1.1. SARS-CoV-2
The COVID-19 pandemic pointed out the importance of rapid, accurate, and on-demand detection of viruses such as the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Reverse-transcription quantitative real-time polymerase chain reaction (RT-qPCR) is still the gold standard test for the detection of this positive single-stranded RNA (ssRNA) virus responsible for more than 4.8 million deaths worldwide
[1]. This is while rapid detection tests based on the clustered regularly interspaced palindromic repeats of (CRISPR)/Cas proteins in combination with microfluidics are shown to be new promising approaches for pathogen detection
[2].
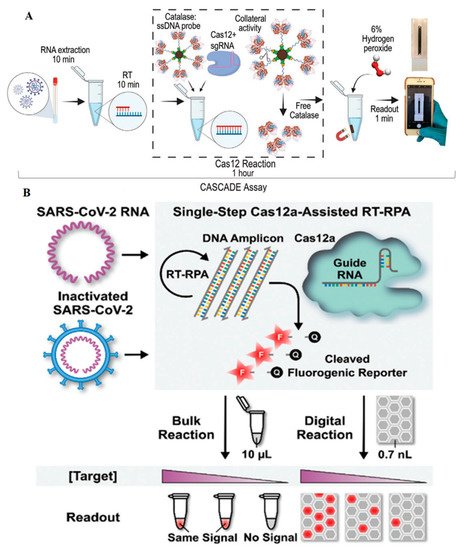
In this regard, Silva et al. developed a cellphone-based amplification-free system with CRISPR/CAS-dependent enzymatic (CASCADE) assay (
Figure 1A)
[3]. After RNA extraction from nasal swabs, the prepared sample was combined with reverse transcriptase (RT) for about 10 min to produce RNA/DNA hybrids. Afterward, the catalase-ssDNA probe bonded to the magnetic beads, and CRISPR/Cas12 protein was added to the reaction chamber for 1 h. Following the specific binding of CRISPR/Cas12 to the SARS-CoV-2 genome, the trans cleavage ability of Cas12a caused the cleavage of ssDNA and production of free catalase. After separating the catalase-ssDNA probes using magnetic beads, 6% hydrogen peroxidase solution was added to generate bubbles, which could be recorded by a smartphone camera. The optical signal generated by these bubbles appeared in 1 min and was simply and quantitatively measured by the smartphone without any need for external devices. CASCADE was capable of detecting down to 50 RNA copies per µL without any amplification; this capacity was reduced to five copies per reaction when an amplification step was added. It was concluded that CASCADE is a smartphone-based amplification-free detection platform with the minimum hardware needs. Removing the extraction step and providing an artificial intelligence (AI)-based image databank can help provide faster and more sensitive detection results.
Figure 1. (
A) CASCADE assay based on the trans cleavage activity of Cas12, in which the catalase enzyme produces gas bubbles for visual detection
[3]. (
B) deCOViD assay in which fluorophore-quencher probes break after the activation of Cas12. Cas12 trans cleavage activity and the microfluidics provided the digitized fluorescent signals
[4].
Another smartphone-based pathogen detection resource multiplier was developed using adversarial networks (SPyDERMAN) for intact and viral nucleic acid detection
[5]. The CRISPR/dCas9 protein used in this platform has catalytical inactivated cas proteins that selectively bind to the target sequence without any endonuclease activities. This platform, using a dataset library of adversarial neural network algorithms, could specifically detect five different viruses. These algorithms helped not only to standardize the shape and size of bubbles but also to reduce the differences in various phone cameras. An example of reaction fundamentals for SARS-CoV-2 will be discussed later in the paper. The microfluidic platform was made of poly (methyl methacrylate) sheets, which is a great idea for automated applications with lengthy extraction and amplification processes requiring smaller amounts of reagents. After RNA extraction and cDNA amplification, the mixture was added to a CRISPR/dCas9 solution. After proper incubation, platinum nanoparticles (PtNPs) bonded to monoclonal antibodies against Cas9 and a 6% hydrogen peroxidase solution was added. Later, the gas bubbles produced by the catalase-like activity of PtNPs were photographed.
