 0.01, ### p < 0.001 vs. LPS group (one-way ANOVA followed by Tukey’s Kramer multiple comparisons test).
6. TE Suppressed LPS-Induced Activation of NF-κB/NLRP3 Signaling and Its Downstream Pro-Inflammatory Markers in Lung
As shown in Figure 7, injecting LPS remarkably enhanced the gene and protein expression of NF-κB, as well as the gene expression of NLRP3 and caspase-1 as compared to the control group. Additionally, LPS potentiated the gene expression of iNOS, as well as the gene expression of inflammatory mediators (IL-1β and -6 and TNF-α), compared to the control group. The levels of NOx, IL-6 and -1β, and TNF-α were remarkably elevated in the LPS group, in comparison to the control group.
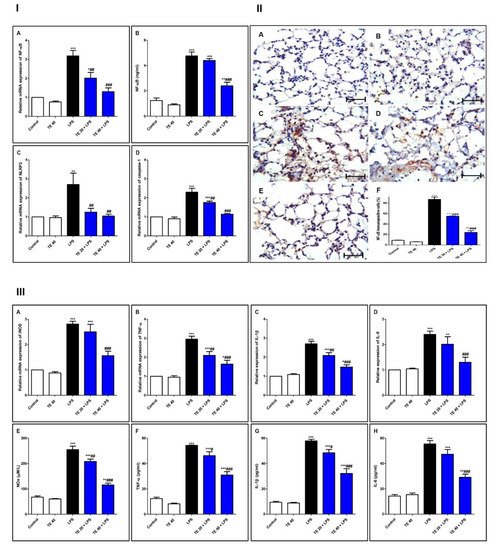
Figure 7. Terretonin (TE) suppressed the LPS-induced activation of NF-κB/NLRP3 signaling and its downstream pro-inflammatory markers in the lung. (I) (A) Gene expression; (B) level of NF-κB; (C) gene expression of NLRP3; (D) gene expression of caspase-1. (II) Immuno-expression of NF-κB protein: (A,B) lung sections of both the control and TE 40 groups, showing minimal cytoplasmic brownish staining; (C) LPS group showing marked nuclear and cytoplasmic brown immunostaining of inflamed lung tissue; (D) TE 20 + LPS and (E) TE 40 + LPS groups exhibiting marked improvement with moderate to minimal nuclear and cytoplasmic brown staining; NF-ĸB immunostain ×400, scale bar 25 µm; (F) % of immunopositive cells among different groups. (III) (A–D) Gene expression of iNOS, TNF-α, IL-1β, and IL-6; (E–H) levels of NOx, TNF-α, IL-1β, and IL-6. Data are the mean ± SE (n = 8). * p < 0.05, ** p < 0.01, *** p < 0.001 vs. control group; # p < 0.05, ## p < 0.01, ### p < 0.001 vs. LPS group (one-way ANOVA followed by Tukey’s Kramer multiple comparisons test).
Nevertheless, TE-pretreated groups revealed a remarkable inhibition of gene and immuno-expression of NF-κB, as well as its level. TE also inhibited LPS-caused activation of the NLRP3 inflammasome (NLRP3, caspase-1, and IL-1β). Furthermore, the gene expression and the level of inflammatory mediators were attenuated in TE-pretreated groups in comparison to the LPS group.
7. TE Decreased LPS-Induced Apoptosis
As shown in Figure 8, LPS administration decreased the level of the anti-apoptotic protein Bcl2 and increased the levels of the pro-apoptotic protein, Bax, as well as caspase-3 compared to normal mice. In contrast, TE pretreatment suppressed the apoptotic changes, as presented by a significant increase in Bcl2 and significant decreases in Bax and caspase-3 compared to the LPS group.
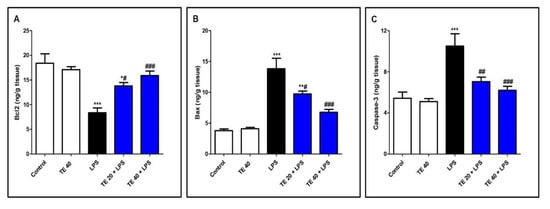
Figure 8. Terretonin (TE) decreased LPS-induced pulmonary apoptosis. (A) B-cell leukemia/lymphoma 2(Bcl-2); (B) Bcl-2-associated X protein (Bax); (C) caspase 3. Data are the mean ± SE (n = 8). * p < 0.05, ** p < 0.01, *** p < 0.001 vs. control group; # p < 0.05, ## p < 0.01, ### p < 0.001 vs. LPS group (one-way ANOVA followed by Tukey’s Kramer multiple comparisons test).
