Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Irtiza Naseer Sheikh | + 2694 word(s) | 2694 | 2021-12-06 03:40:39 | | | |
| 2 | Dean Liu | Meta information modification | 2694 | 2022-01-11 08:47:11 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Sheikh, I. Sleep Disturbances in Pediatric Cancer Patients. Encyclopedia. Available online: https://encyclopedia.pub/entry/18019 (accessed on 08 February 2026).
Sheikh I. Sleep Disturbances in Pediatric Cancer Patients. Encyclopedia. Available at: https://encyclopedia.pub/entry/18019. Accessed February 08, 2026.
Sheikh, Irtiza. "Sleep Disturbances in Pediatric Cancer Patients" Encyclopedia, https://encyclopedia.pub/entry/18019 (accessed February 08, 2026).
Sheikh, I. (2022, January 11). Sleep Disturbances in Pediatric Cancer Patients. In Encyclopedia. https://encyclopedia.pub/entry/18019
Sheikh, Irtiza. "Sleep Disturbances in Pediatric Cancer Patients." Encyclopedia. Web. 11 January, 2022.
Copy Citation
Sleep disturbances represent an understudied yet common source of distress among pediatric cancer patients and survivors, with deleterious effects on quality of life. Sleep issues stem from multiple risk factors, yet individual contributors are difficult to isolate, consequently impeding the identification of targets for intervention.
sleep disturbance
pediatric cancer
excessive daytime sleepiness
insomnia
1. Introduction
Cancer in the pediatric and adolescent population (1–19 years old) remains one of the three leading causes of death in the United States [1]. However, advances in the treatment of pediatric cancer have led to significant increases in long-term survival, highlighting the need to address not only survival but treatment-associated morbidity and improved quality of life as well [2][3]. Among quality-of-life manifestations, children, adolescents, and young adults report sleep disturbances during and after treatment [4][5][6][7]. Indeed, sleep issues and their sequelae appear to be common in pediatric cancer patients and survivors, with almost half experiencing some form of sleep disturbance [8][9]. The most common etiologies of poor sleep in patients and survivors include increased daytime sleepiness and insomnia, followed by increased time to sleep onset, decreased sleep duration, and overall poor sleep quality, issues that may be manageable or treatable [8][9][10][11]. Sleep disturbances may directly result from cancer and its treatment, as well as from multiple other risk factors, many of which lead to a bidirectional relationship between sleep and physical and psychological distress [12][13][14]. This multifactorial relationship between pediatric cancer and sleep disturbances is illustrated in Figure 1.
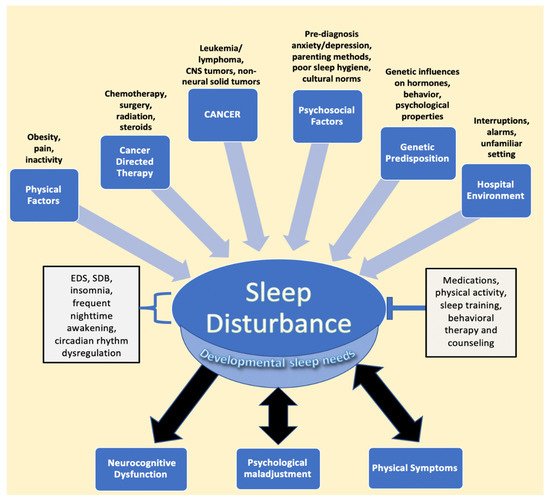
Figure 1. Factors impacting sleep in children diagnosed with cancer. The figure describes the various entities described in the literature that lead to sleep disturbance, followed by the resulting sequalae of negatively impacted sleep. EDS, SDB, frequent nighttime awakening, and circadian rhythm dysregulation are components of disturbed sleep, while interventions such as medications, physical activity, sleep training, and counseling represent mitigating factors that relieve sleep issues. Developmental sleep needs are affected globally by sleep disturbances, despite the fact that children of different ages require different amounts of sleep. Psychological maladjustment and physical symptoms, such as pain, have been found to have a bidirectional relationship with sleep issues. Abbreviations: CNS, central nervous system; EDS, excessive daytime sleepiness; SDB, sleep disordered breathing.
2. Relationship between Cancer and Treatment and Sleep in Pediatric Cancer
EDS and SDB are the most common sleep issues seen in patients and survivors of hematological malignancies, as well as solid tumors, such as sarcomas, impacting as many as 60% and 40% of patients, respectively, and they are even more common in those with CNS tumors [2]. While the direct role that these cancers play in causing sleep issues is unclear, sleep disturbances occur past the treatment phase, at times more than 5 years after treatment, as demonstrated in a study by Clanton et al. of adult survivors of childhood cancer [14]. Chemotherapy, steroids, and radiation therapy are the mainstays of pediatric cancer therapy and also play a significant role in altering sleep patterns [15]. Changes in sleep during treatment have been observed in a wide array of malignancies, including non-neural solid tumors, hematological cancers, and CNS tumors, despite the differences in the specific chemotherapies administered [15][16]. Hospitalization is also a component of treatment in which initial or subsequent admissions have been shown to negatively affect sleep in pediatric cancer patients [17][18]. Although patients treated in the outpatient setting tend to have better sleep quality than those in the inpatient setting, they still have an elevated risk for poor sleep quality during active treatment. This is evidenced in a study by Zupanec et al., where greater than 80% of ALL patients between the ages of 4 to 18 years old on outpatient maintenance chemotherapy described poor sleep characterized by increased nighttime awakenings and wake time after sleep onset when compared to pediatric norms [16].
