| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Unai Mendibil | + 1748 word(s) | 1748 | 2020-08-11 09:43:25 |
Video Upload Options
The extracellular matrix (ECM) is a complex network with multiple functions, including specific functions during tissue regeneration. Precisely, the properties of the ECM have been thoroughly used in tissue engineering and regenerative medicine research, aiming to restore the function of damaged or dysfunctional tissues. Tissue decellularization is gaining momentum as a technique to obtain potentially implantable decellularized extracellular matrix (dECM) with well-preserved key components. Interestingly, the tissue-specific dECM is becoming a feasible option to carry out regenerative medicine research, with multiple advantages compared to other approaches. We recently published an overview of the most common methods used to obtain the dECM from specific tissues[1]. Here we provide a summary from that report as a helpful guide for future research development.
1. Introduction
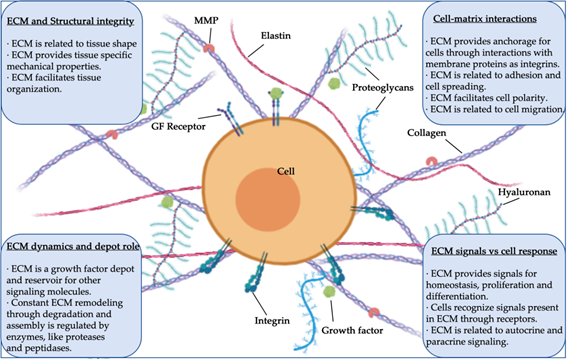
In many adult animal tissues, the main component in terms of volume is not the cells, but the cell-secreted three-dimensional (3D) structure known as the extracellular matrix (ECM). Structural and specialized proteins and proteoglycans are some of the ECM’s macromolecular components, and they interact in this network with multiple key roles and functions. In this way, the ECM serves as the environment in which tissue-resident cells attach, communicate, and interact, thereby regulating cell dynamics and behavior, and contributing to the maintenance of tissue-specific cell phenotypes and functions (Figure 1).
Figure 1. Structure, components, and functions of extracellular matrix (ECM) (MMP, matrix metalloprotease; GF, growth factor).
The properties of the ECM have been thoroughly used in tissue engineering and regenerative medicine research, aiming to restore the function of damaged or dysfunctional tissues. In this context, applications of ECM-derived components are multiple, from in vitro stem cell basic research to clinical settings.Often, strategies are based on specific ECM components, such as collagens, fibrin, hyaluronic acid, or even cell-culture-derived ECM. Using these materials, multiple fabrication techniques have been implemented to generate successful ECM-derived biomimetic structures such as hydrogels, or even more sophisticated engineering approaches such as micropatterned surfaces, electrospinning, 3D printing, and bioprinting-designed scaffolds, among others.
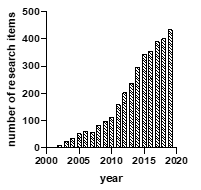
Interestingly, tissue decellularization is becoming a common technique to obtain decellularized extracellular matrix (dECM) and it is a research field gaining momentum in recent years (Figure 2). dECM is usually obtained by chemical, enzymatic, and/or physical decellularization methods, developed to eliminate the cells and their waste, mainly DNA. dECM-related advantages are often associated with better performance and applicability as implants for tissue repair, and also with better mechanical/biochemical properties for the intended use. Initially, research in this field was focused precisely on developing proper decellularization methods and techniques, while more recently, the field is moving to implement approaches related to bioengineering and which tackle applied research aims.
Figure 2. Research items per year with the words “decellularization” or “decellularized” in the title (Source: https://scholar.google.es "allintitle: decellularized OR decellularization").
Notably, regenerative medicine research can now take advantage of approaches based on the use of the targeted tissue-specific dECM. The ECM is different from one tissue to another, and therefore, decellularization methods and techniques to obtain dECM have been extensively studied and tissue-specifically improved, aiming to preserve the ECM molecules and structures relevant for the intended use.