A new CRISPR-based sensor known as digitization-enhanced CRISPR/Cas-assisted one-pot virus detection (deCOViD) was developed by Park et al. (
Figure 1B)
[4]. deCOViD is based on reverse transcription and recombinase polymerase amplification (RT-RPA), which uses fluorophore probes like Alexa647. After the production of DNA amplicons, CRISPR/Cas12a specifically cleaved the target DNA so that the fluorescent signals could be observed. Integrating this into a digitized microfluidic chip, besides minimizing the reagents and materials, increased the reaction speed thanks to the locally high concentration of nucleic acids in the small microfluidic wells. Using RT-RPA instead of traditional RT-PCR and transforming the platform into a microfluidic platform also reduced the detection time by up to 15 min. The system was also capable of reading the heat-inactivated virus without extracting RNA in 30 min. Since RNA extraction and purification were no longer needed, the detection time became shorter and LOD improved. CRISPR/Cas12a with a proper crRNA used in this research was responsible for the high specificity of the sensor. deCOViD has the lowest LOD in comparison with similar CRISPR-based platforms. The LOD was reported to be one genome equivalent (GE) per µL for SARS-CoV-2 genome detection and 20 GE per µL for the heat-inactivated SARS-CoV-2 samples.
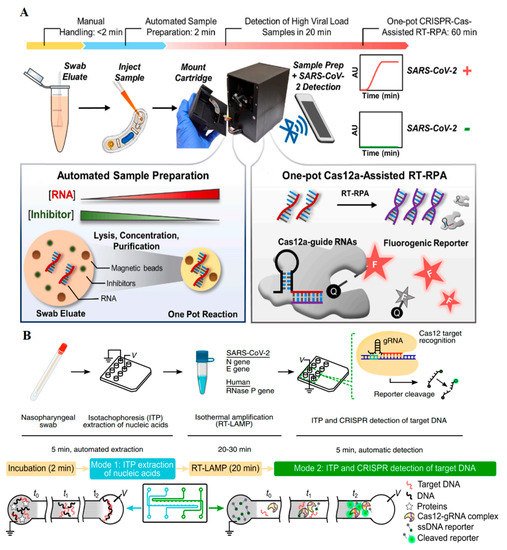
A novel SARS-CoV-2 POC, introduced by Chen et al., composed of miniaturized magnetic arms, fluorescent detector, and heating module (
Figure 2A)
[6]. The sensor known as POC-CRISPR worked in three main steps. Firstly, a nasopharyngeal swab was inserted into an elution tube containing detergent and magnetic beads buffers. The detergent caused virus decomposition and then the negatively-charged RNAs bonded to the positively-charged magnetic beads. Next the solution was transferred to the working cartridge and was placed in the droplet magneto-fluidic (DM) device, where the extraction, purification, and concentration processes happened all together. In this step, the magnetic beads separated the RNAs from other cell lysates and concentrated them by moving them to a new area in the microfluidic device. Following these processes, the purified RNA was produced using the RT-RPA and DNA amplicons. In this step, fluorescent signals were generated when fluorophore probes were released through DNA cleavage by the CRISPR/Cas12a. The signals were then detected by the DM device, and signals were wirelessly sent to a cellphone. The microprocessors embedded in the DM device accelerated this step. The POC-CRISPR was reported to have an acceptable LOD (1 GE per µL), require a low (100 µL) starting solution, and have a short duration of less than 30 min. In this system, the magnetic beads acted as the fundamentals for RNA purification and concentration along with shortening the purification time; they also enhanced the sensitivity thanks to their concentrating ability. Therefore, their applicability in high-tech sensors to increase the performance of CRISPR-based assays, especially in microfluidic devices, was shown.
Figure 2. (
A) POC-CRISPR assay with droplet microfluidics, in which RNAs are separated and concentrated using electrostatistical attachment to magnetic beads and fluorescent signals again using Cas12 trans cleavage
[6]. (
B) An electrokinetic microfluidic chip that creates isotachophoresis mobility in the microfluidic device for RNA separation and sensitivity enhancement
[7].
Electrokinetic microfluidic sensor coupled with CRISPR/Cas12 and reverse transcription-loop-mediated isothermal amplification (RT-LAMP) is another example (
Figure 2B)
[7]. This sensor was modified for SARS-CoV-2 detection using isotachophoresis (ITP) technique and an electrokinetic microfluidic chip. First, ITP-based ion mobility was generated in the microchannel embedded in the chip. The coexistence of a high-mobility leading (LE) and a low-mobility trailing (TE) buffer in a microfluidic channel resulted in different ion mobility in the sample following the application of an electric field. This phenomenon caused negatively charged nucleic acids to be fully separated from other parts of a cell lysate. After the nucleic acid separation and purification steps in a nasopharyngeal sample, the SARS-CoV-2 RNA genome was transformed into cDNA using the RT-LAMP system in the chip. After the amplification process, CRISPR/Cas12 specifically recognized the target sequence. Then ssDNA, which was attached to a fluorophore and a quencher, was cleaved by the trans cleavage ability of the CRISPR/Cas12 protein. By separating the fluorophore and the quencher pair, the fluorescent signals representing the desired genome, this process happened in 30–40 min with an LOD of 10 copies per µL. Automated reagent mixing, need for a low amount of reagents, and accurate detection are the main advantages of this sensor.