In the sections below, the effects of cancer on sleep during treatment and into survivorship are explored, followed by a discussion on the role treatment itself plays in contributing to sleep issues in patients and survivors.
2.1. Effects of Hematological Malignancies and Non-Central Nervous System (CNS) Solid Tumor Cancers on Sleep
Nearly 40%, and, in some cases, greater than 80%, of pediatric leukemia patients report some form of sleep disturbance, with insomnia being the most prevalent; however, there is a need to describe the direct impact of pediatric health conditions, such as cancer, on sleep [2][16][19][20]. Researchers have explored the biology of the circadian clock in leukemia cells and propose that alterations in the circadian rhythm of cells allow for unchecked replication [21][22][23][24][25]. There is a need for further research to determine the direct effects of hematological malignancies on sleep; the relationship between circadian gene expression in cancer cells and circadian-rhythm-dysregulation-related sleep disruption may represent a starting point for such an investigation.
In contrast to hematological malignancies, non-neural solid tumors may play a more direct and measurable role in causing sleep disturbances. They can result in OSA or SDB because of their anatomic location relative to the upper and lower airways and may necessitate surgical removal to relieve the obstruction [26]. However, outside of a case report of a spinal osteochondroma leading to sleep apnea or studies with mixed cancer diagnoses, no significant studies have isolated the role of pediatric sarcomas in sleep issues [10][26][27]. Nunes et al. presented one of the few studies that recognized that patients with sarcoma have greater difficulty with sleep and fatigue, possibly due to associated bone pain [28].
In pediatric leukemia survivorship, there is wide variation in the reported prevalence of sleep disturbances, with rates ranging from 13% to 50% in acute lymphoblastic leukemia survivors [27][29]. These differences in rates are compounded by reports of pediatric leukemia survivors who do not experience clinically poorer sleep than their sibling controls [27][30]; this contrasts with reports that indicate that leukemia survivors report disturbed sleep more than a decade after treatment [29]. Conflicting data on sleep in leukemia survivors lack a satisfactory explanation and highlight the need for further research to discover the true changes in sleep.
Similar to its hematological counterpart, the effects of lymphoma on sleep in survivorship represent another opportunity for further investigation. Research from the Childhood Cancer Survivor study looking at the factors that influence fatigue and poor sleep in adult survivors of childhood Hodgkin lymphoma (HL) demonstrated that survivors with a high body mass index (BMI) and significant bodily pain were more likely to experience EDS [31]. In that study, those with at least “medium” pain were five times more likely to experience poor sleep quality. In reviewing the effect of BMI on sleep, the effect on HL survivors is similar to those of obese survivors of CNS tumors, with higher BMI scores correlating with higher risks of sleep issues [32]. This highlights that BMI is a predictive factor that affects the sleep quality of lymphoma survivors and may be extrapolated to be a cause of disturbed sleep in other survivors, regardless of cancer type.
There is a significant lack of research on the direct effects of non-CNS cancers on sleep in both patients on treatment and those in survivorship. The results of current studies highlight that non-CNS cancers can directly impact patients’ physiological, psychosomatic, and anatomical features to disrupt sleep. Each point of disruption, such as pain, BMI, and physical-activity limitations, may serve as a modifiable target for interventions to improve sleep quality in patients and survivors [31][33][34][35][36][37]. Moreover, as further explained below, the type of cancer and its location may help clinicians predict the various types of sleep disturbances in these individuals.
2.2. Effects of CNS Tumors on Sleep
Nearly 80% of patients with CNS tumors experience sleep issues, with EDS being the most common in survivorship, as described in a study by Rosen et al., where greater than 60% of CNS tumor patients experienced EDS in survivorship [2][38]. Those with tumors of the brainstem, thalamus, or hypothalamus are the most frequent cancer patients referred for sleep studies; brain tumors are the second leading cause of secondary narcolepsy [39]. More than a quarter of survivors of pediatric brain tumors are also noted to suffer from insomnia [40]. However, research into the causes of sleep disturbances in pediatric CNS tumor patients is lacking compared to that in pediatric CNS tumor survivors. Examples indicate that the location of CNS tumors and disruption of neural structures that regulate sleep through hormonal control are the strongest examples of the direct effects of brain tumors on sleep [41][42][43]. Craniopharyngioma, a relatively common pediatric brain tumor that typically affects the hypothalamus, can disrupt melatonin secretion due to damage to the suprachiasmatic nucleus, leading to daytime sleepiness and poorer quality of life [44][45][46][47]. Posterior fossa tumors, including those at the brainstem, can result in apneic episodes during sleep, narcolepsy, or SDB, due to infiltration of respiratory centers, leading to EDS [2][39][48][49][50]. Interestingly, tumors in areas that are not ordinarily associated with sleep, such as the cerebellum, can lead to sleep disturbances, with potentially fatal consequences stemming from respiratory failure due to central sleep apnea, indicating that the cerebellum, primarily thought to direct motor function, may also have a role to play in controlling respiration, and the disruption of that respiration control leads to central sleep apnea [42][48][51]. Moreover, as Lee et al. describe, the effects of therapies, such as chemotherapy and radiation, on the cerebellum in contributing to sleep apnea is unclear but is probably a contributing factor to the observed central sleep apnea [51]. The effects of various pediatric CNS tumors indicate that, taking into account different locations and comparing their impact on sleep during treatment represent fruitful areas of investigation. This is especially important in pediatric oncology, considering that medulloblastomas, which occur in the cerebellum, are among the most common CNS tumors in children [52].