References
- Ibrahim, S.R.M.; Ahmed, N.; Almalki, S.; Alharbi, N.; El-Agamy, D.S.; Alahmadi, L.A.; Saubr, M.K.; Elkablawy, M.; Elshafie, R.M.; Mohamed, G.A.; et al. Vitex agnus-castus safeguards the lung against lipopolysaccharide-induced toxicity in mice. J. Food Biochem. 2019, 43, e12750.
- Ammar, E.A.; Sharawy, M.H.; Shalaby, A.A.; El-Agamy, D.S. Effects of methyl palmitate and lutein on LPS–induced acute lung injury in rats. World J. Respirol. 2013, 3, 20–28.
- El-Agamy, D.S. Nilotinib ameliorates lipopolysaccharide induced acute lung injury in rats. Toxicol. Appl. Pharmacol. 2011, 253, 153–160.
- Shao, L.; Meng, D.; Yang, F.; Song, H.; Tang, D. Irisin-mediated protective effect on LPS-induced acute lung injury via suppressing inflammation and apoptosis of alveolar epithelial cells. Biochem. Biophys. Res. Commun. 2017, 487, 194–200.
- Ahmed, N.; Aljuhani, N.; Salamah, S.; Surrati, H.; El-Agamy, D.S.; Elkablawy, M.A.; Ibrahim, S.R.M.; Mohamed, G.A. Pulicaria petiolaris effectively attenuates lipopolysaccharide (LPS)-induced acute lung injury in mice. Arch. Biol. Sci. 2018, 70, 699–706.
- Abdallah, H.M.; El-Agamy, D.S.; Ibrahim, S.R.M.; Mohamed, G.A.; Elsaed, W.M.; Elghamdi, A.A.; Safo, M.K.; Malebari, A.M. Euphorbia cuneata represses LPS-induced acute lung injury in mice via its antioxidative and anti-inflammatory activities. Plants 2020, 9, 1620.
- Righetti, R.F.; Dos Santos, T.M.; Camargo, L.D.N.; Aristóteles, L.R.C.R.B.; Fukuzaki, S.; de Souza, F.C.R.; Santana, F.P.R.; de Agrela, M.V.R.; Cruz, M.M.; Alonso-Vale, M.I.C.; et al. Protective effects of anti-IL17 on acute lung injury induced by LPS in mice. Front. Pharmacol. 2018, 9, 1021.
- Shaaban, A.A.; El-Kashef, D.H.; Hamed, M.F.; El-Agamy, D.S. Protective effect of pristimerin against LPS-induced acute lung injury in mice. Int. Immunopharmacol. 2018, 59, 31–39.
- Zhang, H.; Chen, S.; Zeng, M.; Lin, D.; Wang, Y.; Wen, X.; Xu, C.; Yang, L.; Fan, X.; Gong, Y.; et al. Apelin-13 administration protects against LPS-induced acute lung injury by inhibiting NF-κB pathway and NLRP3 inflammasome activation. Cell Physiol. Biochem. 2018, 49, 1918–1932.
- El-Agamy, D.S.; Mohamed, G.A.; Ahmed, N.; Elkablawy, M.A.; Elfaky, M.A.; Elsaed, W.M.; Mohamed, S.G.A.; Ibrahim, S.R.M. Protective anti-inflammatory activity of tovophyllin A against acute lung injury and its potential cytotoxicity to epithelial lung and breast carcinomas. Inflammopharmacology 2020, 28, 153–163.
- Liu, X.; Jin, X.; Yu, D.; Liu, G. Suppression of NLRP3 and NF-κB signaling pathways by alpha-Cyperone via activating SIRT1 contributes to attenuation of LPS-induced acute lung injury in mice. Int. Immunopharmacol. 2019, 76, 105886.