Puig et al. developed a fluorescent sensor called miSHERLOCK (minimally instrumented SHERLOCK). It worked based on the trans cleavage ability of the CRISPR/Cas13
[8]. This specific enzymatic test was implemented in a miniatured device that extracted and concentrated the viral RNA, needed for SARS-CoV-2 detection, in the saliva samples. The device consisted of two parts. In the first part, the saliva sample was inserted, and then the RNA molecules were extracted and concentrated using polyethersulfone (PES) membrane. After this step, the concentrated RNA met the CRISPR/Cas13 enzyme and other related cocktail factors in the second part of the two-chambered holder containing the fluorescent laser. Around 1 h later, the device was connected to a mobile as the fluorescent readout. This platform could detect three different variants of SARS-CoV-2 successfully with an average LOD of 1200 copies per mL for the universal variant. Albeit the sensitive and smart idea behind the miSHERLOCK, the need for 4 mL of saliva sample and the 55-min reaction time were among its shortcomings.
1.2. Ebola Virus
Ebola is a deadly virus from the Filoviridae family with a negative ssRNA genome. It was responsible for a deadly outbreak of a hemorrhagic fever syndrome between the years 2013 and 2020
[9]. While sequencing and PCR methods are the main detection methods of Ebola virus, POC devices with a need for minimum reagents and inexpensive materials seem to be required for possible future endemics.
A POC sensor for Ebola detection based on the CRISPR/Cas13a trans cleavage ability was developed by Qin et al.
[10]. In this attempt, a microfluidic chip with 24 parallel assays for the Ebola RNA and CRISPR-crRNA combinations was fabricated. During the free-amplification process, CRISPR/Cas13a-crRNA targeted the Ebola genome and after Cas13 activation, due to the trans cleavage ability of the Cas protein, the quenched RNA probes were cleaved, resulting in fluorescent signals. The reported LOD was 5.45 × 10
7 copies per mL in 5 min. The inexpensive POC sensor needed low sample volume. The short detection time, little background signals, and the free-amplification process were among its advantages. The high cross-reactivity reported in this platform could be due to the off-targets of CRISPR/Cas13a, and could be reduced by developing recombinant Cas’s proteins with fewer off-targets.
Smart nucleic acid response materials [PEG hydrogel with ssDNA linkers (PEG-ssDNA) and polyacrylamide hydrogel with DNA linkers (PA-DNA)] could also be fabricated for this purpose. PEG-ssDNA hydrogels absorb fluorophores and enzymes, while PA-DNA hydrogels entrap gold nanoparticles (AuNPs) and cells. When CRISPR/Cas12a proteins in the hydrogel structure meet the target sequence, their trans cleavage activity breaks the DNA linkers, changing the morphology of the hydrogels. It also results in the release of the fluorophores bonded to the linkers or entrapped AuNPs, producing color signals. Based on these fundamentals, a paper-based microfluidic system (µPAD) was fabricated for Ebola detection
[11]. The target genome was amplified by RT-RPA and inserted into the µPAD with embedded hydrogels. Based on the lateral flow (LF) mobility of the reaction molecules, different signals were obtained. The color- (fluorescent signals for the fluorophores, and visual ones for the AuNPs), electric- (by combining the µPAD system with an electrode-based chip for conductivity measurement), and visual microscopy-(morphological changes of hydrogels) based signals are various output signals leveraged from the described microfluidic system. Although the sensor could detect the Ebola genome at concentrations as low as 11 aM, the hydrogel alteration process was time-consuming and thus the response signals only appeared after a few hours, which is not suitable for rapid detection tests. Furthermore, due to the sensitivity of CRISPR/Cas proteins to temperature, this material may not work properly for long hours at room temperature.
1.3. Human Immunodeficiency (HIV)
HIV is a positive ssRNA virus that belongs to the Retrovirus family. The virus causes human acute immunodeficiency syndrome (AIDS) and has been responsible for a global pandemic with almost 30 million deaths during the past four decades. Although lifelong antiretroviral therapy (ART) is still its main treatment, there is still no efficient drug or vaccine on the market. As a result, fast and reliable detection methods remain crucial for such widespread syndrome to reduce its complications
[12].