In adult survivors of pediatric CNS tumors, EDS continues to remain the main source of sleep disruption, followed by issues such as increased sleep onset latency, insomnia, and SDB [2][6][53][38][54][15][39][40][44][45][55][56][57]. Moreover, CNS tumor survivors with high BMI scores had lower levels of melatonin and were at a higher risk of hypersomnia, narcolepsy, and SDB, indicating that hormonal disruption continues into survivorship and is a significant component of sleep disturbance, especially in those with high BMIs in the range of obesity [27][45][55][58].
While associations have been documented between EDS and brain tumors in the pediatric cancer population, the etiologies of certain sleep disturbances are not always clear. For example, resolution of SDB does not always lead to an improvement in EDS, suggesting that EDS is a multifactorial problem due to inefficient sleep, significant night awakenings, or circadian rhythm disorders that may manifest from varying sources [57][59].
Overall, the literature examining the impact of brain tumors on sleep indicates that the location of a brain tumor, its effect on hormone disruption, and its structural disruption of the brain contribute to sleep disturbances [44][46][48][51]. Moreover, EDS is a significant source of distress in patients and can result due to radiation, chemotherapy, or surgery, and as Rosen et al. demonstrate, it can continue into survivorship [38]. Pediatric CNS tumor patients and survivors represent an important population for research into the direct effects of cancer on sleep, especially as pediatric CNS tumors are the most frequent pediatric cancer referred for sleep studies [2].
2.3. Effects of Chemotherapy on Sleep
Patients undergoing chemotherapy report significant sleep disturbances, characterized by increased nighttime awakenings and restlessness [16][60][61]. In a sample of mainly leukemia and non-Hodgkin lymphoma patients, 95% of adolescents undergoing chemotherapy experienced disrupted or low-quality sleep at least three times a week that was associated with tiredness, decreased alertness, and decreased satisfaction with the prior night’s sleep quality [62].
In terms of specific risk factors and mechanisms, some patients who report sleep disturbances during chemotherapy have been found to have gene polymorphisms in genes encoding Interleukin-6 (IL-6) and tumor necrosis factor (TNF) that lead to elevated cytokine levels in the context of inadequate sleep [63]. As shown by Cheung et al. in a study looking at ALL survivors at a median of 7 years following diagnosis, the effects of chemotherapy on disturbed sleep, such as increased nighttime awakenings, may persist well into survivorship, as a result of an increase in cortisol, cytokines, and the associated physiologic cascade of immune and inflammatory responses [64][65]. These sleep issues have been shown to lead to worsened neurobehavioral outcomes, such as aggression, attention difficulties, and learning issues [66][67].
Chemotherapy also plays a role in increasing the risk of developing psychological disturbances associated with impaired sleep, including anxiety, mood disorders, and behavioral issues [64][65]. Further research is needed to identify the extent to which chemotherapy independently affects sleep during treatment and survivorship, as well as to improve our understanding of the underlying risk factors and mechanisms of sleep disturbances that result from chemotherapy [60].
2.4. Effects of Steroid Treatment on Sleep
Steroids such as dexamethasone and prednisone are commonly used in the treatment of cancer patients and have been found to contribute to insomnia in adolescent acute lymphoblastic leukemia patients, some of whom require sleep aids [2]. Dexamethasone, in particular, is associated with poorer sleep quality compared to those on prednisone, longer time spent napping to compensate for inefficient nighttime sleep than prednisone, and disruption of REM sleep [68][69][70][71]. As evidenced by a study in ALL patients between the ages of 5 and 18 years old, dexamethasone is also associated with changes in circadian rhythm activity and an increased feeling of fatigue [72]. Sanford et al. also demonstrated, through the evaluation of patients with ALL on maintenance treatment, that gender differences may exist in experienced sleep issues, considering that inadequate sleep duration and quality due to dexamethasone appeared to be exaggerated in females [73].
More research is needed to fully understand the mechanisms of sleep disturbance that result from steroid treatment and why some agents (e.g., dexamethasone) appear to have more impact than others (e.g., prednisone). The differing effects of the two steroids may be attributable to the nearly three-fold longer half-life, stronger potency, and increased CNS penetration of dexamethasone when compared to prednisone [74][75]. Further, in a study exploring the mechanism behind dexamethasone’s effects on sleep, pediatric leukemia patients on dexamethasone with certain genotypes for the genes IL-6, polymerase delta-interacting protein 3 (POLDIP3), or α2-Heremans-Schmid glycoprotein (AHSG), which encodes a hepatic protein, were affected most negatively in terms of sleep duration and efficiency, again indicating that patient-specific characteristics increase vulnerability to sleep difficulties under certain conditions and treatments [76].