- Wei, J.; Chen, G.; Shi, X.; Zhou, H.; Liu, M.; Chen, Y.; Feng, D.; Zhang, P.; Wu, L.; Lv, X. Nrf2 activation protects against intratracheal LPS induced mouse/murine acute respiratory distress syndrome by regulating macrophage polarization. Biochem. Biophys. Res. Commun. 2018, 500, 790–796.
- Yang, H.; Lv, H.; Li, H.; Ci, X.; Peng, L. Oridonin protects LPS-induced acute lung injury by modulating Nrf2-mediated oxidative stress and Nrf2-independent NLRP3 and NF-κB pathways. Cell Commun. Signal. 2019, 17, 62.
- Fu, C.; Hao, S.; Xu, X.; Zhou, J.; Liu, Z.; Lu, H.; Wang, L.; Jin, W.; Li, S. Activation of SIRT1 ameliorates LPS-induced lung injury in mice via decreasing endothelial tight junction permeability. Acta. Pharmacol. Sin. 2019, 40, 630–641.
- Gong, Q.; Xue, Y.; Li, X.; Song, L.; Zhu, L. DL-3-n-butylphthalide attenuates lipopolysaccharide-induced acute lung injury via SIRT1-dependent and -independent regulation of Nrf2. Int. Immunopharmacol. 2019, 74, 105658.
- Han, S.; Li, Z.; Han, F.; Jia, Y.; Qi, L.; Wu, G.; Cai, W.; Xu, Y.; Li, C.; Zhang, W.; et al. ROR alpha protects against LPS–induced inflammation by down-regulating SIRT1/NF-κ B pathway. Arch. Biochem. Biophys. 2019, 668, 1–8.
- Peng, X.P.; Li, X.H.; Li, Y.; Huang, X.T.; Luo, Z.Q. The protective effect of oleanolic acid on NMDA-induced MLE-12 cells apoptosis and lung injury in mice by activating SIRT1 and reducing NF-κB acetylation. Int. Immunopharmacol. 2019, 70, 520–529.
- Ibrahim, S.R.M.; Mohamed, S.G.A.; Sindi, I.A.; Mohamed, G.A. Biologically active secondary metabolites and biotechnological applications of species of the family Chaetomiaceae (Sordariales): An updated review from 2016 to 2021. Mycol. Prog. 2021, 20, 595–639.
- Ibrahim, S.R.M.; Altyar, A.E.; Mohamed, S.G.A.; Mohamed, G.A. Genus Thielavia: Phytochemicals, industrial importance and biological relevance. Nat. Prod. Res. 2021.
- Ibrahim, S.R.M.; Mohamed, S.G.A.; Altyar, A.E.; Mohamed, G.A. Natural products of the fungal genus Humicola: Diversity, biological activity, and industrial importance. Curr. Microbiol. 2021, 78, 2488–2509.
- Ibrahim, S.R.M.; Sirwi, A.; Eid, B.G.; Mohamed, S.G.A.; Mohamed, G.A. Fungal depsides—Naturally inspiring molecules: Biosynthesis, structural characterization, and biological activities. Metabolites 2021, 11, 683.
- Khayat, M.T.; Ibrahim, S.R.M.; Mohamed, G.A.; Abdallah, H.M. Anti-inflammatory metabolites from endophytic fungus Fusarium sp. Phytochem. Lett. 2019, 29, 104–109.
- Ibrahim, S.R.M.; Mohamed, G.A.; Khedr, A.I.M. γ-Butyrolactones from Aspergillus species: Structures, biosynthesis, and biological activities. Nat. Prod. Commun. 2017, 12, 791–800.
- Ibrahim, S.R.M.; Mohamed, G.A.; Ross, S.A. Aspernolides L and M, new butyrolactones from the endophytic fungus Aspergillus versicolor. Z. Naturforsch C. 2017, 72, 155–160.
- Noor, A.O.; Almasri, D.M.; Bagalagel, A.A.; Abdallah, H.M.; Mohamed, S.G.A.; Mohamed, G.A.; Ibrahim, S.R.M. Naturally occurring isocoumarins derivatives from endophytic fungi: Sources, isolation, structural characterization, biosynthesis, and biological activities. Molecules 2020, 25, 395.