2. Organ Decellularization and Tissue Decellularization Approaches for Biomedical Applications
Whole tissue or intact tissue pieces or sections are a common starting point for decellularization, especially when the final purpose is whole organ decellularization for bioengineering purposes. In these cases, the resulting dECM is a tissue scaffold generally created to keep its structure as intact as possible. The bigger the tissue pieces, the longer it takes to make sure that they are completely decellularized. Moreover, the longer the period of chemicals and enzymatic reactions, the higher the chance of damaging the ECM components.
In tissue pieces, it is easy to assess by histology the decellularization and integrity of the remaining ECM. It is important to quantify the DNA content by molecular techniques, with the safe limit of DNA content in a decellularized tissue established as below 50 pg DNA per milligram of dry tissue. Regarding macromolecules, they need to be assessed both in quantity and quality by histology, spectrophotometry, and other techniques, while the other ECM components as growth factors may need to be assessed and quantified as well.
Tissue decellularization is achieved using as starting material tissues treated with mechanical or chemical methods for tissue grinding, pulverization, or homogenization before decellularization. This approach is gaining relevance, especially in strategies aiming to use the dECM in postprocessing fabrication approaches (hydrogels, 3D printing, electrospinning, and similar). The outcome of decellularization in these cases is dECM powder, an intermediate product mainly used to artificially generate further ECM coatings or 3D structures. This aims to use the properties of the relevant ECM components to improve biocompatibility, adhesion, differentiation, and/or other properties or purposes.The applications of decellularized materials and matrices in regenerative medicine context are multiple, including clinically used implantable materials, and continue to expand.
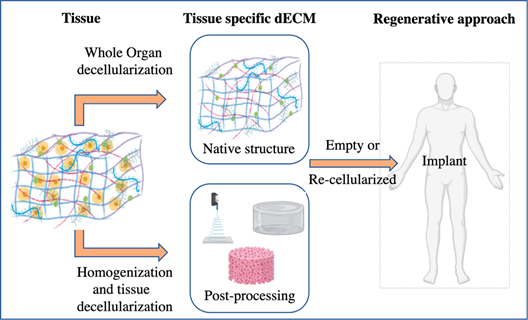
In any case, the decellularization procedure selected and the further characterization needs depend on the final aim and approach (Figure 3). Whole organ, tissue pieces, or powdered ECM are the most common starting materials and multiple possible decellularization methods can be applied, all of them with advantages and disadvantages to be taken into account in light of the specific aim and context.
Figure 3. Schematic of organ decellularization and tissue decellularization approaches.
3. ECM Decellularization and Sterilization Methods
Most protocols describe combinatorial and sequential use of different physical, chemical, and enzymatic techniques in order to achieve tissue-specific decellularization. In general, chemical and enzymatic techniques are mainly responsible for successful decellularization in most protocols. Physical techniques are generally used to complement chemical and enzymatic techniques and therefore increase the decellularization effects (Table 1). Physical techniques can produce damage in the matrix, while chemical techniques can produce reactions that change the chemical composition of the ECM. For this reason, setting up the decellularization protocol is of paramount importance in each specific approach.
3.1. Chemical and Enzymatic Methods for Decellularization
Detergents are chemical agents used to solubilize cell membranes and to dissociate their inner structure. Among them, Triton X-100 is the most commonly used detergent in decellularization processes. It is an effective detergent to eliminate cells from many tissues, but it is generally avoided in tissues with glycosaminoglycans (GAGs) as a key component in their matrix.
Enzymes are also used in most decellularization protocols, mainly to eliminate cell waste and other undesirable components of the ECM. However enzymatic treatments can often lead to additional problems related to enzyme removal. Among such enzymes, trypsin is the most commonly used in decellularization procedures.
The treatment of the DNA waste is of paramount relevance in all decellularization processes, due to the tendency of the nuclear material to remain stuck to ECM proteins. In this sense, endonucleases and exonucleases are other kinds of enzymes that are of great use to eliminate the waste of nuclear components.