There is a consensus that steroids lead to increased daytime napping and nighttime insomnia; as they are a mainstay of cancer treatment, practitioners should be aware of the sleep disruption that they can cause. As with chemotherapy, more research is necessary to ascertain the impact of individual steroid agents on sleep independent of other factors, as well as to better identify patient characteristics that may lead to greater vulnerability to sleep disturbance with steroids.
2.5. Effects of Radiation Therapy on Sleep
Few works in the literature exist regarding the direct effects of radiation therapy on sleep in pediatric cancer patients. Cranial radiation affects brain structures, such as the suprachiasmatic nucleus that regulates sleep and wakefulness, through direct injury to those structures [77][32][78][79]. Effects of such radiation-induced injuries to the sleep-regulating structures in the hypothalamic–pituitary axis, including the SCN, may persist into adulthood [32][55][78][79][80].
Endocrine changes secondary to radiation therapy may also have a role in affecting sleep years after treatment. For example, adults who were treated as children with cranial radiation therapy (CRT) and reported difficulty with wakefulness after sleep demonstrated lower growth-hormone peak levels after an insulin challenge [78]. Considering that the hypothalamus regulates growth hormone release and sleep, both functions that are potentially affected by CRT, further research is needed to determine the interplay not only between the disruption of growth hormone release due to CRT and sleep but the long-term impact of CRT on the brain structures involved in the hormonal control of sleep [81][82].
2.6. Effects of the Hospital Environment on Sleep
The hospital environment leads to shorter total sleep duration and difficulty remaining asleep during inpatient stays [17][60][83][84][85]. Hospitalized pediatric cancer patients with disrupted sleep experience greater levels of fatigue and tend to sleep longer during the day to compensate for nighttime disruptions that are at least partly related to nighttime room entries and exits, excessive lights, and noise [17][18][86]. When comparing specific cancers, Graef et al. described that hospitalized pediatric patients between the ages of 4 and 19 years old with medulloblastoma exhibited drastically shorter sleep durations and higher sleep onset latency than pediatric patients with ALL or non-CNS solid tumor patients [87]. Such studies highlight the potential for optimizing the inpatient environment and clinical practices as intervention targets to improve sleep in pediatric cancer inpatients.
References
- Cunningham, M.R.; Walton, M.A.; Carter, P.M. The Major Causes of Death in Children and Adolescents in the United States. New Engl. J. Med. 2018, 379, 2468–2475.
- Rosen, G.; Brand, S.R. Sleep in children with cancer: Case review of 70 children evaluated in a comprehensive pediatric sleep center. Support. Care Cancer 2011, 19, 985–994.
- O’Leary, M.; Krailo, M.; Anderson, J.R.; Reaman, G.H. Progress in Childhood Cancer: 50 Years of Research Collaboration, a Report from the Children’s Oncology Group. Semin. Oncol. 2008, 35, 484–493.
- Hart, N.C.; Palermo, T.M.; Rosen, C.L. Health-related quality of life among children presenting to a pediatric sleep disorders clinic. Behav. Sleep. Med. 2005, 3, 4–17.
- Walker, A.J.; Johnson, K.P.; Miaskowski, C.; Lee, K.A.; Gedaly-Duff, V. Sleep quality and sleep hygiene behaviors of adolescents during chemotherapy. J. Clin. Sleep Med. 2010, 6, 439–444.
- Mandrell, B.N.; Wise, M.; Schoumacher, R.A.; Pritchard, M.; West, N.; Ness, K.K.; Crabtree, V.M.; Merchant, T.E.; Morris, B. Excessive daytime sleepiness and sleep-disordered breathing disturbances in survivors of childhood central nervous system tumors. Pediatr. Blood Cancer 2012, 58, 746–751.
- Paruthi, S.; Brooks, L.J.; D’Ambrosio, C.; Hall, W.A.; Kotagal, S.; Lloyd, R.M.; Malow, B.A.; Maski, K.; Nichols, C.; Quan, S.F.; et al. Consensus Statement of the American Academy of Sleep Medicine on the Recommended Amount of Sleep for Healthy Children: Methodology and Discussion. J. Clin. Sleep Med. 2016, 12, 1549–1561.
- Daniel, C.L.; Aggarwal, R.; Schwartz, L.A. Sleep in Adolescents and Young Adults in the Year After Cancer Treatment. J. Adolesc. Young Adult Oncol. 2017, 6, 560–567.
- Chen-Lim, L.M.; Davis, K.F. Children With Cancer: Should You be Concerned About Their Sleep? J. Pediatr. Nurs. Nurs. Care Child. Fam. 2010, 25, e15.
- Wright, M. Children receiving treatment for cancer and their caregivers: A mixed methods study of their sleep characteristics. Pediatr. Blood Cancer 2011, 56, 638–645.
- Fortmann, J.; Fisher, A.; Hough, R.; Gregory, A.; Pugh, G. Sleep Quality Among Teenagers and Young Adults with Cancer. Cancer Nurs. 2021, 44, 13–19.
- Allen, J.M.; Graef, D.M.; Ehrentraut, J.H.; Tynes, B.L.; Crabtree, V.M. Sleep and Pain in Pediatric Illness: A Conceptual Review. CNS Neurosci. Ther. 2016, 22, 880–893.