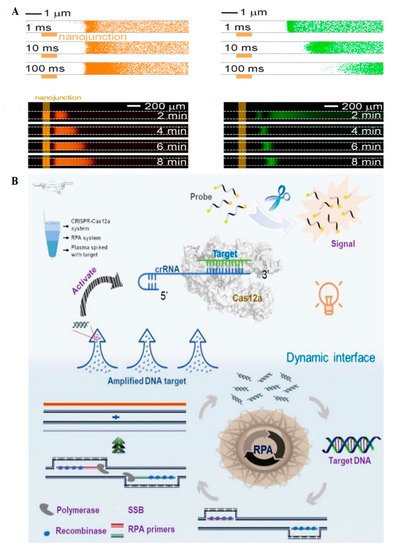
A sensor for C-C chemokine receptor type 5 (CCR5) gene detection related to the human immunodeficiency virus (HIV) was developed as a model by Lee et al. (
Figure 3A)
[13]. This gene expresses one of the most important entry receptors for HIV. The sensor contained a microfluidic platform with a microchannel creating ion concentration polarization (ICP). The detection process in the sensor was based on different mobility behaviors monitored by fluorophores. Following the ICP generation in the microchannels and based on different hydrodynamic behavior of negatively charged moieties that are targeted DNA in this case, two different mobility behaviors were reported. In the presence of free DNA (non-target sequence) in the microchannel, the propagation mobility behavior was reported. This is while the stacking mobility was observed in the presence of DNA-CRISPR/dCas9 (target sequence), which was later coupled with fluorophores (FAM and cy3). The sensor was able to detect 3 pM DNA in 100 min by a 18.4 pM/min concentration rate. It, however, has not been tested on real physiological samples, as the development of proper ICP in such complex samples may be challenging or affected by different factors. Moreover, unbalanced DNA and CRISPR/dCas9 molar ratios (for example, in the presence of very low or very high amounts of target DNA in the sample) could lead to false-negative and overload responses, respectively. Additionally, the preconcentration process reported along with the stacking behavior overcame the need for amplification in this sensor.
Figure 3. (
A) The microfluidic platform with a microchannel creating ion concentration polarization (ICP) shows two different mobility behaviors of bound and unbound DNA-CRISPR complexes. Green and orange colors represent the stacking and propagation mobility behaviors, respectively
[13]. (
B) The DAMR platform represents the dynamic diffusion of cleaved nucleic acid products after Cas12 trans cleavage activity through the multiphase sucrose solutions with different concentrations
[14].
1.4. Human Papillomavirus (HPV)
HPV is a circular double-stranded DNA widespread virus with nearly 200 subtypes. HPV infects basal epithelial cells, leading to certain cancers such as cervical cancer. More than 90% of cervical cancers are believed to be HPV related. HPV16 and HPV18 are the most common subtypes, mainly detected through sequencing or PCR methods
[15]. Despite the approval of a vaccine against HPV, early detection using rapid and non-invasive methods is still needed and yet challenging.
In an attempt by Yin et al., a POC device consisted of a three-chambered-microfluidic chip and a dynamic aqueous multiphase reaction (DAMR) system conjugated with RPA (for amplification), and CRISPR/Cas12a (for selective detection) was developed (
Figure 3B)
[14]. The dynamic aqueous multiphase was generated using different sucrose concentrations: DNA sample in the 40% sucrose concentration in the bottom phase, RPA reagent in 10% sucrose in the middle phase, and CRISPR/Cas12a with fluorophore-quencher DNA probes in the top phase. In this regard, DNA was dynamically diffused from the bottom phase (with higher sucrose concentrations) to the top one (with lower sucrose concentrations). It was amplified in the middle phase with RPA before being diffused to the third phase, where the fluorescent signals appeared thanks to the trans cleavage properties of CRISPR/Cas12a. In this phase, the fluorophores were released from the fluorophore-quencher probes following the selective DNA binding to the crRNA. This sensor detected HPV16 and HPV18 with 10 and 100 copies per mL, respectively, in 1 h. The use of multiphase dynamic mobility reduced the reaction time by about 100 times compared with the single-phase reaction methods. This novel multiplex sensor was easily fabricated. Pretreatment steps like extraction and purification, however, are still needed for real samples.