3.2. Physical Methods for Decellularization
Physical techniques are not enough to decellularize the tissue, but they can help in combination with chemical and enzymatic processes. The most commonly used physical technique is snap freezing, or freeze–thawing, as the first step of a decellularization process. By freezing tissue, intracellular ice crystals are formed, thereby disrupting cellular membranes and causing cell lysis. Mechanical agitation and sonication are also useful in combination with a chemical treatment to assist in cell lysis and the removal of cellular debris.
Table 1. Methods used in decellularization processes.
|
Methods |
Mechanism |
Side Effects on the ECM |
|
Chemical |
|
|
|
Acid; Base |
Solubilizes cytoplasmic components, disrupts nucleic acids |
Damages collagen and GAG |
|
Triton X-100 |
Breaks lipid–lipid and lipid–protein unions, while leaving the protein interactions untouched |
Not recommended for ECM where the lipids and GAG are important components |
|
SDS |
Liquefies the internal and external cell membranes |
Tends to denaturalize proteins and may induce nuclear and cytoplasmic waste in the remaining matrix |
|
Triton X-200 |
Similar to its X-100 counterpart. Very effective in some tissues |
Needs to be combined with a zwitterionic detergent to be effective. Damages the matrix in a similar way that SDS does. |
|
CHAPS |
Properties of ionic and nonionic detergents |
Similar damage level compared to Triton X-100 |
|
TBP |
Disrupts protein–protein interactions |
Variable results, collagen degradation but keeping the mechanical properties |
|
Hypertonic and hypotonic solutions |
Osmotic pressure to make the membrane explode |
High amount of cell waste in the remaining matrix |
|
Enzymes |
|
|
|
EDTA, EGTA |
Breaks cell adhesion to matrix. It is usually combined with trypsin |
Does not actually kill the cells |
|
Trypsin |
Digestion of membrane proteins leading to cell dead |
Can damage the proteins in the ECM, in particular laminin and GAG |
|
Pepsin |
It targets peptide bounds |
Causes high damage in the ECM proteins if left for too long |
|
Endonucleases and Exonucleases |
Degradation of the nuclear material inside and outside of the nucleus |
Further cleaning and enzyme removal is required, as they may promote immune response |
|
Physical |
|
|
|
Freezing |
Crystals created in the freezing process destroy the cell membrane |
The overall protein structure of the ECM may be compromised |
|
Force |
Mechanical pressure can be enough to induce the lysis in some tissues |
Limited to tissues with hard structures, as it can greatly damage the ECM structure |
|
Agitation |
Commonly used to facilitate chemical agent infiltration and to induce cell lysis |
Aggressive processes like sonication can greatly damage the ECM |
|
Vacuum-assisted decellularization (VAD) |
Enables chemical agents to reach the more inner parts of the tissue |
It is not a decellularization method but a facilitator |
|
Hydrostatic pressure |
Applies high pressure to the tissue and induces cell lysis |
Excessive pressure can damage the structure |
3.3. Sterilization Methods
The dECM is commonly used in in vivo implantable approaches. Therefore, in order to prevent the transmission of pathogens, the dECM needs to be sterilized to eliminate any microorganisms and to prevent infections. There are some useful physical and chemical dECM sterilization techniques, mainly those using heat and those using radiation, although their suitability depends on multiple factors, which may need to be considered in each specific approach.
4. Decellularization methods by tissue
In order to choose a decellularization method, the tissue itself and the strategy to be followed have to be taken into consideration. Since each tissue has a different structure and composition, the decellularization methods have to be specifically selected and empirically tested. Therefore, the literature shows trends in the use of specific combinatorial physical and chemical approaches for each specific tissue and application.
The specific treatments in the cases of the following tissues: Bone, cartilage, adipose, skeletal muscle and tendons, cardiovascular, vascular, dermal, related to respiratory system, related to gastrointestinal tract, nervous, cornea and thymus can be found in the complete review[1].
References
- Unai Mendibil; Raquel Ruiz-Hernandez; Sugoi Retegi-Carrion; Nerea Garcia-Urquia; Beatriz Olalde-Graells; Ander Abarrategi; Tissue-Specific Decellularization Methods: Rationale and Strategies to Achieve Regenerative Compounds. International Journal of Molecular Sciences 2020, 21, 5447, 10.3390/ijms21155447.