- Alvaro, P.K.; Roberts, R.; Harris, J.K. A Systematic Review Assessing Bidirectionality between Sleep Disturbances, Anxiety, and Depression. Sleep 2013, 36, 1059–1068.
- Clanton, N.R.; Klosky, J.L.; Li, C.; Jain, N.; Srivastava, D.K.; Mulrooney, D.; Zeltzer, L.; Stovall, M.; Robison, L.L.; Krull, K.R. Fatigue, vitality, sleep, and neurocognitive functioning in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Cancer 2011, 117, 2559–2568.
- Kaleyias, J.; Manley, P.; Kothare, S.V. Sleep Disorders in Children with Cancer. Semin. Pediatr. Neurol. 2012, 19, 25–34.
- Zupanec, S.; Jones, H.; Stremler, R. Sleep Habits and Fatigue of Children Receiving Maintenance Chemotherapy for All and Their Parents. J. Pediatr. Oncol. Nurs. 2010, 27, 217–228.
- Lee, S.; Narendran, G.; Tomfohr-Madsen, L.; Schulte, F. A systematic review of sleep in hospitalized pediatric cancer patients. Psycho-Oncology 2017, 26, 1059–1069.
- Hinds, P.S.; Hockenberry, M.; Rai, S.N.; Zhang, L.; Razzouk, B.I.; McCarthy, K.; Cremer, L.; Rodriguez-Galindo, C. Nocturnal awakenings, sleep environment interruptions, and fatigue in hospitalized children with cancer. Oncol. Nurs. Forum. 2007, 34, 393–402.
- Steur, L.M.H.; Kolk, R.H.E.; Mooij, F.; de Vries, R.; Grootenhuis, M.A.; Kaspers, G.J.L.; Van Litsenburg, R.R.L. The prevalence and risk factors of sleep problems in pediatric oncology: Its effect on quality of life during and after cancer treatment. Expert Rev. Qual. Life Cancer Care 2016, 1, 153–171.
- Daniel, L.C.; Schwartz, L.A.; Mindell, J.A.; Tucker, C.A.; Barakat, L.P. Initial Validation of the Sleep Disturbances in Pediatric Cancer Model. J. Pediatric Psychol. 2016, 41, 588–599.
- Rahman, S.; Al-Hallaj, A.S.; Nedhi, A.; Gmati, G.; Ahmed, K.; Jama, H.A.; Trivilegio, T.; Mashour, A.; Askar, A.A. Differential Expression of Circadian Genes in Leukemia and a Possible Role for Sirt1 in Restoring the Circadian Clock in Chronic Myeloid Leukemia. J. Circadian Rhythm. 2017, 15, 3.
- Padmanabhan, K.; Billaud, M. Desynchronization of Circadian Clocks in Cancer: A Metabolic and Epigenetic Connection. Front. Endocrinol. 2017, 8, 136.
- Fu, L.; Kettner, N.M. The circadian clock in cancer development and therapy. Prog. Mol. Biol. Transl. Sci. 2013, 119, 221–282.
- Savvidis, C.; Koutsilieris, M. Circadian rhythm disruption in cancer biology. Mol. Med. 2012, 18, 1249–1260.
- Haus, E.L.; Smolensky, M.H. Shift work and cancer risk: Potential mechanistic roles of circadian disruption, light at night, and sleep deprivation. Sleep Med. Rev. 2013, 17, 273–284.
- Wang, V.; Chou, D. Anterior C1-2 osteochondroma presenting with dysphagia and sleep apnea. J. Clin. Neurosci. 2009, 16, 581–582.
- Mulrooney, D.A.; Ness, K.K.; Neglia, J.P.; Whitton, J.A.; Green, D.M.; Zeltzer, L.K.; Robison, L.L.; Mertens, A.C. Fatigue and sleep disturbance in adult survivors of childhood cancer: A report from the childhood cancer survivor study (CCSS). Sleep 2008, 31, 271–281.
- Nunes, M.D.R.; Jacob, E.; Adlard, K.; Secola, R.; Nascimento, L. Fatigue and Sleep Experiences at Home in Children and Adolescents With Cancer. Oncol. Nurs. Forum. 2015, 42, 498–506.
- Meeske, K.A.; Siegel, S.E.; Globe, D.R.; Mack, W.J. Prevalence and Correlates of Fatigue in Long-Term Survivors of Childhood Leukemia. J. Clin. Oncol. 2005, 23, 5501–5510.
- Russell, K.B.; Merz, E.L.; Reynolds, K.; Schulte, F.; Tomfohr-Madsen, L. Sleep Disturbances in Survivors of Pediatric Acute Lymphoblastic Leukemia and Their Siblings. J. Pediatr. Psychol. 2020, 45, 707–716.
- Rach, A.M.; Crabtree, V.M.; Brinkman, T.M.; Zeltzer, L.; Marchak, J.G.; Srivastava, D.; Tynes, B.; Lai, J.S.; Robison, L.L. Predictors of fatigue and poor sleep in adult survivors of childhood Hodgkin’s lymphoma: A report from the Childhood Cancer Survivor Study. J. Cancer Surviv. 2017, 11, 256–263.
- Nolan, V.G.; Gapstur, R.; Gross, C.R.; DeSain, L.A.; Neglia, J.P.; Gajjar, A.; Klosky, J.L.; Merchant, T.E.; Stovall, M.; Ness, K.K. Sleep disturbances in adult survivors of childhood brain tumors. Qual. Life Res. 2013, 22, 781–789.