- Mohamed, G.A.; Ibrahim, S.R.M.; Asfour, H.Z. Antimicrobial metabolites from endophytic fungus Aspergillus versicolor. Phytochem. Lett. 2020, 35, 152–155.
- El-Agamy, D.S.; Ibrahim, S.R.M.; Ahmed, N.; Khoshhal, S.; Abo-Haded, H.M.; Elkablawy, M.A.; Aljuhani, N.; Mohamed, G.A. Aspernolide F, as a new cardioprotective butyrolactone against doxorubicin–induced cardiotoxicity. Int. Immunopharmacol. 2019, 72, 429–436.
- Ibrahim, S.R.M.; Mohamed, G.A.; Al Haidari, R.A.; El-Kholy, A.A.; Zayed, M.F. Potential anti-malarial agents from endophytic fungi: A review. Mini Rev. Med. Chem. 2018, 18, 1110–1132.
- Ibrahim, S.R.M.; Mohamed, G.A.; Asfour, H.Z.; Al Haidari, R.A.; El–Kholy, A.A.; Mohamed, F. Zayed. Fusaristerol A: A new cytotoxic and antifungal ergosterol fatty acid ester from the endophytic fungus Fusarium sp. associated with Mentha longifolia roots. Pharmacogn. Mag. 2018, 14, 308–311.
- Ibrahim, S.R.M.; Mohamed, G.A.; Al Haidaria, R.A.; Zayed, M.F.; El-Kholy, A.A.; Elkhayat, E.S.; Ross, S.A.; Fusarithioamide, B. a new benzamide derivative from the endophytic fungus Fusarium chlamydosporium with potent cytotoxic and antimicrobial activities. Bioorganic Med. Chem. 2018, 26, 786–790.
- Ibrahim, S.R.M.; Mohamed, G.A.; Al Haidari, R.A.; El-Kholy, A.A.; Zayed, M.F.; Khayat, M.T. Biologically active fungal depsidones: Chemistry, biosynthesis, structural characterization, and bioactivities. Fitoterapia 2018, 129, 317–365.
- Liu, X.H.; Miao, F.P.; Qiao, M.F.; Cichewicz, R.H.; Ji, N.Y. Terretonin, ophiobolin, and drimane terpenes with absolute configurations from an algicolous Aspergillus ustus. RSC Adv. 2013, 3, 588–595.
- Hamed, A.; Abdel-Razek, A.S.; Frese, M.; Stammler, H.G.; El-Haddad, A.F.; Ibrahim, T.A.; Sewald, N.; Shaaban, M. Terretonin N: A new meroterpenoid from Nocardiopsis sp. Molecules 2018, 23, 299.
- Elkhayat, E.S.; Ibrahim, S.R.; Mohamed, G.A.; Ross, S.A.; Terrenolide, S. A new anti–leishmanial butenolide from the endophytic fungus Aspergillus terreus. Nat. Prod. Res. 2016, 30, 814–820.
- Ibrahim, S.R.M.; Elkhayat, E.S.; Mohamed, G.A.; Khedr, A.I.; Fouad, M.A.; Kotb, M.H.R.; Ross, S.A. Aspernolides F and G, new butyrolactones from the endophytic fungus Aspergillus terreus. Phytochem. Lett. 2015, 14, 84–90.
- Ibrahim, S.R.M.; Mohamed, G.A.; Kamal, H.M.K.; Mohamed, S.G.A.; Khedr, A.I.M. Terretonins from Aspergillus genus: Structures, biosynthesis, bioactivities, and structural elucidation. Mini Rev. Org. Chem. 2021, 18, 1–13.
- Fukuda, T.; Kurihara, Y.; Kanamoto, A.; Tomoda, H.; Terretonin, G. a new sesterterpenoid antibiotic from marine-derived Aspergillus sp. OPMF00272. J. Antibiot. 2014, 67, 593–595.
- López-Gresa, M.P.; Cabedo, N.; González-Mas, M.C.; Ciavatta, M.A.; Avila, C.; Primo, J. Terretonins E and F, inhibitors of the mitochondrial respiratory chain from the marine-derived fungus Aspergillus insuetus. J. Nat. Prod. 2009, 72, 1348–1351.
More
Information
Subjects:
Biology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
662
Revisions:
2 times
(View History)
Update Date:
11 Jan 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