- Tutelman, P.R.; Chambers, C.T.; Stinson, J.N.; Parker, J.A.; Fernandez, C.V.; Witteman, H.O.; Nathan, P.C.; Barwick, M.; Campbell, F.; Jibb, L.A.; et al. Pain in Children with Cancer: Prevalence, Characteristics, and Parent Management. Clin. J. Pain 2018, 34, 198–206.
- Oeffinger, K.C.; Mertens, A.C.; Sklar, C.A.; Yasui, Y.; Fears, T.; Stovall, M.; Vik, T.A.; Inskip, P.D.; Robison, L.L. Obesity in Adult Survivors of Childhood Acute Lymphoblastic Leukemia: A Report from the Childhood Cancer Survivor Study. J. Clin. Oncol. 2003, 21, 1359–1365.
- Nathan, P.C.; Nachman, A.; Sutradhar, R.; Kurdyak, P.; Pole, J.D.; Lau, C.; Gupta, S. Adverse mental health outcomes in a population-based cohort of survivors of childhood cancer. Cancer 2018, 124, 2045–2057.
- Lu, Q.; Krull, K.R.; Leisenring, W.; Owen, J.E.; Kawashima, T.; Tsao, J.C.I.; Zebrack, B.; Mertens, A.; Armstrong, G.T.; Stovall, M.; et al. Pain in long-term adult survivors of childhood cancers and their siblings: A report from the Childhood Cancer Survivor Study. Pain 2011, 152, 2616–2624.
- Zhang, F.F.; Parsons, S.K. Parsons, Obesity in Childhood Cancer Survivors: Call for Early Weight Management. Adv. Nutr. 2015, 6, 611–619.
- Rosen, G.M.; Bendel, A.E.; Neglia, J.P.; Moertel, C.L.; Mahowald, M. Sleep in children with neoplasms of the central nervous system: Case review of 14 children. Pediatrics 2003, 112, e46–e54.
- Rosen, G.M.; Shor, A.C.; Geller, T.J. Sleep in children with cancer. Curr. Opin. Pediatr. 2008, 20, 676–681.
- Zhou, E.S.; Recklitis, C.J. Insomnia in adult survivors of childhood cancer: A report from project REACH. Support. Care Cancer 2014, 22, 3061–3069.
- DelRosso, L.M.; Martin, K.; Ferri, R. A not so incidental finding in a 12-year old with sleepiness and headaches. Sleep Med. 2018, 43, 31–33.
- Fujimoto, K.; Kasai, H.; Kunii, R.; Terada, J.; Tatsumi, K. Obstructive Sleep Apnea in a Severely Obese Child with Combined Central Sleep Apnea and Sleep-Related Hypoventilation Disorder Caused by a Medullary Tumor. J. Clin. Sleep Med. 2018, 14, 1071–1074.
- Ferri, L.; Filardi, M.; Moresco, M.; Pizza, F.; Vandi, S.; Antelmi, E.; Toni, F.; Zucchelli, M.; Pierangeli, G.; Plazzi, G. Non-24-Hour Sleep-Wake Rhythm Disorder and Melatonin Secretion Impairment in a Patient With Pineal Cyst. J. Clin. Sleep Med. 2017, 13, 1355–1357.
- Jacola, L.M.; Conklin, H.M.; Scoggins, M.A.; Ashford, J.M.; Merchant, T.E.; Mandrell, B.N.; Ogg, R.J.; Curtis, E.; Wise, M.S.; Indelicato, D.J.; et al. Investigating the Role of Hypothalamic Tumor Involvement in Sleep and Cognitive Outcomes Among Children Treated for Craniopharyngioma. J. Pediatr. Psychol. 2016, 41, 610–622.
- Gapstur, R.; Gross, C.R.; Ness, K. Factors associated with sleep-wake disturbances in child and adult survivors of pediatric brain tumors: A review. Oncol. Nurs. Forum. 2009, 36, 723–731.
- Müller, H.L.; Handwerker, G.; Wollny, B.; Faldum, A.; Sörensen, N. Melatonin secretion and increased daytime sleepiness in childhood craniopharyngioma patients. J. Clin. Endocrinol. Metab. 2002, 87, 3993–3996.
- Pickering, L.; Jennum, P.; Gammeltoft, S.; Poulsgaard, L.; Feldt-Rasmussen, U.; Klose, M. Sleep-wake and melatonin pattern in craniopharyngioma patients. Eur. J. Endocrinol. 2014, 170, 873–884.
- Ito, K.; Murofushi, T.; Mizuno, M.; Semba, T. Pediatric brain stem gliomas with the predominant symptom of sleep apnea. Int. J. Pediatr. Otorhinolaryngol. 1996, 37, 53–64.
- Marcus, C.L.; Trescher, W.H.; Halbower, A.C.; Lutz, J. Secondary narcolepsy in children with brain tumors. Sleep 2002, 25, 435–439.
- Weil, A.G.; Muir, K.; Hukin, J.; Desautels, A.; Martel, V.; Perreault, S. Narcolepsy and Hypothalamic Region Tumors: Presentation and Evolution. Pediatr. Neurol. 2018, 84, 27–31.
- Lee, A.; Chen, M.L.; Abeshaus, S.; Poliakov, A.; Ojemann, J.G. Posterior fossa tumors and their impact on sleep and ventilatory control: A clinical perspective. Respir. Physiol. Neurobiol. 2013, 189, 261–271.
- Packer, R.J. Childhood brain tumors: Accomplishments and ongoing challenges. J. Child. Neurol. 2008, 23, 1122–1127.
- Kathy, R.; Anna, G.; Gallicchio, L.; Gamaldo, C. Sleep disordered breathing risk in childhood cancer survivors: An exploratory study. Pediatr. Blood Cancer 2015, 62, 693–697.
- Fisher, R.S.; Rausch, J.R.; Ferrante, A.C.; Prussien, K.V.; Olshefski, R.S.; Vannatta, K.A.; Compas, B.E.; Gerhardt, C.A. Trajectories of health behaviors across early childhood cancer survivorship. Psychooncology 2019, 28, 68–75.
- Manley, P.E.; McKendrick, K.; McGillicudy, M.; Chi, S.N.; Kieran, M.W.; Cohen, L.E.; Kothare, S.; Michael Scott, R.; Goumnerova, L.C.; Sun, P.; et al. Sleep dysfunction in long term survivors of craniopharyngioma. J. Neurooncol. 2012, 108, 543–549.
- Verberne, L.M.; Maurice-Stam, H.; Grootenhuis, M.A.; Van Santen, H.M.; Schouten-Van Meeteren, A.Y.N. Sleep disorders in children after treatment for a CNS tumour. J. Sleep Res. 2012, 21, 461–469.
- Brimeyer, C.; Adams, L.; Zhu, L.; Srivastava, D.K.; Wise, M.; Hudson, M.M.; Crabtree, V.M. Sleep complaints in survivors of pediatric brain tumors. Support Care Cancer 2016, 24, 23–31.
- O’Gorman, C.S.; Simoneau-Roy, J.; Pencharz, P.; MacFarlane, J.; MacLusky, I.; Narang, I.; Adeli, K.; Daneman, D.; Hamilton, J. Sleep-Disordered Breathing Is Increased in Obese Adolescents with Craniopharyngioma Compared with Obese Controls. J. Clin. Endocrinol. Metab. 2010, 95, 2211–2218.
- Lipton, J.; Megerian, J.T.; Kothare, S.V.; Cho, Y.J.; Shanahan, T.; Chart, H.; Ferber, R.; Adler-Golden, L.; Cohen, L.E.; Czeisler, C.A.; et al. Melatonin deficiency and disrupted circadian rhythms in pediatric survivors of craniopharyngioma. Neurology 2009, 73, 323–325.
- Hockenberry, M.J.; Hooke, M.C.; Gregurich, M.A.; McCarthy, K.; Sambuco, G.; Krull, K. Symptom clusters in children and adolescents receiving cisplatin, doxorubicin, or ifosfamide. Oncol. Nurs. Forum. 2010, 37, E16–E27.
- Ameringer, S.; Elswick, R.K., Jr.; Shockey, D.P.; Dillon, R. A pilot exploration of symptom trajectories in adolescents with cancer during chemotherapy. Cancer Nurs. 2013, 36, 60–71.
- Erickson, J.M.; Beck, S.L.; Christian, B.R.; Dudley, W.; Hollen, P.J.; Albritton, K.A.; Sennett, M.; Dillon, R.L.; Godder, K. Fatigue, sleep-wake disturbances, and quality of life in adolescents receiving chemotherapy. J. Pediatr. Hematol. Oncol. 2011, 33, e17–e25.
- Vallance, K.; Yang, J.; Li, J.; Crabtree, V.M.; Hinds, P.S.; Mandrell, B.N. Disturbed sleep in pediatric patients with leukemia: The potential role of interleukin-6(-174GC) and tumor necrosis factor (-308GA) polymorphism. Oncol. Nurs. Forum. 2011, 38, E365–E372.
- Cheung, Y.T.; Brinkman, T.M.; Mulrooney, D.A.; Mzayek, Y.; Liu, W.; Banerjee, P.; Panoskaltsis-Mortari, A.; Srivastava, D.; Pui, C.H.; Robison, L.L.; et al. Impact of sleep, fatigue, and systemic inflammation on neurocognitive and behavioral outcomes in long-term survivors of childhood acute lymphoblastic leukemia. Cancer 2017, 123, 3410–3419.
- Cheung, Y.T.; Lim, S.R.; Ho, H.K.; Chan, A. Cytokines as mediators of chemotherapy-associated cognitive changes: Current evidence, limitations and directions for future research. PLoS ONE 2013, 8, e81234.
- Astill, R.G.; Van der Heijden, K.B.; Van Ijzendoorn, M.H.; Van Someren, E.J. Sleep, cognition, and behavioral problems in school-age children: A century of research meta-analyzed. Psychol. Bull. 2012, 138, 1109–1138.
- Millman, R.P. Excessive sleepiness in adolescents and young adults: Causes, consequences, and treatment strategies. Pediatrics 2005, 115, 1774–1786.
- Moser, N.J.; Phillips, B.A.; Guthrie, G.; Barnett, G. Effects of dexamethasone on sleep. Pharmacol. Toxicol. 1996, 79, 100–102.
- Born, J.; DeKloet, E.R.; Wenz, H.; Kern, W.; Fehm, H.L. Gluco-and antimineralocorticoid effects on human sleep: A role of central corticosteroid receptors. Am. J. Physiol. 1991, 260, E183–E188.
- Rosen, G.; Harris, A.K.; Liu, M.; Dreyfus, J.; Krueger, J.; Messinger, Y.H. The effects of dexamethasone on sleep in young children with acute lymphoblastic leukemia. Sleep Med. 2015, 16, 503–509.
- Daniel, L.C.; Li, Y.; Kloss, J.D.; Reilly, A.F.; Barakat, L.P. The impact of dexamethasone and prednisone on sleep in children with acute lymphoblastic leukemia. Support. Care Cancer 2016, 24, 3897–3906.
- Rogers, V.E.; Zhu, S.; Ancoli-Israel, S.; Hinds, P.S. Impairment in circadian activity rhythms occurs during dexamethasone therapy in children with leukemia. Pediatr. Blood Cancer 2014, 61, 1986–1991.
- Sanford, S.D.; Okuma, J.O.; Pan, J.; Srivastava, D.K.; West, N.; Farr, L.; Hinds, P.S. Gender differences in sleep, fatigue, and daytime activity in a pediatric oncology sample receiving dexamethasone. J. Pediatr. Psychol. 2008, 33, 298–306.
- Zoorob, R.; Cender, D. A different look at corticosteroids. Am. Fam. Physician 1998, 58, 443–450.
- Balis, F.M.; Lester, C.M.; Chrousos, G.P.; Heideman, R.L.; Poplack, D.G. Differences in cerebrospinal fluid penetration of corticosteroids: Possible relationship to the prevention of meningeal leukemia. J. Clin. Oncol. 1987, 5, 202–207.
- Vallance, K.; Liu, W.; Mandrell, B.N.; Panetta, J.C.; Gattuso, J.S.; Hockenberry, M.; Zupanec, S.; Yang, L.; Yang, J.; Hinds, P.S. Mechanisms of dexamethasone-induced disturbed sleep and fatigue in paediatric patients receiving treatment for ALL. Eur. J. Cancer 2010, 46, 1848–1855.
- Zeltzer, L.K.; Recklitis, C.; Buchbinder, D.; Zebrack, B.; Casillas, J.; Tsao, J.C.; Lu, Q.; Krull, K. Psychological status in childhood cancer survivors: A report from the Childhood Cancer Survivor Study. J. Clin. Oncol. 2009, 27, 2396–2404.
- Van Someren, E.J.; Swart-Heikens, J.; Endert, E.; Bisschop, P.H.; Swaab, D.F.; Bakker, P.J.; Romijn, J.A.; Fliers, E. Long-term effects of cranial irradiation for childhood malignancy on sleep in adulthood. Eur. J. Endocrinol. 2004, 150, 503–510.
- Khan, R.B.; Merchant, T.E.; Sadighi, Z.S.; Bello, M.S.; Lu, Z.; Sykes, A.; Wise, M.S.; Crabtree, V.M.; Zabrowski, J.; Simmons, A.; et al. Prevalence, risk factors, and response to treatment for hypersomnia of central origin in survivors of childhood brain tumors. J. Neurooncol. 2018, 136, 379–384.
- Follin, C.; Erfurth, E.M. Long-Term Effect of Cranial Radiotherapy on Pituitary-Hypothalamus Area in Childhood Acute Lymphoblastic Leukemia Survivors. Curr. Treat Options Oncol. 2016, 17, 50.
- Merchant, T.E.; Rose, S.R.; Bosley, C.; Wu, S.; Xiong, X.; Lustig, R.H. Growth hormone secretion after conformal radiation therapy in pediatric patients with localized brain tumors. J. Clin. Oncol. 2011, 29, 4776–4780.
- Steiger, A.; Holsboer, F. Neuropeptides and human sleep. Sleep 1997, 20, 1038–1052.
- Cowherd, E.L.; Sutton, A.G.; Vincent, J.O.; Humphries, M.S.; Ritter, V.; Fine, J.; Steiner, M.J. Timing and Duration of Sleep in Hospitalized Children: An Observational Study. Hosp. Pediatr. 2019, 9, 333–339.
- Meltzer, L.J.; Davis, K.F.; Mindell, J.A. Patient and parent sleep in a children’s hospital. Pediatr. Nurs. 2012, 38, 64–71.
- Linder, L.A.; Christian, B.J. Nighttime sleep disruptions, the hospital care environment, and symptoms in elementary school-age children with cancer. Oncol. Nurs. Forum. 2012, 39, 553–561.
- Setoyama, A.; Ikeda, M.; Kamibeppu, K. Objective assessment of sleep status and its correlates in hospitalized children with cancer: Exploratory study. Pediatr. Int. 2016, 58, 842–849.
- Graef, D.M.; Crabtree, V.M.; Srivastava, D.K.; Li, C.; Pritchard, M.; Hinds, P.S.; Mandrell, B. Sleep and mood during hospitalization for high-dose chemotherapy and hematopoietic rescue in pediatric medulloblastoma. Psychooncology 2018, 27, 1847–1853.
More
Information
Subjects:
Oncology; Pediatrics
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
11 Jan 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




